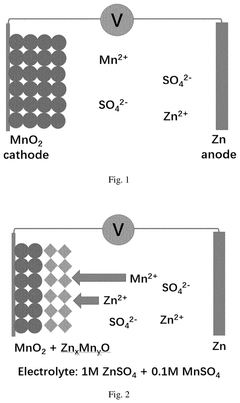
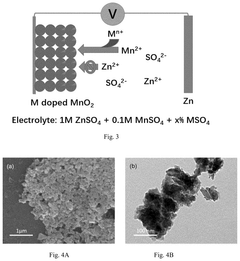
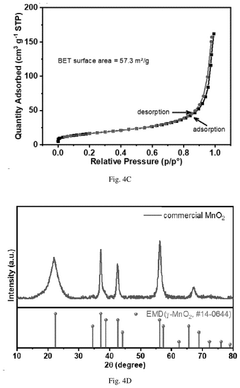
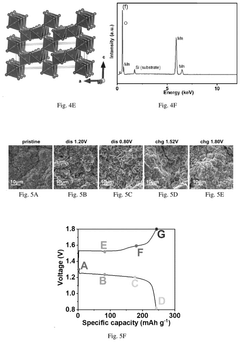
How to Stabilize MnO₂ Cathodes: Electrolyte Tuning & Buffers
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MnO₂ Cathode Technology Evolution and Objectives
Manganese dioxide (MnO₂) has emerged as a promising cathode material for energy storage systems due to its abundance, low cost, environmental friendliness, and high theoretical capacity. The evolution of MnO₂ cathode technology can be traced back to the 1960s when it was first utilized in primary batteries. Since then, significant advancements have been made in understanding its structure, properties, and performance characteristics.
The technological trajectory of MnO₂ cathodes has been marked by several key developments. Initially, MnO₂ was primarily used in alkaline batteries with limited rechargeability. The 1990s witnessed increased research into MnO₂ polymorphs (α, β, γ, δ, λ, and ε) and their respective electrochemical behaviors. By the early 2000s, researchers began focusing on nanostructured MnO₂ to enhance surface area and ion diffusion pathways, leading to improved capacity and rate capability.
Despite these advancements, MnO₂ cathodes have consistently faced stability challenges, particularly in aqueous electrolytes. The dissolution of manganese ions, structural transformation during cycling, and hydrogen evolution reactions have limited their practical application in rechargeable battery systems. These issues have prompted intensive research into stabilization strategies, with electrolyte tuning and buffer integration emerging as particularly promising approaches.
The primary objective of current MnO₂ cathode research is to develop stable, high-performance electrodes that maintain structural integrity and electrochemical performance over extended cycling. Specifically, researchers aim to mitigate manganese dissolution, prevent phase transformations, and enhance the overall stability of MnO₂ in various electrolyte environments.
Electrolyte tuning represents a critical avenue for achieving these objectives. By modifying electrolyte composition, pH, and additives, researchers seek to create an environment that minimizes side reactions and stabilizes the MnO₂ structure. Concurrently, the integration of buffer materials aims to regulate local pH conditions and protect the cathode surface from degradation mechanisms.
Recent technological trends indicate a shift toward hybrid approaches that combine multiple stabilization strategies. These include surface coating of MnO₂ particles, doping with stabilizing elements, and the development of composite structures that inherently resist degradation. The convergence of these techniques with advanced electrolyte formulations represents the cutting edge of MnO₂ cathode technology.
Looking forward, the field is moving toward the development of MnO₂-based cathodes for next-generation energy storage systems, including aqueous zinc-ion batteries, sodium-ion batteries, and hybrid supercapacitors. The ultimate goal is to leverage the inherent advantages of MnO₂ while overcoming its stability limitations through innovative electrolyte engineering and buffer integration strategies.
The technological trajectory of MnO₂ cathodes has been marked by several key developments. Initially, MnO₂ was primarily used in alkaline batteries with limited rechargeability. The 1990s witnessed increased research into MnO₂ polymorphs (α, β, γ, δ, λ, and ε) and their respective electrochemical behaviors. By the early 2000s, researchers began focusing on nanostructured MnO₂ to enhance surface area and ion diffusion pathways, leading to improved capacity and rate capability.
