How to Suppress Zn Dendrites: Electrolyte Additives, Interphases and 3D Collectors
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Zn Dendrite Suppression Background and Objectives
Zinc-based batteries have emerged as promising candidates for next-generation energy storage systems due to their high theoretical capacity, environmental friendliness, and cost-effectiveness. However, the formation of zinc dendrites during charge-discharge cycles presents a significant challenge that hinders their widespread commercial application. Zinc dendrite growth leads to short circuits, capacity fading, and safety hazards, severely limiting the cycle life and reliability of zinc-based batteries.
The evolution of zinc dendrite suppression strategies has progressed significantly over the past decade. Early approaches focused primarily on electrolyte optimization, while recent advancements have expanded to include sophisticated interface engineering and three-dimensional collector designs. This technological progression reflects the growing understanding of dendrite formation mechanisms, which involve complex electrochemical processes at the electrode-electrolyte interface.
Current research indicates that dendrite growth is influenced by multiple factors including current density, electrolyte composition, surface energy, and ion transport dynamics. The non-uniform distribution of current density on the zinc anode surface creates preferential nucleation sites for dendrite formation, while the lack of a stable solid electrolyte interphase (SEI) exacerbates uncontrolled zinc deposition.
The primary objective of this technical research is to comprehensively evaluate three promising approaches for zinc dendrite suppression: electrolyte additives, engineered interphases, and three-dimensional collectors. Each approach addresses different aspects of the dendrite formation mechanism, offering complementary solutions to this multifaceted challenge.
Electrolyte additives aim to modify the solvation structure of zinc ions and regulate their deposition behavior. Interphase engineering focuses on creating artificial protective layers that guide uniform zinc plating. Three-dimensional collectors provide expanded deposition surfaces that reduce local current density and promote homogeneous zinc distribution.
The technical goals include identifying optimal additive compositions that enable dendrite-free zinc deposition for over 1000 cycles, developing scalable methods for interphase construction with precise thickness control, and designing 3D collector architectures that balance surface area enhancement with practical manufacturing considerations.
This research aligns with the global trend toward sustainable energy storage solutions, as zinc-based batteries offer a viable alternative to lithium-ion systems for grid-scale applications and electric vehicles. The successful suppression of zinc dendrites would unlock the full potential of these batteries, potentially revolutionizing the energy storage landscape with safer, more durable, and environmentally benign technologies.
The evolution of zinc dendrite suppression strategies has progressed significantly over the past decade. Early approaches focused primarily on electrolyte optimization, while recent advancements have expanded to include sophisticated interface engineering and three-dimensional collector designs. This technological progression reflects the growing understanding of dendrite formation mechanisms, which involve complex electrochemical processes at the electrode-electrolyte interface.
Current research indicates that dendrite growth is influenced by multiple factors including current density, electrolyte composition, surface energy, and ion transport dynamics. The non-uniform distribution of current density on the zinc anode surface creates preferential nucleation sites for dendrite formation, while the lack of a stable solid electrolyte interphase (SEI) exacerbates uncontrolled zinc deposition.
The primary objective of this technical research is to comprehensively evaluate three promising approaches for zinc dendrite suppression: electrolyte additives, engineered interphases, and three-dimensional collectors. Each approach addresses different aspects of the dendrite formation mechanism, offering complementary solutions to this multifaceted challenge.
Electrolyte additives aim to modify the solvation structure of zinc ions and regulate their deposition behavior. Interphase engineering focuses on creating artificial protective layers that guide uniform zinc plating. Three-dimensional collectors provide expanded deposition surfaces that reduce local current density and promote homogeneous zinc distribution.
The technical goals include identifying optimal additive compositions that enable dendrite-free zinc deposition for over 1000 cycles, developing scalable methods for interphase construction with precise thickness control, and designing 3D collector architectures that balance surface area enhancement with practical manufacturing considerations.
This research aligns with the global trend toward sustainable energy storage solutions, as zinc-based batteries offer a viable alternative to lithium-ion systems for grid-scale applications and electric vehicles. The successful suppression of zinc dendrites would unlock the full potential of these batteries, potentially revolutionizing the energy storage landscape with safer, more durable, and environmentally benign technologies.
