How to Build Quasi-Solid ZIBs: Gel Polymer Electrolytes — Recipes & Tests
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
ZIB Technology Evolution and Development Goals
Zinc-ion batteries (ZIBs) have emerged as a promising alternative to lithium-ion batteries due to their inherent safety, environmental friendliness, and cost-effectiveness. The evolution of ZIB technology can be traced back to the early 2000s when researchers began exploring zinc-based electrochemical systems as potential energy storage solutions. Initially, these systems faced significant challenges including zinc dendrite formation, limited cycle life, and poor rate capability, which hindered their practical applications.
The technological trajectory of ZIBs has witnessed several pivotal advancements over the past decade. The first generation of ZIBs primarily utilized aqueous electrolytes, which offered good ionic conductivity but suffered from water decomposition and electrode corrosion issues. The second generation introduced various additives and electrode modifications to mitigate these problems, extending battery lifespan and improving performance metrics.
A paradigm shift occurred with the introduction of quasi-solid electrolytes, particularly gel polymer electrolytes (GPEs), representing the third generation of ZIB technology. These GPEs combine the high ionic conductivity of liquid electrolytes with the mechanical stability of solid electrolytes, effectively addressing the dendrite growth problem while maintaining satisfactory electrochemical performance.
Current research trends are focused on developing advanced GPEs with optimized compositions, enhanced mechanical properties, and superior electrochemical stability. The integration of novel polymeric matrices, zinc salts, and functional additives has shown promising results in laboratory settings, pushing the boundaries of ZIB performance metrics.
The primary technological goals for quasi-solid ZIBs using GPEs include achieving energy densities exceeding 200 Wh/kg, power densities of over 500 W/kg, and cycle lives of more than 2000 cycles at 80% capacity retention. Additionally, there is a strong emphasis on developing GPEs that can operate effectively across a wide temperature range (-20°C to 60°C) to enable diverse applications from consumer electronics to grid-scale energy storage.
Future development trajectories are likely to explore bio-derived polymers for environmentally sustainable GPEs, multi-functional additives that simultaneously address multiple performance limitations, and composite electrolyte systems that synergistically combine the advantages of different materials. The ultimate goal is to create ZIB systems that can compete with or surpass lithium-ion batteries in specific application scenarios while offering superior safety profiles and lower environmental impact.
The convergence of materials science, electrochemistry, and manufacturing innovations is expected to accelerate the evolution of quasi-solid ZIBs, potentially leading to commercial viability within the next 5-10 years, particularly for applications where safety and cost considerations outweigh the need for extremely high energy density.
The technological trajectory of ZIBs has witnessed several pivotal advancements over the past decade. The first generation of ZIBs primarily utilized aqueous electrolytes, which offered good ionic conductivity but suffered from water decomposition and electrode corrosion issues. The second generation introduced various additives and electrode modifications to mitigate these problems, extending battery lifespan and improving performance metrics.
A paradigm shift occurred with the introduction of quasi-solid electrolytes, particularly gel polymer electrolytes (GPEs), representing the third generation of ZIB technology. These GPEs combine the high ionic conductivity of liquid electrolytes with the mechanical stability of solid electrolytes, effectively addressing the dendrite growth problem while maintaining satisfactory electrochemical performance.
Current research trends are focused on developing advanced GPEs with optimized compositions, enhanced mechanical properties, and superior electrochemical stability. The integration of novel polymeric matrices, zinc salts, and functional additives has shown promising results in laboratory settings, pushing the boundaries of ZIB performance metrics.
The primary technological goals for quasi-solid ZIBs using GPEs include achieving energy densities exceeding 200 Wh/kg, power densities of over 500 W/kg, and cycle lives of more than 2000 cycles at 80% capacity retention. Additionally, there is a strong emphasis on developing GPEs that can operate effectively across a wide temperature range (-20°C to 60°C) to enable diverse applications from consumer electronics to grid-scale energy storage.
Future development trajectories are likely to explore bio-derived polymers for environmentally sustainable GPEs, multi-functional additives that simultaneously address multiple performance limitations, and composite electrolyte systems that synergistically combine the advantages of different materials. The ultimate goal is to create ZIB systems that can compete with or surpass lithium-ion batteries in specific application scenarios while offering superior safety profiles and lower environmental impact.
The convergence of materials science, electrochemistry, and manufacturing innovations is expected to accelerate the evolution of quasi-solid ZIBs, potentially leading to commercial viability within the next 5-10 years, particularly for applications where safety and cost considerations outweigh the need for extremely high energy density.
Market Analysis for Quasi-Solid ZIB Applications
The global market for zinc-ion batteries (ZIBs) is experiencing significant growth, driven by increasing demand for sustainable energy storage solutions. The quasi-solid ZIB segment, particularly those utilizing gel polymer electrolytes, represents a promising niche with substantial market potential. Current market valuations estimate the overall zinc-based battery market at approximately $7.3 billion in 2023, with projections to reach $12.5 billion by 2028, growing at a CAGR of 11.4%.