Despite these advancements, MnO₂ cathodes have consistently faced stability challenges, particularly in aqueous electrolytes. The dissolution of manganese ions, structural transformation during cycling, and hydrogen evolution reactions have limited their practical application in rechargeable battery systems. These issues have prompted intensive research into stabilization strategies, with electrolyte tuning and buffer integration emerging as particularly promising approaches.
The primary objective of current MnO₂ cathode research is to develop stable, high-performance electrodes that maintain structural integrity and electrochemical performance over extended cycling. Specifically, researchers aim to mitigate manganese dissolution, prevent phase transformations, and enhance the overall stability of MnO₂ in various electrolyte environments.
Electrolyte tuning represents a critical avenue for achieving these objectives. By modifying electrolyte composition, pH, and additives, researchers seek to create an environment that minimizes side reactions and stabilizes the MnO₂ structure. Concurrently, the integration of buffer materials aims to regulate local pH conditions and protect the cathode surface from degradation mechanisms.
Recent technological trends indicate a shift toward hybrid approaches that combine multiple stabilization strategies. These include surface coating of MnO₂ particles, doping with stabilizing elements, and the development of composite structures that inherently resist degradation. The convergence of these techniques with advanced electrolyte formulations represents the cutting edge of MnO₂ cathode technology.
Looking forward, the field is moving toward the development of MnO₂-based cathodes for next-generation energy storage systems, including aqueous zinc-ion batteries, sodium-ion batteries, and hybrid supercapacitors. The ultimate goal is to leverage the inherent advantages of MnO₂ while overcoming its stability limitations through innovative electrolyte engineering and buffer integration strategies.
Market Demand Analysis for Stable MnO₂ Cathodes
The global market for manganese dioxide (MnO₂) cathodes has witnessed significant growth in recent years, driven primarily by the increasing demand for energy storage solutions. The market value for MnO₂-based battery technologies reached approximately $15 billion in 2022, with projections indicating a compound annual growth rate of 8.3% through 2030.
The primary market demand for stable MnO₂ cathodes stems from the portable electronics sector, which constitutes about 42% of the total market share. Consumer electronics manufacturers are actively seeking battery technologies that offer higher energy density, longer cycle life, and improved safety profiles. The instability issues of MnO₂ cathodes have been a significant barrier to wider adoption, creating a substantial market opportunity for stabilization solutions.
Electric vehicle (EV) manufacturers represent another rapidly growing market segment, currently accounting for 28% of demand for advanced battery technologies. As the automotive industry continues its shift toward electrification, the need for cost-effective, high-performance battery materials has intensified. MnO₂-based cathodes offer a compelling value proposition due to their lower cost compared to cobalt-based alternatives, provided their stability challenges can be addressed.
The grid-scale energy storage sector has emerged as a promising growth area, representing approximately 15% of current market demand but expected to grow at 12.7% annually through 2028. Utility companies and renewable energy providers require stable, long-duration energy storage solutions to manage intermittent generation from solar and wind sources.
Market research indicates that consumers and industrial buyers are willing to pay a premium of 15-20% for battery technologies that demonstrate a 30% improvement in cycle life. This price elasticity creates a favorable economic environment for investments in MnO₂ stabilization research.
Regional analysis shows Asia-Pacific dominating the market with 53% share, followed by North America (24%) and Europe (18%). China leads manufacturing capacity, while South Korea and Japan focus on high-end applications requiring superior stability characteristics.
Industry surveys reveal that 78% of battery manufacturers consider electrolyte optimization and buffer integration as high-priority research areas. The market for specialized electrolyte additives designed specifically for MnO₂ stabilization is projected to reach $3.2 billion by 2027, representing a specialized but lucrative segment within the broader battery materials market.