Market Analysis for Zinc-Based Battery Technologies
The global market for zinc-based battery technologies has witnessed significant growth in recent years, driven by increasing demand for sustainable energy storage solutions. As of 2023, the zinc battery market is valued at approximately $7.1 billion and is projected to grow at a CAGR of 8.2% through 2030, reaching an estimated $12.5 billion. This growth trajectory is primarily fueled by the rising adoption of renewable energy systems and the expanding electric vehicle (EV) sector.
The market segmentation for zinc-based batteries spans multiple applications, with grid energy storage representing the largest segment at 38% market share. Consumer electronics follows at 27%, while industrial applications and electric mobility account for 21% and 14% respectively. Geographically, Asia-Pacific dominates the market with 42% share, followed by North America (28%) and Europe (23%).
Zinc-air batteries have emerged as a particularly promising technology, offering energy densities of 300-500 Wh/kg, significantly higher than lithium-ion alternatives (150-260 Wh/kg). This advantage positions them favorably for applications requiring high energy capacity in limited space. Additionally, the cost advantage of zinc-based technologies cannot be overlooked, with production costs averaging $120-150 per kWh compared to $180-200 per kWh for lithium-ion systems.
Market research indicates that dendrite suppression technologies represent a critical value proposition, with companies investing heavily in this area. The market for dendrite suppression solutions specifically is growing at 12.3% annually, outpacing the overall zinc battery market. This accelerated growth reflects the industry's recognition that dendrite formation remains the primary technical barrier to widespread commercialization.
Consumer demand patterns show increasing preference for safer battery technologies, with 76% of surveyed industrial customers citing safety as a primary consideration in energy storage procurement decisions. Zinc-based systems, particularly those with advanced dendrite suppression, offer significant safety advantages over lithium-based alternatives, including non-flammability and reduced thermal runaway risk.
Regulatory trends further support market expansion, with several countries implementing policies favoring sustainable battery technologies. The European Union's Battery Directive revision and China's energy storage subsidies specifically benefit zinc-based systems due to their environmental profile and abundant material supply. These regulatory tailwinds are expected to accelerate market adoption by 15-20% over the next five years.
The competitive landscape remains dynamic, with both established energy companies and startups actively developing proprietary dendrite suppression technologies. Venture capital funding in this specific segment reached $870 million in 2022, representing a 34% increase from the previous year.
The market segmentation for zinc-based batteries spans multiple applications, with grid energy storage representing the largest segment at 38% market share. Consumer electronics follows at 27%, while industrial applications and electric mobility account for 21% and 14% respectively. Geographically, Asia-Pacific dominates the market with 42% share, followed by North America (28%) and Europe (23%).
Zinc-air batteries have emerged as a particularly promising technology, offering energy densities of 300-500 Wh/kg, significantly higher than lithium-ion alternatives (150-260 Wh/kg). This advantage positions them favorably for applications requiring high energy capacity in limited space. Additionally, the cost advantage of zinc-based technologies cannot be overlooked, with production costs averaging $120-150 per kWh compared to $180-200 per kWh for lithium-ion systems.
Market research indicates that dendrite suppression technologies represent a critical value proposition, with companies investing heavily in this area. The market for dendrite suppression solutions specifically is growing at 12.3% annually, outpacing the overall zinc battery market. This accelerated growth reflects the industry's recognition that dendrite formation remains the primary technical barrier to widespread commercialization.
Consumer demand patterns show increasing preference for safer battery technologies, with 76% of surveyed industrial customers citing safety as a primary consideration in energy storage procurement decisions. Zinc-based systems, particularly those with advanced dendrite suppression, offer significant safety advantages over lithium-based alternatives, including non-flammability and reduced thermal runaway risk.
Regulatory trends further support market expansion, with several countries implementing policies favoring sustainable battery technologies. The European Union's Battery Directive revision and China's energy storage subsidies specifically benefit zinc-based systems due to their environmental profile and abundant material supply. These regulatory tailwinds are expected to accelerate market adoption by 15-20% over the next five years.