Quasi-solid ZIBs address several critical market needs that conventional lithium-ion and liquid electrolyte zinc batteries cannot fully satisfy. The primary market drivers include safety concerns, as gel polymer electrolytes significantly reduce leakage risks compared to liquid electrolytes. This safety advantage opens applications in consumer electronics, wearable devices, and medical implants where safety is paramount.
The renewable energy sector presents another substantial market opportunity. Grid-scale energy storage systems require safe, cost-effective solutions with long cycle life. Quasi-solid ZIBs offer advantages in these parameters, positioning them as competitive alternatives to existing technologies. Market analysis indicates that this sector could represent 35% of the total addressable market for quasi-solid ZIBs by 2027.
Regional market distribution shows Asia-Pacific leading in both production and consumption, with China accounting for 42% of global manufacturing capacity. North America and Europe follow with growing adoption rates, particularly in renewable energy applications and electric mobility solutions. Developing markets in Africa and South America show increasing interest due to the technology's potential for off-grid applications.
Consumer electronics represents the most immediate market opportunity, with an estimated market share of 28% for zinc-based battery technologies. The wearable technology segment is growing at 18% annually, creating significant demand for safe, flexible energy storage solutions that quasi-solid ZIBs can provide.
Market barriers include competition from established lithium-ion technologies and emerging alternatives such as sodium-ion batteries. Price sensitivity remains a challenge, though economies of scale are expected to improve cost competitiveness as production volumes increase. Current production costs for quasi-solid ZIBs are approximately 15-20% higher than conventional zinc batteries, but this gap is narrowing.
Customer adoption analysis reveals that industrial users prioritize cycle life and total cost of ownership, while consumer applications focus on safety and form factor flexibility. Both segments value the environmental benefits of zinc-based technologies, which contain no toxic materials and utilize abundant resources, aligning with growing market demand for sustainable solutions.
Quasi-solid ZIBs address several critical market needs that conventional lithium-ion and liquid electrolyte zinc batteries cannot fully satisfy. The primary market drivers include safety concerns, as gel polymer electrolytes significantly reduce leakage risks compared to liquid electrolytes. This safety advantage opens applications in consumer electronics, wearable devices, and medical implants where safety is paramount.
The renewable energy sector presents another substantial market opportunity. Grid-scale energy storage systems require safe, cost-effective solutions with long cycle life. Quasi-solid ZIBs offer advantages in these parameters, positioning them as competitive alternatives to existing technologies. Market analysis indicates that this sector could represent 35% of the total addressable market for quasi-solid ZIBs by 2027.
Regional market distribution shows Asia-Pacific leading in both production and consumption, with China accounting for 42% of global manufacturing capacity. North America and Europe follow with growing adoption rates, particularly in renewable energy applications and electric mobility solutions. Developing markets in Africa and South America show increasing interest due to the technology's potential for off-grid applications.
Consumer electronics represents the most immediate market opportunity, with an estimated market share of 28% for zinc-based battery technologies. The wearable technology segment is growing at 18% annually, creating significant demand for safe, flexible energy storage solutions that quasi-solid ZIBs can provide.
Market barriers include competition from established lithium-ion technologies and emerging alternatives such as sodium-ion batteries. Price sensitivity remains a challenge, though economies of scale are expected to improve cost competitiveness as production volumes increase. Current production costs for quasi-solid ZIBs are approximately 15-20% higher than conventional zinc batteries, but this gap is narrowing.
Customer adoption analysis reveals that industrial users prioritize cycle life and total cost of ownership, while consumer applications focus on safety and form factor flexibility. Both segments value the environmental benefits of zinc-based technologies, which contain no toxic materials and utilize abundant resources, aligning with growing market demand for sustainable solutions.
Current Challenges in Gel Polymer Electrolyte ZIBs
Despite significant advancements in zinc-ion battery (ZIB) technology utilizing gel polymer electrolytes (GPEs), several critical challenges continue to impede their widespread commercial adoption and optimal performance. These challenges span multiple technical domains and require innovative solutions to overcome.
The ionic conductivity of current GPE formulations remains suboptimal compared to liquid electrolytes, typically ranging from 10^-4 to 10^-3 S/cm at room temperature. This limitation directly impacts battery power density and rate capability, particularly at high discharge rates. The trade-off between mechanical strength and ionic conductivity presents a persistent engineering dilemma, as enhancing one property often compromises the other.
Interface stability between the GPE and zinc metal anode represents another significant hurdle. Uneven zinc deposition during cycling leads to dendrite formation, which can penetrate the GPE, causing internal short circuits and safety hazards. Current GPE formulations have not fully resolved this critical safety concern, limiting the practical application of these batteries in commercial settings.