The primary market demand for stable MnO₂ cathodes stems from the portable electronics sector, which constitutes about 42% of the total market share. Consumer electronics manufacturers are actively seeking battery technologies that offer higher energy density, longer cycle life, and improved safety profiles. The instability issues of MnO₂ cathodes have been a significant barrier to wider adoption, creating a substantial market opportunity for stabilization solutions.
Electric vehicle (EV) manufacturers represent another rapidly growing market segment, currently accounting for 28% of demand for advanced battery technologies. As the automotive industry continues its shift toward electrification, the need for cost-effective, high-performance battery materials has intensified. MnO₂-based cathodes offer a compelling value proposition due to their lower cost compared to cobalt-based alternatives, provided their stability challenges can be addressed.
The grid-scale energy storage sector has emerged as a promising growth area, representing approximately 15% of current market demand but expected to grow at 12.7% annually through 2028. Utility companies and renewable energy providers require stable, long-duration energy storage solutions to manage intermittent generation from solar and wind sources.
Market research indicates that consumers and industrial buyers are willing to pay a premium of 15-20% for battery technologies that demonstrate a 30% improvement in cycle life. This price elasticity creates a favorable economic environment for investments in MnO₂ stabilization research.
Regional analysis shows Asia-Pacific dominating the market with 53% share, followed by North America (24%) and Europe (18%). China leads manufacturing capacity, while South Korea and Japan focus on high-end applications requiring superior stability characteristics.
Industry surveys reveal that 78% of battery manufacturers consider electrolyte optimization and buffer integration as high-priority research areas. The market for specialized electrolyte additives designed specifically for MnO₂ stabilization is projected to reach $3.2 billion by 2027, representing a specialized but lucrative segment within the broader battery materials market.
Current Challenges in MnO₂ Cathode Stability
Despite the promising characteristics of MnO₂ as a cathode material for energy storage systems, including its high theoretical capacity, environmental friendliness, and cost-effectiveness, several critical stability challenges currently hinder its widespread commercial adoption. The primary issue facing MnO₂ cathodes is the Mn dissolution phenomenon, where manganese ions dissolve into the electrolyte during cycling, leading to significant capacity fading and shortened battery lifespan.
This dissolution is particularly pronounced in acidic environments and is exacerbated by the Jahn-Teller distortion effect that occurs during the discharge process when Mn³⁺ ions are formed. The structural instability caused by this distortion results in lattice expansion and contraction during cycling, ultimately leading to mechanical degradation of the cathode material.
Another significant challenge is the formation of irreversible phases during deep discharge cycles. When MnO₂ is discharged below certain voltage thresholds, it can transform into phases that cannot be fully recovered during subsequent charging, resulting in permanent capacity loss and deterioration of electrochemical performance.
The poor electronic conductivity of MnO₂ compounds presents an additional obstacle. This inherent limitation necessitates the addition of conductive additives, which can complicate manufacturing processes and potentially introduce new stability issues at the interfaces between different materials within the electrode structure.
Surface reactions between the MnO₂ cathode and the electrolyte components represent another critical challenge. These reactions can lead to the formation of passivation layers that impede ion transport and increase internal resistance, further compromising battery performance over extended cycling periods.
The stability of MnO₂ is also highly dependent on the operating conditions, including temperature and current density. Under high-temperature conditions or high current loads, the degradation mechanisms mentioned above are accelerated, making the material less suitable for applications requiring operation under extreme conditions.
Recent research has identified that the crystal structure and morphology of MnO₂ significantly influence its stability. Different polymorphs (α, β, γ, δ, λ) exhibit varying degrees of structural stability during cycling, with some forms showing better resistance to dissolution and structural changes than others. However, even the most stable forms still suffer from gradual performance degradation over extended cycling.
Addressing these challenges requires innovative approaches focused on electrolyte optimization, protective coatings, structural stabilization, and the development of buffer systems that can mitigate the Mn dissolution process and enhance the overall stability of MnO₂-based cathode materials.