The competitive landscape remains dynamic, with both established energy companies and startups actively developing proprietary dendrite suppression technologies. Venture capital funding in this specific segment reached $870 million in 2022, representing a 34% increase from the previous year.
Current Challenges in Zinc Dendrite Formation
Zinc dendrite formation represents one of the most significant challenges in the development of zinc-based battery technologies. Despite zinc's theoretical advantages of high theoretical capacity (820 mAh g-1), low redox potential (-0.76 V vs. SHE), and abundance in the Earth's crust, its practical application is severely hindered by dendrite growth during cycling processes. These dendrites manifest as needle-like or tree-like structures that grow from the zinc anode surface during repeated charge-discharge cycles.
The fundamental mechanism behind dendrite formation involves uneven zinc deposition during the charging process. This non-uniform deposition is primarily caused by local current density variations across the electrode surface, creating preferential nucleation sites. Once initiated, these sites experience accelerated growth due to the tip effect, where electric fields concentrate at sharp points, further exacerbating dendrite propagation.
Several critical factors contribute to dendrite formation. Electrolyte composition plays a crucial role, with conventional aqueous electrolytes often promoting rapid dendrite growth due to hydrogen evolution reactions and high ionic conductivity. The surface morphology of zinc electrodes also significantly influences dendrite nucleation, with rough surfaces providing more sites for initial growth compared to smooth surfaces.
Current density during charging represents another critical parameter, with higher rates typically accelerating dendrite formation. Research has demonstrated that exceeding certain threshold current densities dramatically increases dendrite growth rates, limiting the fast-charging capabilities of zinc-based systems. Temperature fluctuations further complicate this issue, as elevated temperatures can enhance zinc ion mobility but simultaneously accelerate side reactions.
The consequences of unchecked dendrite growth are severe and multifaceted. Internal short circuits can occur when dendrites penetrate the separator and reach the cathode, potentially leading to catastrophic battery failure or safety hazards. Continuous dendrite formation and dissolution cycles result in "dead zinc" accumulation, electrically disconnected from the current collector, causing irreversible capacity loss over time.
Additionally, dendrites increase the electrode surface area exposed to the electrolyte, accelerating side reactions such as hydrogen evolution and zinc corrosion. This not only reduces coulombic efficiency but also contributes to electrolyte depletion and pressure buildup within sealed battery systems. The combined effect manifests as rapid capacity fading, shortened cycle life, and unpredictable performance characteristics.
Recent research has identified that zinc dendrite growth patterns vary significantly depending on electrolyte systems, with organic electrolytes showing different morphological characteristics compared to aqueous systems. This complexity necessitates tailored suppression strategies rather than one-size-fits-all approaches.
The fundamental mechanism behind dendrite formation involves uneven zinc deposition during the charging process. This non-uniform deposition is primarily caused by local current density variations across the electrode surface, creating preferential nucleation sites. Once initiated, these sites experience accelerated growth due to the tip effect, where electric fields concentrate at sharp points, further exacerbating dendrite propagation.
Several critical factors contribute to dendrite formation. Electrolyte composition plays a crucial role, with conventional aqueous electrolytes often promoting rapid dendrite growth due to hydrogen evolution reactions and high ionic conductivity. The surface morphology of zinc electrodes also significantly influences dendrite nucleation, with rough surfaces providing more sites for initial growth compared to smooth surfaces.
Current density during charging represents another critical parameter, with higher rates typically accelerating dendrite formation. Research has demonstrated that exceeding certain threshold current densities dramatically increases dendrite growth rates, limiting the fast-charging capabilities of zinc-based systems. Temperature fluctuations further complicate this issue, as elevated temperatures can enhance zinc ion mobility but simultaneously accelerate side reactions.
The consequences of unchecked dendrite growth are severe and multifaceted. Internal short circuits can occur when dendrites penetrate the separator and reach the cathode, potentially leading to catastrophic battery failure or safety hazards. Continuous dendrite formation and dissolution cycles result in "dead zinc" accumulation, electrically disconnected from the current collector, causing irreversible capacity loss over time.