Water management within GPE systems poses a complex challenge. While water molecules facilitate ion transport, excessive water content accelerates zinc corrosion and hydrogen evolution, reducing coulombic efficiency and battery lifespan. Conversely, insufficient water content severely restricts ionic mobility. Finding the optimal water content balance remains elusive in current GPE formulations.
Long-term cycling stability presents persistent difficulties, with capacity retention typically declining after several hundred cycles. This degradation stems from multiple factors including zinc dendrite growth, electrolyte depletion, and cathode material dissolution. The complex interplay of these degradation mechanisms makes addressing cycling stability particularly challenging.
Manufacturing scalability and cost-effectiveness represent significant barriers to commercialization. Current laboratory-scale synthesis methods for GPEs often involve complex procedures and expensive materials that are difficult to scale up for mass production. The lack of standardized manufacturing protocols further complicates industrial adoption.
Temperature sensitivity of GPE-based ZIBs remains problematic, with performance deteriorating significantly at temperature extremes. Most current GPE formulations show optimal performance only within a narrow temperature window (10-40°C), limiting their application in diverse environmental conditions.
Environmental stability and shelf life of GPE-based ZIBs require improvement, as many formulations experience performance degradation during storage due to water evaporation, polymer degradation, or zinc salt crystallization. These issues significantly impact the commercial viability of GPE-ZIB technology.
The ionic conductivity of current GPE formulations remains suboptimal compared to liquid electrolytes, typically ranging from 10^-4 to 10^-3 S/cm at room temperature. This limitation directly impacts battery power density and rate capability, particularly at high discharge rates. The trade-off between mechanical strength and ionic conductivity presents a persistent engineering dilemma, as enhancing one property often compromises the other.
Interface stability between the GPE and zinc metal anode represents another significant hurdle. Uneven zinc deposition during cycling leads to dendrite formation, which can penetrate the GPE, causing internal short circuits and safety hazards. Current GPE formulations have not fully resolved this critical safety concern, limiting the practical application of these batteries in commercial settings.
Water management within GPE systems poses a complex challenge. While water molecules facilitate ion transport, excessive water content accelerates zinc corrosion and hydrogen evolution, reducing coulombic efficiency and battery lifespan. Conversely, insufficient water content severely restricts ionic mobility. Finding the optimal water content balance remains elusive in current GPE formulations.
Long-term cycling stability presents persistent difficulties, with capacity retention typically declining after several hundred cycles. This degradation stems from multiple factors including zinc dendrite growth, electrolyte depletion, and cathode material dissolution. The complex interplay of these degradation mechanisms makes addressing cycling stability particularly challenging.
Manufacturing scalability and cost-effectiveness represent significant barriers to commercialization. Current laboratory-scale synthesis methods for GPEs often involve complex procedures and expensive materials that are difficult to scale up for mass production. The lack of standardized manufacturing protocols further complicates industrial adoption.
Temperature sensitivity of GPE-based ZIBs remains problematic, with performance deteriorating significantly at temperature extremes. Most current GPE formulations show optimal performance only within a narrow temperature window (10-40°C), limiting their application in diverse environmental conditions.
Environmental stability and shelf life of GPE-based ZIBs require improvement, as many formulations experience performance degradation during storage due to water evaporation, polymer degradation, or zinc salt crystallization. These issues significantly impact the commercial viability of GPE-ZIB technology.
Gel Polymer Electrolyte Formulation Methods
01 Gel polymer electrolytes for quasi-solid ZIBs
Gel polymer electrolytes (GPEs) are key components in quasi-solid zinc-ion batteries, providing a balance between liquid and solid electrolytes. These GPEs typically consist of a polymer matrix that immobilizes the liquid electrolyte, offering improved safety and mechanical stability while maintaining good ionic conductivity. Common polymers used include PVA, PEO, and PMMA, which are combined with zinc salts to create flexible, leak-proof electrolyte systems that enable stable zinc plating/stripping processes.- Gel polymer electrolytes for quasi-solid ZIBs: Gel polymer electrolytes (GPEs) are key components in quasi-solid zinc-ion batteries, providing ionic conductivity while maintaining mechanical stability. These electrolytes typically consist of a polymer matrix infused with zinc salt solutions, offering advantages such as reduced leakage risk and improved safety compared to liquid electrolytes. Common polymers used include PVA, PEO, and PMMA, which can be modified to enhance zinc ion transport properties while maintaining structural integrity.
- Polymer matrix modifications for enhanced performance: Various modifications to the polymer matrix can significantly improve the performance of quasi-solid ZIBs. These include cross-linking strategies to enhance mechanical strength, incorporation of nanofillers to improve ionic conductivity, and chemical modifications to increase zinc ion transport. Advanced polymer blends and composite structures can be designed to optimize the balance between mechanical properties and electrochemical performance, resulting in batteries with higher capacity, better cycling stability, and improved rate capability.