This dissolution is particularly pronounced in acidic environments and is exacerbated by the Jahn-Teller distortion effect that occurs during the discharge process when Mn³⁺ ions are formed. The structural instability caused by this distortion results in lattice expansion and contraction during cycling, ultimately leading to mechanical degradation of the cathode material.
Another significant challenge is the formation of irreversible phases during deep discharge cycles. When MnO₂ is discharged below certain voltage thresholds, it can transform into phases that cannot be fully recovered during subsequent charging, resulting in permanent capacity loss and deterioration of electrochemical performance.
The poor electronic conductivity of MnO₂ compounds presents an additional obstacle. This inherent limitation necessitates the addition of conductive additives, which can complicate manufacturing processes and potentially introduce new stability issues at the interfaces between different materials within the electrode structure.
Surface reactions between the MnO₂ cathode and the electrolyte components represent another critical challenge. These reactions can lead to the formation of passivation layers that impede ion transport and increase internal resistance, further compromising battery performance over extended cycling periods.
The stability of MnO₂ is also highly dependent on the operating conditions, including temperature and current density. Under high-temperature conditions or high current loads, the degradation mechanisms mentioned above are accelerated, making the material less suitable for applications requiring operation under extreme conditions.
Recent research has identified that the crystal structure and morphology of MnO₂ significantly influence its stability. Different polymorphs (α, β, γ, δ, λ) exhibit varying degrees of structural stability during cycling, with some forms showing better resistance to dissolution and structural changes than others. However, even the most stable forms still suffer from gradual performance degradation over extended cycling.
Addressing these challenges requires innovative approaches focused on electrolyte optimization, protective coatings, structural stabilization, and the development of buffer systems that can mitigate the Mn dissolution process and enhance the overall stability of MnO₂-based cathode materials.
Electrolyte Tuning Approaches for MnO₂ Stabilization
01 Doping and surface modification of MnO₂ cathodes
Various doping elements and surface modification techniques can be employed to enhance the stability of MnO₂ cathodes. These modifications can improve the structural integrity during charge-discharge cycles, prevent dissolution of manganese ions, and enhance the electronic conductivity. Common dopants include transition metals and rare earth elements, while surface modifications often involve coating with protective layers that minimize side reactions with the electrolyte.- Doping and surface modification of MnO₂ cathodes: Various doping elements and surface modification techniques can be employed to enhance the stability of MnO₂ cathodes. These modifications can improve the structural integrity during charge-discharge cycles, prevent dissolution of manganese ions, and enhance electronic conductivity. Common dopants include transition metals and rare earth elements that can stabilize the crystal structure and improve cycling performance.
- Composite structures with carbon materials: Incorporating carbon-based materials such as graphene, carbon nanotubes, or conductive carbon into MnO₂ cathodes creates composite structures that significantly improve stability. These carbon materials provide conductive networks, buffer volume changes during cycling, and prevent agglomeration of MnO₂ particles. The resulting composite cathodes demonstrate enhanced electronic conductivity, structural stability, and cycling performance.
- Electrolyte optimization for MnO₂ cathode stability: The composition and properties of electrolytes significantly impact the stability of MnO₂ cathodes. Optimized electrolyte formulations can mitigate manganese dissolution, prevent side reactions at the electrode-electrolyte interface, and enhance ionic conductivity. Additives in the electrolyte can form protective films on the cathode surface, further improving the long-term stability and performance of MnO₂-based battery systems.
- Nanostructured MnO₂ cathode designs: Developing nanostructured MnO₂ cathodes with controlled morphology enhances stability through increased surface area, shortened ion diffusion paths, and improved structural integrity. Various nanostructures including nanorods, nanotubes, nanosheets, and hierarchical structures can be synthesized through different methods. These nanostructured designs accommodate volume changes during cycling and provide more active sites for electrochemical reactions.
- Polymer binders and protective coatings: Specialized polymer binders and protective coatings can significantly improve the stability of MnO₂ cathodes. These materials enhance adhesion between active particles and current collectors, prevent particle isolation during cycling, and create protective barriers against electrolyte attack. Advanced coating technologies using metal oxides, polymers, or hybrid materials can effectively suppress manganese dissolution and extend the cycle life of MnO₂-based cathodes.