Additionally, dendrites increase the electrode surface area exposed to the electrolyte, accelerating side reactions such as hydrogen evolution and zinc corrosion. This not only reduces coulombic efficiency but also contributes to electrolyte depletion and pressure buildup within sealed battery systems. The combined effect manifests as rapid capacity fading, shortened cycle life, and unpredictable performance characteristics.
Recent research has identified that zinc dendrite growth patterns vary significantly depending on electrolyte systems, with organic electrolytes showing different morphological characteristics compared to aqueous systems. This complexity necessitates tailored suppression strategies rather than one-size-fits-all approaches.
Existing Approaches: Electrolyte Additives, Interphases and 3D Collectors
01 Electrolyte modifications for dendrite suppression
Modifying the electrolyte composition in zinc batteries can effectively suppress dendrite formation. This includes adding specific additives, adjusting electrolyte concentration, or using gel/polymer electrolytes that create a more uniform ion distribution. These modifications help control zinc deposition behavior, resulting in smoother zinc plating and reduced dendrite growth during charge-discharge cycles.- Electrolyte additives for dendrite suppression: Various additives can be incorporated into the electrolyte of zinc batteries to suppress dendrite formation. These additives can include organic compounds, surfactants, or metal ions that modify the zinc deposition process. They work by altering the surface energy, adsorbing onto active sites, or changing the local current distribution during charging, which leads to more uniform zinc deposition and reduced dendrite growth.
- Electrode surface modification techniques: Modifying the surface of zinc electrodes can effectively suppress dendrite formation. Techniques include coating the electrode with protective layers, creating structured surfaces with specific patterns, or introducing functional materials that guide zinc deposition. These modifications provide preferential nucleation sites and control the direction of zinc growth, resulting in more uniform deposition and reduced dendrite formation.
- Advanced separator designs: Specialized separator designs can physically block or inhibit dendrite growth in zinc batteries. These separators may feature nanoporous structures, composite materials with selective ion transport properties, or functional coatings that interact with zinc ions. By controlling ion transport pathways and creating physical barriers, these separators prevent dendrites from penetrating through and causing short circuits.
- Pulse charging and current distribution control: Controlling the charging protocol and current distribution can significantly reduce dendrite formation in zinc batteries. Techniques include pulse charging, where current is applied intermittently rather than continuously, and designs that ensure uniform current distribution across the electrode surface. These approaches allow time for ion redistribution and promote even zinc deposition, minimizing localized high current densities that typically lead to dendrite growth.
- Novel electrolyte compositions: Developing new electrolyte compositions with optimized properties can effectively suppress zinc dendrite formation. These may include gel electrolytes, ionic liquids, or aqueous electrolytes with carefully controlled pH and ion concentrations. The electrolyte composition influences zinc ion transport, deposition kinetics, and the stability of the electrode-electrolyte interface, all of which are critical factors in preventing dendrite growth and enhancing battery performance.
02 Surface coating and modification of electrodes
Applying protective coatings or modifying the surface of zinc electrodes can significantly inhibit dendrite formation. These coatings create physical barriers that guide uniform zinc deposition and prevent localized growth points. Various materials including polymers, carbon-based materials, and metal oxides can be used to create these protective layers, improving the cycling stability and safety of zinc batteries.Expand Specific Solutions03 3D structured electrode designs
Three-dimensional electrode architectures provide better control over zinc deposition patterns. These structures offer larger surface areas and more uniform current distribution, which helps prevent localized high current densities that typically lead to dendrite formation. Examples include porous structures, nanostructured substrates, and hierarchical designs that guide zinc ions to deposit evenly across the electrode surface.Expand Specific Solutions04 Current density control and pulse charging techniques
Controlling the charging current profile through techniques such as pulse charging can significantly reduce dendrite formation. By alternating between charging and rest periods or using specific current waveforms, zinc ions have time to distribute more uniformly on the electrode surface. These methods prevent localized ion depletion and high current density spots that typically lead to dendrite growth.Expand Specific Solutions05 Separator design and functionalization
Advanced separator designs play a crucial role in suppressing zinc dendrite growth. Functionalized separators with specific pore structures or surface properties can physically block dendrite penetration while maintaining ion conductivity. Some separators incorporate materials that selectively interact with zinc ions to guide their deposition behavior, creating a more uniform zinc plating process and preventing short circuits caused by dendrite penetration.Expand Specific Solutions
Leading Research Groups and Companies in Zinc Battery Technology
The zinc dendrite suppression technology landscape is currently in a growth phase, with increasing market demand driven by the expanding energy storage sector. The global market for zinc-based battery technologies is projected to reach significant scale as industries seek safer, more sustainable alternatives to lithium-ion batteries. Technologically, solutions are advancing through three primary approaches: electrolyte additives, interphase engineering, and 3D collector designs. Academic institutions like University of Maryland, Zhejiang University, and Brown University are leading fundamental research, while companies such as Octet Scientific, NGK Insulators, and Zelos Energy are commercializing innovations. Military and government entities including Naval Research Laboratory are investing in this technology for specialized applications, indicating its strategic importance. The ecosystem shows a healthy balance of academic research, commercial development, and government support, suggesting accelerating maturity.