- Zinc salt complexes and additives in gel electrolytes: The selection and optimization of zinc salts and additives play crucial roles in quasi-solid ZIB performance. Common zinc salts include ZnSO4, Zn(CF3SO3)2, and ZnCl2, which can be combined with additives such as ionic liquids, organic solvents, or water-in-salt systems to enhance ionic conductivity and electrochemical stability. These additives can mitigate zinc dendrite formation, expand the electrochemical window, and improve the compatibility between the electrolyte and electrodes, leading to batteries with longer cycle life and higher energy density.
- Interface engineering in quasi-solid ZIBs: Interface engineering between the gel polymer electrolyte and electrodes is critical for optimizing quasi-solid ZIB performance. Strategies include surface modification of electrodes, creation of artificial solid electrolyte interphases, and development of gradient-structured electrolytes. These approaches can reduce interfacial resistance, suppress side reactions, and enhance zinc ion transport across interfaces. Proper interface design helps mitigate issues such as zinc dendrite growth and electrode degradation, resulting in batteries with improved safety and longer operational lifetimes.
- Novel cathode materials compatible with gel electrolytes: Development of cathode materials specifically designed to work with gel polymer electrolytes is essential for high-performance quasi-solid ZIBs. Materials such as manganese oxides, vanadium oxides, Prussian blue analogs, and organic compounds can be engineered to facilitate zinc ion insertion/extraction in the quasi-solid state environment. These cathodes can be modified through nanostructuring, doping, or composite formation to enhance their compatibility with gel electrolytes, resulting in batteries with higher capacity, better rate performance, and improved cycling stability.
02 Polymer matrix compositions and additives
Various polymer matrices and additives are employed to enhance the performance of gel polymer electrolytes in quasi-solid ZIBs. These compositions often include natural polymers like cellulose derivatives, synthetic polymers such as PVDF, or composite systems combining multiple polymers. Additives such as ionic liquids, ceramic fillers, and cross-linking agents are incorporated to improve ionic conductivity, mechanical strength, and interfacial stability. These formulations help suppress zinc dendrite formation and extend battery cycle life while maintaining flexibility and processability.Expand Specific Solutions03 Zinc salt complexes and electrolyte formulations
Specialized zinc salt complexes and electrolyte formulations are critical for quasi-solid ZIBs. These typically include zinc sulfate, zinc chloride, or zinc triflate dissolved in aqueous or non-aqueous solvents before gelation. The concentration and type of zinc salts significantly affect ionic conductivity, zinc ion transport, and electrode compatibility. Some formulations incorporate chelating agents or pH modifiers to control zinc ion coordination and prevent side reactions, resulting in improved electrochemical stability and reduced corrosion of zinc anodes.Expand Specific Solutions04 Electrode-electrolyte interface engineering
Interface engineering between electrodes and gel polymer electrolytes is essential for quasi-solid ZIBs. This involves surface modifications of electrodes, incorporation of functional interlayers, or development of specialized coatings to improve wettability and adhesion. These approaches minimize interfacial resistance, enhance zinc ion transport across interfaces, and prevent undesired side reactions. Advanced interface designs also help mitigate issues like electrolyte depletion, electrode volume changes, and contact loss during cycling, leading to batteries with improved rate capability and cycling stability.Expand Specific Solutions05 Manufacturing processes and device integration
Specialized manufacturing processes and device integration techniques are developed for quasi-solid ZIBs with gel polymer electrolytes. These include in-situ gelation methods, solution casting, electrospinning, and 3D printing approaches to create uniform, defect-free electrolyte layers. Assembly techniques focus on ensuring good contact between components while maintaining mechanical integrity. Advanced packaging solutions address challenges related to moisture sensitivity, mechanical stress, and thermal management, enabling the production of flexible, compact, and durable zinc-ion batteries suitable for various applications including wearable electronics and grid storage.Expand Specific Solutions
Leading Research Groups and Companies in ZIB Development
The quasi-solid zinc-ion battery (ZIB) market using gel polymer electrolytes is currently in an early growth phase, characterized by intensive R&D activities across academic institutions and commercial entities. The global market size for ZIBs is expanding rapidly, driven by increasing demand for safer, more sustainable energy storage solutions. Leading academic players including City University of Hong Kong, Northeast Normal University, and South China University of Technology are advancing fundamental research, while commercial entities like LG Energy Solution, Samsung Electronics, and BASF are focusing on scalable manufacturing processes. The technology maturity remains moderate, with companies like Ruipu Energy and Capchem Technology developing proprietary gel polymer electrolyte formulations to overcome challenges in ionic conductivity and mechanical stability, positioning ZIBs as promising alternatives to lithium-ion technologies.