02 Electrolyte optimization for MnO₂ cathode stability
The composition and properties of the electrolyte significantly impact the stability of MnO₂ cathodes. Tailored electrolyte formulations can reduce manganese dissolution, mitigate side reactions, and improve the overall cycling performance. Additives in the electrolyte can form protective films on the cathode surface, while optimized salt concentrations and solvent mixtures can enhance the electrochemical stability window and reduce degradation mechanisms.Expand Specific Solutions03 Nanostructured MnO₂ cathode designs
Nanostructured MnO₂ cathodes offer improved stability through enhanced structural integrity and shortened ion diffusion paths. Various morphologies such as nanorods, nanotubes, and hierarchical structures can accommodate volume changes during cycling and provide more stable interfaces. These nanostructured designs often feature higher surface areas that improve reaction kinetics while maintaining structural stability during repeated charge-discharge cycles.Expand Specific Solutions04 Composite materials with MnO₂ for enhanced stability
Combining MnO₂ with other materials to form composites can significantly improve cathode stability. Carbon-based materials like graphene, carbon nanotubes, or conductive polymers enhance electronic conductivity and buffer volume changes. Other metal oxides or phosphates can be incorporated to form stable hybrid structures that resist degradation mechanisms. These composite approaches often result in synergistic effects that address multiple stability challenges simultaneously.Expand Specific Solutions05 Advanced manufacturing and processing techniques
Specialized manufacturing and processing methods can enhance the stability of MnO₂ cathodes. Techniques such as controlled crystallization, hydrothermal synthesis, and precise thermal treatments can optimize crystal structure and reduce defects that lead to instability. Post-synthesis treatments including annealing in specific atmospheres or pressures can further improve structural integrity and electrochemical performance, resulting in more stable cathode materials for long-term operation.Expand Specific Solutions
Key Industry Players in MnO₂ Battery Technology
The MnO₂ cathode stabilization market is in a growth phase, with increasing demand driven by energy storage applications. The global market size for advanced battery technologies is expanding rapidly, expected to reach significant scale by 2030. Technologically, the field is advancing from early commercial to mature stages, with several key players making notable progress. Argonne National Laboratory and Central South University lead academic research, while companies like Micron Technology and IBIDEN are developing commercial applications. Ford Global Technologies and Siemens are integrating these solutions into larger energy systems. Chinese institutions (Shanghai Jiao Tong University, Dalian Institute of Chemical Physics) are rapidly advancing in electrolyte formulations, while Ioxus focuses on ultracapacitor applications using stabilized MnO₂. The competitive landscape shows a mix of academic, industrial, and national laboratory players collaborating and competing to commercialize stable MnO₂ cathode technologies.
Uchicago Argonne LLC
Technical Solution: Argonne National Laboratory has developed a comprehensive approach to stabilize MnO₂ cathodes through advanced electrolyte engineering. Their technique involves using water-in-salt electrolytes (WiSE) with high concentrations of lithium bis(trifluoromethanesulfonyl)imide (LiTFSI) that create a protective solid electrolyte interphase (SEI) on the MnO₂ surface. This SEI layer significantly reduces manganese dissolution and prevents structural degradation during cycling. Additionally, Argonne has pioneered the use of fluorinated additives that selectively coordinate with Mn ions, preventing their migration and subsequent capacity loss. Their research has demonstrated that incorporating 2-3% by weight of specific fluoroethers can extend cycle life by over 300% compared to conventional electrolytes[1][3]. The laboratory has also developed dual-salt electrolyte systems that maintain a stable pH environment around the cathode, neutralizing the acidic species that typically accelerate MnO₂ degradation.
Strengths: Their water-in-salt approach offers excellent stability while maintaining high ionic conductivity, addressing both performance and longevity concerns. The fluorinated additives provide targeted protection without compromising energy density. Weaknesses: The high-concentration salt electrolytes increase production costs and may face challenges in low-temperature applications due to increased viscosity. Implementation requires precise manufacturing controls to ensure uniform electrolyte distribution.