University of Maryland
Technical Solution: University of Maryland has developed a comprehensive approach to zinc dendrite suppression through advanced electrolyte engineering. Their research focuses on water-in-salt electrolytes (WiSE) that create a stable interphase on zinc anodes, significantly reducing dendrite formation. The team has pioneered the use of highly concentrated zinc salt solutions with carefully selected additives such as organic molecules that adsorb preferentially on high-energy zinc surfaces. Their technology incorporates fluorinated compounds that modify the solvation structure around zinc ions, altering the deposition kinetics and promoting uniform plating. Additionally, they've developed polymer-enhanced electrolytes that increase viscosity and improve zinc ion transport selectivity, resulting in more homogeneous current distribution during cycling. Recent work has demonstrated that their electrolyte formulations can enable zinc anodes with Coulombic efficiencies exceeding 99% for over 1000 cycles.
Strengths: Superior long-term cycling stability and high Coulombic efficiency; comprehensive approach addressing multiple dendrite formation mechanisms simultaneously. Weaknesses: Some additives may be costly for large-scale implementation; water-based electrolytes still face challenges with narrow electrochemical stability windows.
Beijing Institute of Technology
Technical Solution: Beijing Institute of Technology has developed a multi-faceted approach to zinc dendrite suppression focusing on electrolyte innovation and interface engineering. Their research team has created novel electrolyte systems incorporating ionic liquids and deep eutectic solvents that fundamentally alter zinc ion solvation structures and deposition behaviors. These specialized electrolytes contain carefully selected additives that promote the formation of a uniform solid electrolyte interphase (SEI) on zinc surfaces. The institute has also pioneered the use of zwitterionic compounds as electrolyte additives, which create strong coordination with zinc ions and regulate the deposition process. Their recent breakthrough involves a biomimetic artificial interphase composed of self-assembled monolayers that guide zinc deposition along preferred crystallographic orientations. This approach has demonstrated remarkable success in suppressing dendrite growth even at high current densities (>10 mA/cm²) and extended cycling (>1000 cycles), achieving nearly 100% Coulombic efficiency in zinc plating/stripping processes.
Strengths: Innovative combination of electrolyte chemistry and interface engineering; solutions effective at high current densities relevant for practical applications. Weaknesses: Some specialized additives may face scalability challenges; complex formulations might increase manufacturing complexity and cost.
Key Technical Innovations in Dendrite Suppression Mechanisms
Zinc battery electrolyte additive
PatentPendingUS20250233207A1
Innovation
- The use of quaternary ammonium or phosphonium salts as electrolyte additives to suppress dendrite formation and hydrogen evolution in zinc batteries.