City University of Hong Kong
Technical Solution: City University of Hong Kong has developed advanced quasi-solid zinc-ion batteries using hydrogel polymer electrolytes based on polyacrylamide (PAM) and polyvinyl alcohol (PVA) matrices. Their approach incorporates zinc salts (ZnSO4, Zn(CF3SO3)2) within these polymer networks to create electrolytes with high ionic conductivity (10^-2 to 10^-3 S/cm) while maintaining mechanical stability. Their recipe typically involves dissolving the polymer (6-10 wt%) in aqueous zinc salt solutions (1-2M), followed by chemical or physical crosslinking processes. The research team has demonstrated batteries with capacity retention of >90% after 1000 cycles and rate capabilities up to 5C. They've also pioneered the incorporation of MXene nanosheets into the gel matrix to enhance conductivity and zinc ion transport properties.
Strengths: Exceptional cycling stability with minimal capacity fading; superior mechanical properties allowing flexible device construction; high ionic conductivity comparable to liquid electrolytes while preventing dendrite formation. Weaknesses: Potential water evaporation issues in hydrogel-based systems affecting long-term stability; relatively complex synthesis procedures compared to liquid electrolytes; possible challenges in large-scale manufacturing.
LG Chem Ltd.
Technical Solution: LG Chem has developed proprietary quasi-solid zinc-ion battery technology using gel polymer electrolytes based on modified polyethylene oxide (PEO) and polyvinylidene fluoride (PVDF) matrices. Their approach incorporates zinc salts (typically Zn(TFSI)2 or ZnCl2) at optimized concentrations (0.5-1.5M) within these polymer networks. The company's manufacturing process involves a solvent-casting technique where the polymer and zinc salts are dissolved in appropriate solvents (acetonitrile or DMF), cast into films, and subjected to controlled drying and thermal treatment. LG Chem's quasi-solid ZIBs demonstrate energy densities of 85-120 Wh/kg, power densities up to 300 W/kg, and capacity retention of approximately 85% after 500 cycles. Their electrolytes achieve ionic conductivities in the range of 10^-4 to 10^-3 S/cm at room temperature.
Strengths: Established large-scale manufacturing capabilities; strong integration with existing battery production lines; excellent safety profile with reduced leakage risk compared to liquid systems. Weaknesses: Lower ionic conductivity compared to some academic research prototypes; potential challenges with low-temperature performance; higher production costs compared to conventional liquid electrolyte systems.
Critical Patents and Literature on Quasi-Solid ZIBs
Gel polymer electrolytes comprising electrolyte additive
PatentActiveUS20190140317A1
Innovation
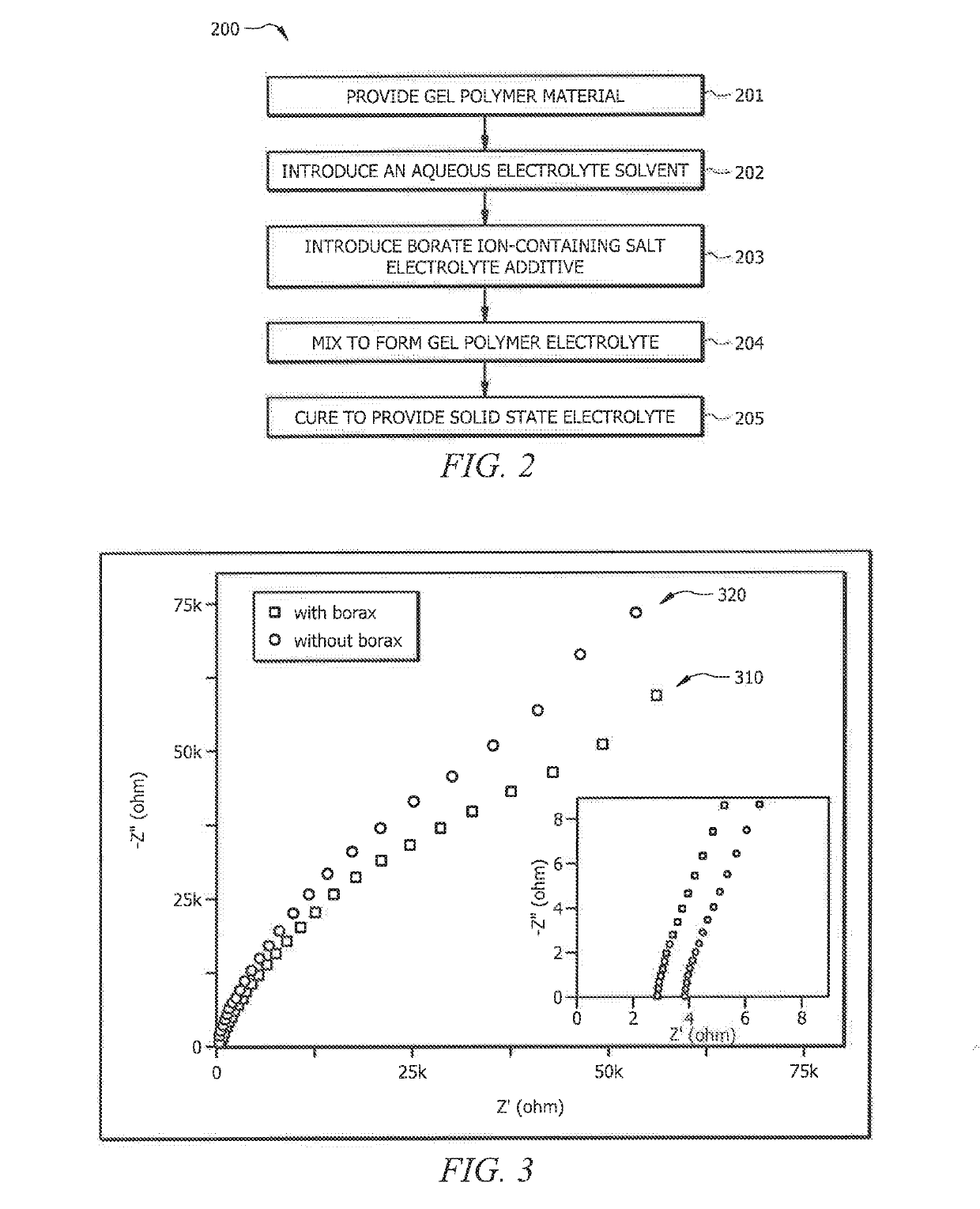
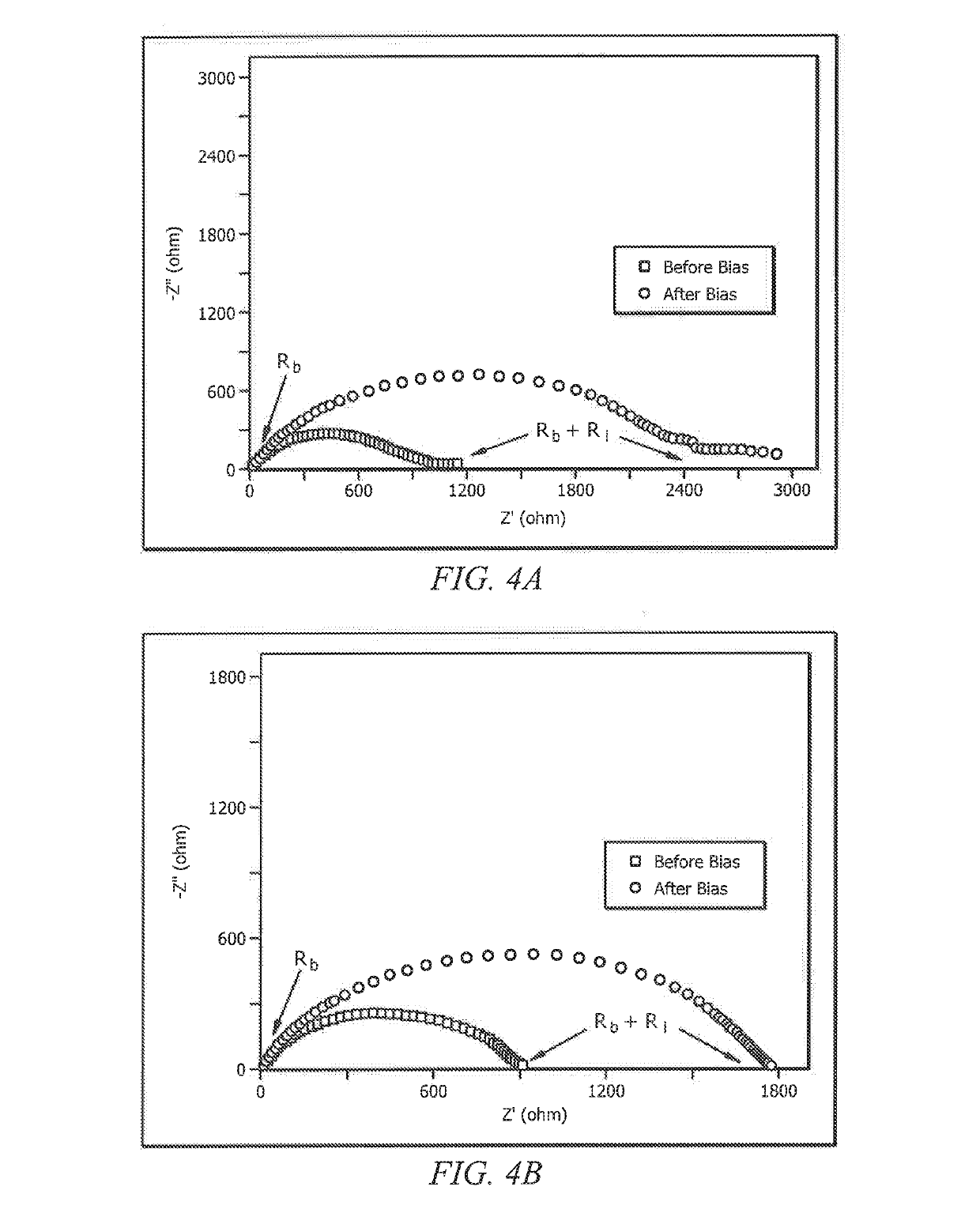
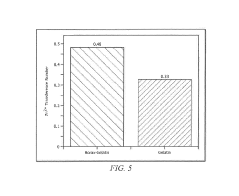
- Incorporating boron-based additives, such as borax or boric acid, into the aqueous gel polymer electrolytes to enhance ionic conductivity and mobile zinc ion transport, facilitating the development of flexible and transparent battery configurations with improved electrochemical performance.