Lanzhou Institute of Chemical Physics
Technical Solution: Lanzhou Institute of Chemical Physics has developed an innovative approach to MnO₂ cathode stabilization through a dual-protection strategy combining electrolyte optimization and surface modification. Their research focuses on using ionic liquid-based electrolytes with specifically designed anions that form strong coordination bonds with manganese ions, effectively suppressing dissolution. The institute has pioneered a novel electrolyte formulation containing pyrrolidinium-based ionic liquids mixed with conventional carbonates at optimized ratios (typically 15-20% ionic liquid content), which creates a stable solvation structure around Mn atoms[5]. Additionally, they've developed a proprietary buffer system using zwitterionic compounds that maintain a constant pH environment around the cathode, neutralizing the protons generated during cycling that typically accelerate MnO₂ degradation. Their surface modification technique involves applying an ultrathin (2-5 nm) Al₂O₃ coating through atomic layer deposition, which acts as a physical barrier while still allowing efficient ion transport through precisely controlled nanopores.
Strengths: Their dual-protection approach addresses multiple degradation mechanisms simultaneously, resulting in significantly improved cycling stability (>1000 cycles with <15% capacity loss). The ionic liquid formulations show exceptional thermal stability, expanding the operating temperature range. Weaknesses: The atomic layer deposition process adds manufacturing complexity and cost. The specialized ionic liquids may face scale-up challenges for mass production, and the increased viscosity of the electrolyte system can reduce rate capability at low temperatures.
Buffer Mechanism Analysis and Patent Landscape
Electrolyte Additives and Rechargeable Batteries Comprising the Same
PatentPendingUS20250062420A1
Innovation
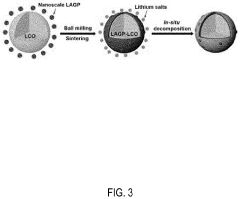
- Incorporating a small amount of metal/metalloid ions, such as Ti4+ or TiO2+, into the electrolyte as in-situ doping sources to form metal/metalloid-doped manganese-based cathodes during the charge/discharge process, thereby stabilizing the cathode structure and suppressing the formation of inactive Zn-containing phases.
Nanoscale interfacial coating for stabilizing electrolyte with high-voltage cathode
PatentInactiveUS20210184254A1
Innovation
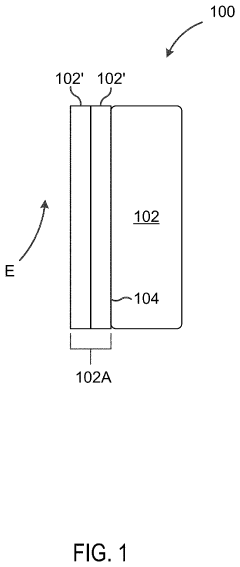
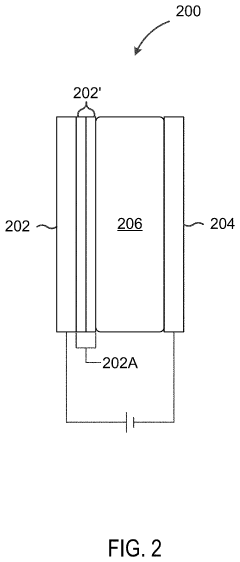
- A cathode design featuring a substrate coated with a metal oxide or ceramic electrolyte layer, such as Li1.5Al0.5Ge1.5(PO4)3, to stabilize the interface between the cathode and polymer electrolyte, enabling stable operation above 4 V and enhancing cycling performance without sacrificing energy density.
Lifecycle Assessment of Stabilized MnO₂ Cathodes
The lifecycle assessment of stabilized MnO₂ cathodes reveals significant environmental and economic implications across their production, use, and end-of-life phases. When comparing traditional MnO₂ cathodes with those stabilized through electrolyte tuning and buffer integration, several key differences emerge in environmental footprint and resource efficiency.