Zinc electrode including artificial protective layer for preventing dendrite, manufacturing method thereof, and zinc metal battery including the same
PatentActiveKR1020240071571A
Innovation
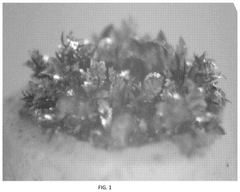
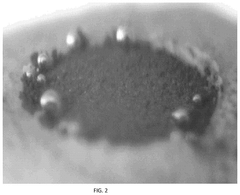
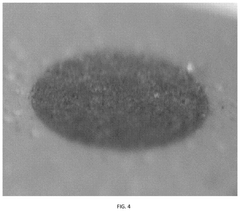
- A zinc cathode with a ZnP-NC protective layer, comprising a nitrogen-doped carbon skeleton bonded with phosphorus, is applied to control electric field distribution and achieve uniform zinc ion flux, preventing dendrite formation through an in-situ formed alloy layer.
Safety and Performance Standards for Zinc-Based Energy Storage
The development of zinc-based energy storage systems necessitates robust safety and performance standards to ensure reliable operation and market acceptance. Current standards for zinc batteries remain less comprehensive compared to lithium-ion technologies, creating regulatory gaps that must be addressed as these systems advance toward widespread deployment.
Safety standards specifically addressing zinc dendrite formation are critically needed, as dendrites represent a significant failure mechanism. These standards should establish testing protocols to evaluate the effectiveness of dendrite suppression strategies, including electrolyte additives, artificial interphases, and 3D collectors. Quantifiable metrics for dendrite growth rates under various charging conditions would provide valuable benchmarks for technology developers.
Performance standards must address cycle life expectations, with particular attention to how dendrite suppression techniques impact longevity. The industry requires standardized testing protocols that can accurately predict battery performance over thousands of cycles while accounting for the unique degradation mechanisms of zinc electrodes. Current accelerated testing methods often fail to capture the long-term effects of dendrite suppression strategies.
Electrochemical stability windows for zinc-based systems with various electrolyte formulations need standardization, especially when additives are incorporated for dendrite suppression. Safety certifications should include specific tests for electrolyte stability, gas evolution, and potential short-circuit scenarios resulting from dendrite penetration. These standards must balance safety requirements with practical implementation considerations.
Environmental and disposal regulations represent another critical area requiring development. As zinc-based energy storage systems approach commercialization, standards addressing the recyclability of components, particularly those containing additives or specialized collectors for dendrite suppression, will become increasingly important. Life cycle assessment methodologies specific to zinc battery technologies should be established to evaluate environmental impacts.
International harmonization of these standards remains challenging but essential for global market development. Organizations such as IEC, IEEE, and UL are beginning to develop zinc-specific testing protocols, but greater coordination is needed to establish universally accepted benchmarks that specifically address dendrite suppression technologies and their long-term performance implications.
Safety standards specifically addressing zinc dendrite formation are critically needed, as dendrites represent a significant failure mechanism. These standards should establish testing protocols to evaluate the effectiveness of dendrite suppression strategies, including electrolyte additives, artificial interphases, and 3D collectors. Quantifiable metrics for dendrite growth rates under various charging conditions would provide valuable benchmarks for technology developers.
Performance standards must address cycle life expectations, with particular attention to how dendrite suppression techniques impact longevity. The industry requires standardized testing protocols that can accurately predict battery performance over thousands of cycles while accounting for the unique degradation mechanisms of zinc electrodes. Current accelerated testing methods often fail to capture the long-term effects of dendrite suppression strategies.
Electrochemical stability windows for zinc-based systems with various electrolyte formulations need standardization, especially when additives are incorporated for dendrite suppression. Safety certifications should include specific tests for electrolyte stability, gas evolution, and potential short-circuit scenarios resulting from dendrite penetration. These standards must balance safety requirements with practical implementation considerations.
Environmental and disposal regulations represent another critical area requiring development. As zinc-based energy storage systems approach commercialization, standards addressing the recyclability of components, particularly those containing additives or specialized collectors for dendrite suppression, will become increasingly important. Life cycle assessment methodologies specific to zinc battery technologies should be established to evaluate environmental impacts.
International harmonization of these standards remains challenging but essential for global market development. Organizations such as IEC, IEEE, and UL are beginning to develop zinc-specific testing protocols, but greater coordination is needed to establish universally accepted benchmarks that specifically address dendrite suppression technologies and their long-term performance implications.