Safety and Stability Assessment Protocols
Safety assessment protocols for quasi-solid zinc-ion batteries (ZIBs) with gel polymer electrolytes (GPEs) must be comprehensive and rigorous to ensure commercial viability. These protocols should begin with thermal stability testing, including differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) to evaluate the thermal decomposition behavior of GPEs under various temperature conditions. Tests should be conducted between room temperature and 300°C to identify potential phase transitions and decomposition points.
Mechanical integrity testing represents another critical aspect, particularly for quasi-solid batteries that may experience physical deformation. Compression, puncture, and bending tests should be performed to evaluate the GPE's ability to maintain structural integrity while preventing internal short circuits. These tests should simulate real-world mechanical stresses that batteries might encounter during transportation or daily use.
Electrochemical stability window (ESW) assessment is fundamental for determining the voltage range within which the GPE remains stable. Cyclic voltammetry and linear sweep voltammetry should be conducted to identify potential decomposition reactions at extreme potentials. For zinc-ion batteries, the ESW should ideally extend beyond 2.0 V to accommodate various cathode materials.
Long-term cycling stability protocols must evaluate capacity retention and coulombic efficiency over extended periods. Accelerated aging tests at elevated temperatures (40-60°C) can provide insights into the GPE's resistance to degradation mechanisms such as zinc dendrite formation and electrolyte decomposition. These tests should run for at least 500 cycles or 1000 hours to generate meaningful data.
Chemical compatibility testing between the GPE and electrode materials is essential to identify potential parasitic reactions. Techniques such as FTIR, XPS, and SEM-EDX should be employed to analyze interfacial regions before and after cycling. Additionally, storage tests at various temperatures should be conducted to evaluate self-discharge rates and shelf-life characteristics.
Safety response testing under abuse conditions represents the final critical protocol. This includes nail penetration tests, overcharge/overdischarge tests, and external short-circuit tests. For quasi-solid ZIBs, particular attention should be paid to zinc dendrite penetration resistance, as this remains a significant safety concern. Standardized testing procedures from organizations like UL, IEC, or UN 38.3 should be adapted specifically for zinc-ion chemistry with gel polymer electrolytes.
Mechanical integrity testing represents another critical aspect, particularly for quasi-solid batteries that may experience physical deformation. Compression, puncture, and bending tests should be performed to evaluate the GPE's ability to maintain structural integrity while preventing internal short circuits. These tests should simulate real-world mechanical stresses that batteries might encounter during transportation or daily use.
Electrochemical stability window (ESW) assessment is fundamental for determining the voltage range within which the GPE remains stable. Cyclic voltammetry and linear sweep voltammetry should be conducted to identify potential decomposition reactions at extreme potentials. For zinc-ion batteries, the ESW should ideally extend beyond 2.0 V to accommodate various cathode materials.
Long-term cycling stability protocols must evaluate capacity retention and coulombic efficiency over extended periods. Accelerated aging tests at elevated temperatures (40-60°C) can provide insights into the GPE's resistance to degradation mechanisms such as zinc dendrite formation and electrolyte decomposition. These tests should run for at least 500 cycles or 1000 hours to generate meaningful data.
Chemical compatibility testing between the GPE and electrode materials is essential to identify potential parasitic reactions. Techniques such as FTIR, XPS, and SEM-EDX should be employed to analyze interfacial regions before and after cycling. Additionally, storage tests at various temperatures should be conducted to evaluate self-discharge rates and shelf-life characteristics.