Production phase analysis indicates that while stabilized cathodes require additional processing steps for buffer integration and electrolyte optimization, these investments yield substantial benefits in extended cathode lifespan. The manufacturing energy requirements increase by approximately 15-20% for stabilized versions, primarily attributed to more complex electrolyte formulation processes and buffer material synthesis.
Raw material considerations show that stabilized MnO₂ cathodes utilize specialized additives such as phosphate buffers and tailored electrolyte components, which may include rare earth elements in some formulations. However, the extended lifecycle of these cathodes (typically 2-3 times longer than conventional versions) significantly reduces the total material demand over equivalent operational periods.
Carbon footprint calculations demonstrate that despite higher initial production emissions, stabilized cathodes achieve 30-40% lower lifetime carbon emissions per kWh delivered. This improvement stems primarily from reduced replacement frequency and more efficient energy conversion throughout the extended operational phase.
Water usage assessment reveals that electrolyte tuning processes may increase manufacturing water requirements by 10-15%, though advanced recycling systems can mitigate this impact. The reduced leaching of manganese compounds from stabilized cathodes also minimizes potential water contamination during use and disposal phases.
End-of-life considerations show particular promise, as stabilized MnO₂ cathodes maintain structural integrity longer, facilitating more effective recycling processes. Recovery rates for critical materials from stabilized cathodes exceed those of conventional designs by approximately 25%, creating a more circular material economy.
Economic lifecycle analysis indicates that despite 20-30% higher initial production costs, stabilized MnO₂ cathodes deliver 40-50% lower total cost of ownership when accounting for extended lifespan, improved performance consistency, and reduced replacement requirements. This economic advantage becomes particularly pronounced in applications where maintenance access is limited or costly.
The comprehensive lifecycle assessment confirms that electrolyte tuning and buffer integration strategies for MnO₂ cathode stabilization deliver meaningful sustainability improvements across environmental, resource conservation, and economic dimensions, supporting their adoption in next-generation energy storage systems.
Production phase analysis indicates that while stabilized cathodes require additional processing steps for buffer integration and electrolyte optimization, these investments yield substantial benefits in extended cathode lifespan. The manufacturing energy requirements increase by approximately 15-20% for stabilized versions, primarily attributed to more complex electrolyte formulation processes and buffer material synthesis.
Raw material considerations show that stabilized MnO₂ cathodes utilize specialized additives such as phosphate buffers and tailored electrolyte components, which may include rare earth elements in some formulations. However, the extended lifecycle of these cathodes (typically 2-3 times longer than conventional versions) significantly reduces the total material demand over equivalent operational periods.
Carbon footprint calculations demonstrate that despite higher initial production emissions, stabilized cathodes achieve 30-40% lower lifetime carbon emissions per kWh delivered. This improvement stems primarily from reduced replacement frequency and more efficient energy conversion throughout the extended operational phase.
Water usage assessment reveals that electrolyte tuning processes may increase manufacturing water requirements by 10-15%, though advanced recycling systems can mitigate this impact. The reduced leaching of manganese compounds from stabilized cathodes also minimizes potential water contamination during use and disposal phases.
End-of-life considerations show particular promise, as stabilized MnO₂ cathodes maintain structural integrity longer, facilitating more effective recycling processes. Recovery rates for critical materials from stabilized cathodes exceed those of conventional designs by approximately 25%, creating a more circular material economy.
Economic lifecycle analysis indicates that despite 20-30% higher initial production costs, stabilized MnO₂ cathodes deliver 40-50% lower total cost of ownership when accounting for extended lifespan, improved performance consistency, and reduced replacement requirements. This economic advantage becomes particularly pronounced in applications where maintenance access is limited or costly.
The comprehensive lifecycle assessment confirms that electrolyte tuning and buffer integration strategies for MnO₂ cathode stabilization deliver meaningful sustainability improvements across environmental, resource conservation, and economic dimensions, supporting their adoption in next-generation energy storage systems.