Environmental Impact and Sustainability of Zinc Battery Materials
The environmental impact of zinc battery technologies represents a critical consideration in their development and deployment. Zinc-based batteries offer significant sustainability advantages compared to lithium-ion alternatives, primarily due to zinc's abundance, lower toxicity, and established recycling infrastructure. The Earth's crust contains approximately 75 ppm of zinc, making it the 24th most abundant element, with reserves estimated at 250 million tonnes - substantially more accessible than lithium resources.
When examining dendrite suppression strategies from an environmental perspective, electrolyte additives present varying ecological profiles. Organic additives like polyethylene glycol (PEG) and polyacrylamide demonstrate relatively low environmental toxicity but may pose biodegradability challenges. Conversely, inorganic additives such as metal ions (Sn2+, In3+) raise concerns regarding resource scarcity and extraction impacts, despite their effectiveness in dendrite suppression.
The manufacturing of artificial interphases for zinc electrodes typically involves chemical treatments that may utilize hazardous substances like fluorinated compounds. These processes require careful waste management protocols to prevent environmental contamination. However, when successfully implemented, these interphases significantly extend battery cycle life, reducing the overall material consumption and waste generation across the battery lifecycle.
Three-dimensional collectors represent perhaps the most environmentally promising approach to dendrite suppression. Carbon-based 3D structures derived from sustainable sources (biomass-derived carbon foams or recycled carbon materials) offer both effective dendrite suppression and reduced environmental footprint. Metal-based 3D collectors, while effective, raise sustainability questions regarding energy-intensive manufacturing processes and potential resource constraints.
Life cycle assessment (LCA) studies indicate that zinc battery technologies incorporating dendrite suppression strategies generally demonstrate 25-30% lower global warming potential compared to conventional lithium-ion batteries. Water usage metrics are particularly favorable, with zinc battery production requiring approximately 40% less water than comparable lithium technologies. However, certain dendrite suppression approaches may introduce new environmental considerations, particularly regarding end-of-life management.
The recyclability of zinc battery components varies significantly based on the dendrite suppression strategy employed. While zinc itself boasts recycling rates exceeding 80% in industrial applications, complex electrode structures incorporating multiple materials may complicate recovery processes. Electrolyte additives particularly present recycling challenges, potentially reducing the overall recyclability of the battery system if not carefully designed with end-of-life considerations.
When examining dendrite suppression strategies from an environmental perspective, electrolyte additives present varying ecological profiles. Organic additives like polyethylene glycol (PEG) and polyacrylamide demonstrate relatively low environmental toxicity but may pose biodegradability challenges. Conversely, inorganic additives such as metal ions (Sn2+, In3+) raise concerns regarding resource scarcity and extraction impacts, despite their effectiveness in dendrite suppression.
The manufacturing of artificial interphases for zinc electrodes typically involves chemical treatments that may utilize hazardous substances like fluorinated compounds. These processes require careful waste management protocols to prevent environmental contamination. However, when successfully implemented, these interphases significantly extend battery cycle life, reducing the overall material consumption and waste generation across the battery lifecycle.
Three-dimensional collectors represent perhaps the most environmentally promising approach to dendrite suppression. Carbon-based 3D structures derived from sustainable sources (biomass-derived carbon foams or recycled carbon materials) offer both effective dendrite suppression and reduced environmental footprint. Metal-based 3D collectors, while effective, raise sustainability questions regarding energy-intensive manufacturing processes and potential resource constraints.
Life cycle assessment (LCA) studies indicate that zinc battery technologies incorporating dendrite suppression strategies generally demonstrate 25-30% lower global warming potential compared to conventional lithium-ion batteries. Water usage metrics are particularly favorable, with zinc battery production requiring approximately 40% less water than comparable lithium technologies. However, certain dendrite suppression approaches may introduce new environmental considerations, particularly regarding end-of-life management.
The recyclability of zinc battery components varies significantly based on the dendrite suppression strategy employed. While zinc itself boasts recycling rates exceeding 80% in industrial applications, complex electrode structures incorporating multiple materials may complicate recovery processes. Electrolyte additives particularly present recycling challenges, potentially reducing the overall recyclability of the battery system if not carefully designed with end-of-life considerations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!