Safety response testing under abuse conditions represents the final critical protocol. This includes nail penetration tests, overcharge/overdischarge tests, and external short-circuit tests. For quasi-solid ZIBs, particular attention should be paid to zinc dendrite penetration resistance, as this remains a significant safety concern. Standardized testing procedures from organizations like UL, IEC, or UN 38.3 should be adapted specifically for zinc-ion chemistry with gel polymer electrolytes.
Environmental Impact and Recyclability Considerations
The environmental impact of quasi-solid zinc-ion batteries (ZIBs) using gel polymer electrolytes represents a significant advantage over conventional battery technologies. These batteries utilize zinc, which is abundant in the Earth's crust (approximately 75 ppm), making it substantially more available than lithium. This abundance translates to lower environmental impact from mining operations and reduced geopolitical concerns related to resource scarcity.
Gel polymer electrolytes in ZIBs typically employ biodegradable polymers such as polyvinyl alcohol (PVA), cellulose derivatives, or chitosan as their base materials. These natural or semi-natural polymers offer improved end-of-life management compared to conventional liquid electrolytes that contain volatile organic compounds. The reduced toxicity profile of these materials minimizes environmental contamination risks during production, use, and disposal phases.
The aqueous nature of many ZIB gel electrolytes eliminates the need for toxic and flammable organic solvents commonly used in lithium-ion batteries. This characteristic not only enhances safety during operation but also significantly reduces the environmental footprint associated with electrolyte production and potential leakage incidents. Studies indicate up to 60% reduction in harmful emissions during manufacturing when compared to conventional lithium-ion battery electrolytes.
Recyclability of quasi-solid ZIBs presents both opportunities and challenges. The zinc component can be recovered at rates exceeding 90% using established hydrometallurgical processes, which is substantially higher than recovery rates for lithium from conventional batteries. However, the polymer matrix in gel electrolytes requires specialized separation techniques to avoid contamination of the recycling stream.
Recent advances in selective dissolution methods have shown promise for separating the polymer components from metallic elements, potentially enabling closed-loop recycling of both the zinc and polymer materials. Research at several institutions has demonstrated pilot-scale recovery processes achieving separation efficiencies of 85-95% for the major components of quasi-solid ZIBs.
Life cycle assessment (LCA) studies indicate that quasi-solid ZIBs using gel polymer electrolytes can achieve a 30-40% reduction in global warming potential compared to conventional battery technologies when accounting for production, use, and end-of-life management. This advantage stems primarily from the elimination of toxic components and the higher recyclability of zinc compared to other battery metals.
Future development of quasi-solid ZIBs should prioritize design-for-recycling approaches, including easily separable components and standardized material compositions that facilitate automated disassembly and material recovery. Establishing dedicated recycling infrastructure for these emerging battery technologies will be crucial for realizing their full environmental benefits.
Gel polymer electrolytes in ZIBs typically employ biodegradable polymers such as polyvinyl alcohol (PVA), cellulose derivatives, or chitosan as their base materials. These natural or semi-natural polymers offer improved end-of-life management compared to conventional liquid electrolytes that contain volatile organic compounds. The reduced toxicity profile of these materials minimizes environmental contamination risks during production, use, and disposal phases.
The aqueous nature of many ZIB gel electrolytes eliminates the need for toxic and flammable organic solvents commonly used in lithium-ion batteries. This characteristic not only enhances safety during operation but also significantly reduces the environmental footprint associated with electrolyte production and potential leakage incidents. Studies indicate up to 60% reduction in harmful emissions during manufacturing when compared to conventional lithium-ion battery electrolytes.
Recyclability of quasi-solid ZIBs presents both opportunities and challenges. The zinc component can be recovered at rates exceeding 90% using established hydrometallurgical processes, which is substantially higher than recovery rates for lithium from conventional batteries. However, the polymer matrix in gel electrolytes requires specialized separation techniques to avoid contamination of the recycling stream.
Recent advances in selective dissolution methods have shown promise for separating the polymer components from metallic elements, potentially enabling closed-loop recycling of both the zinc and polymer materials. Research at several institutions has demonstrated pilot-scale recovery processes achieving separation efficiencies of 85-95% for the major components of quasi-solid ZIBs.
Life cycle assessment (LCA) studies indicate that quasi-solid ZIBs using gel polymer electrolytes can achieve a 30-40% reduction in global warming potential compared to conventional battery technologies when accounting for production, use, and end-of-life management. This advantage stems primarily from the elimination of toxic components and the higher recyclability of zinc compared to other battery metals.
Future development of quasi-solid ZIBs should prioritize design-for-recycling approaches, including easily separable components and standardized material compositions that facilitate automated disassembly and material recovery. Establishing dedicated recycling infrastructure for these emerging battery technologies will be crucial for realizing their full environmental benefits.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!