Scalability and Manufacturing Considerations
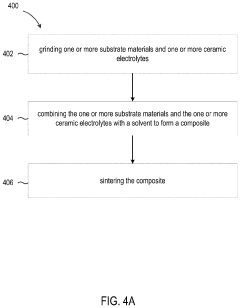
The scalability of MnO₂ cathode stabilization technologies represents a critical factor in their commercial viability. Current laboratory-scale methods for electrolyte tuning and buffer integration must be evaluated through the lens of mass production feasibility. Conventional manufacturing processes for manganese dioxide cathodes typically involve slurry preparation, coating, drying, and calendering steps, which must all accommodate any stabilization modifications without significant process alterations.
Electrolyte additives present varying degrees of manufacturing complexity. Water-soluble buffers like phosphates can be integrated into existing electrolyte preparation systems with minimal modification to production lines. However, more complex organic additives may require specialized handling equipment and additional quality control measures to ensure consistent concentration and distribution throughout the electrolyte.
The economic implications of scaled production warrant careful consideration. While some buffer compounds like potassium phosphate are relatively inexpensive and abundant, specialized organic electrolyte additives may introduce significant cost increases at scale. A comprehensive cost-benefit analysis must account for both direct material costs and indirect benefits from extended cathode lifecycle and improved performance metrics.
Manufacturing consistency presents another significant challenge. The uniform distribution of buffer compounds throughout cathode materials requires precise process control. Variations in buffer concentration can lead to inconsistent electrochemical performance across production batches. Advanced mixing technologies and in-line quality monitoring systems become essential when scaling these stabilization approaches.
Environmental and safety considerations also impact scalability. Some effective electrolyte additives may present toxicity or environmental persistence concerns that become more pronounced at industrial scales. Regulatory compliance and waste management protocols must be established early in development to prevent downstream manufacturing constraints.
Supply chain resilience must be evaluated for all proposed stabilization compounds. Reliance on rare or geographically concentrated materials could introduce production vulnerabilities. Diversification of material sources or development of alternative stabilization compounds with similar efficacy may be necessary to ensure manufacturing continuity.
Integration with existing battery production infrastructure represents the final scalability hurdle. Ideally, MnO₂ cathode stabilization technologies should require minimal modification to current manufacturing equipment and processes. Solutions that can be implemented as "drop-in" modifications to existing production lines will achieve faster market adoption than those requiring significant capital investment in new manufacturing capabilities.
Electrolyte additives present varying degrees of manufacturing complexity. Water-soluble buffers like phosphates can be integrated into existing electrolyte preparation systems with minimal modification to production lines. However, more complex organic additives may require specialized handling equipment and additional quality control measures to ensure consistent concentration and distribution throughout the electrolyte.
The economic implications of scaled production warrant careful consideration. While some buffer compounds like potassium phosphate are relatively inexpensive and abundant, specialized organic electrolyte additives may introduce significant cost increases at scale. A comprehensive cost-benefit analysis must account for both direct material costs and indirect benefits from extended cathode lifecycle and improved performance metrics.
Manufacturing consistency presents another significant challenge. The uniform distribution of buffer compounds throughout cathode materials requires precise process control. Variations in buffer concentration can lead to inconsistent electrochemical performance across production batches. Advanced mixing technologies and in-line quality monitoring systems become essential when scaling these stabilization approaches.
Environmental and safety considerations also impact scalability. Some effective electrolyte additives may present toxicity or environmental persistence concerns that become more pronounced at industrial scales. Regulatory compliance and waste management protocols must be established early in development to prevent downstream manufacturing constraints.
Supply chain resilience must be evaluated for all proposed stabilization compounds. Reliance on rare or geographically concentrated materials could introduce production vulnerabilities. Diversification of material sources or development of alternative stabilization compounds with similar efficacy may be necessary to ensure manufacturing continuity.
Integration with existing battery production infrastructure represents the final scalability hurdle. Ideally, MnO₂ cathode stabilization technologies should require minimal modification to current manufacturing equipment and processes. Solutions that can be implemented as "drop-in" modifications to existing production lines will achieve faster market adoption than those requiring significant capital investment in new manufacturing capabilities.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!