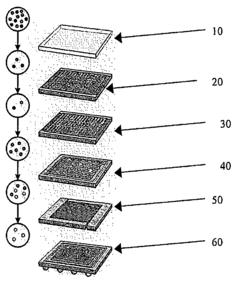
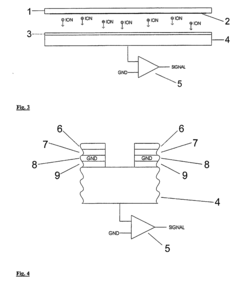
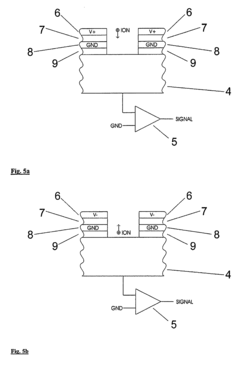
How to Model Ion Transport in Hydrated Channels
AUG 28, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ion Transport Modeling Background and Objectives
Ion transport across hydrated channels represents a fundamental process in biological systems, governing essential functions from neural signaling to cellular homeostasis. The study of ion transport mechanisms has evolved significantly over the past five decades, transitioning from basic electrophysiological observations to sophisticated computational models that incorporate quantum mechanical principles. This technological progression has been driven by advances in computational capabilities, experimental techniques, and theoretical frameworks that collectively enhance our understanding of ion-water interactions within confined spaces.
The field began with simplistic continuum models in the 1950s and has now advanced to include molecular dynamics simulations that can track individual ion movements at femtosecond resolution. Recent breakthroughs in cryo-electron microscopy have provided unprecedented structural insights into ion channels, complementing computational approaches and creating a more comprehensive understanding of transport mechanisms.
Current technological trends point toward multi-scale modeling approaches that bridge quantum mechanical calculations with coarse-grained simulations, allowing researchers to capture both atomic-level interactions and macroscopic transport phenomena. Machine learning algorithms are increasingly being integrated into these models, enabling more accurate predictions of ion selectivity and conductance based on channel structure and environmental conditions.
The primary objective of ion transport modeling is to develop predictive frameworks that accurately represent the behavior of ions in hydrated channels across different spatial and temporal scales. These models aim to elucidate the molecular determinants of ion selectivity, the energetics of ion permeation, and the influence of water molecules on transport kinetics. Additionally, they seek to explain how structural changes in channels affect functional outcomes, providing insights relevant to drug design and biomedical applications.
Secondary goals include establishing standardized protocols for model validation against experimental data, developing user-friendly simulation platforms accessible to researchers without extensive computational expertise, and creating databases of ion channel parameters to facilitate comparative studies across different biological systems.
The ultimate technological target is to achieve computational models capable of predicting ion channel behavior with sufficient accuracy to guide pharmaceutical development, inform bioengineering applications, and enhance our fundamental understanding of cellular physiology. This requires overcoming current limitations in computational efficiency, force field accuracy, and the representation of complex biological environments.
As we advance toward these objectives, the field is witnessing increasing collaboration between computational scientists, structural biologists, and electrophysiologists, creating an interdisciplinary approach that promises to accelerate progress in modeling ion transport phenomena in hydrated channels.
The field began with simplistic continuum models in the 1950s and has now advanced to include molecular dynamics simulations that can track individual ion movements at femtosecond resolution. Recent breakthroughs in cryo-electron microscopy have provided unprecedented structural insights into ion channels, complementing computational approaches and creating a more comprehensive understanding of transport mechanisms.
Current technological trends point toward multi-scale modeling approaches that bridge quantum mechanical calculations with coarse-grained simulations, allowing researchers to capture both atomic-level interactions and macroscopic transport phenomena. Machine learning algorithms are increasingly being integrated into these models, enabling more accurate predictions of ion selectivity and conductance based on channel structure and environmental conditions.
The primary objective of ion transport modeling is to develop predictive frameworks that accurately represent the behavior of ions in hydrated channels across different spatial and temporal scales. These models aim to elucidate the molecular determinants of ion selectivity, the energetics of ion permeation, and the influence of water molecules on transport kinetics. Additionally, they seek to explain how structural changes in channels affect functional outcomes, providing insights relevant to drug design and biomedical applications.
Secondary goals include establishing standardized protocols for model validation against experimental data, developing user-friendly simulation platforms accessible to researchers without extensive computational expertise, and creating databases of ion channel parameters to facilitate comparative studies across different biological systems.
The ultimate technological target is to achieve computational models capable of predicting ion channel behavior with sufficient accuracy to guide pharmaceutical development, inform bioengineering applications, and enhance our fundamental understanding of cellular physiology. This requires overcoming current limitations in computational efficiency, force field accuracy, and the representation of complex biological environments.
As we advance toward these objectives, the field is witnessing increasing collaboration between computational scientists, structural biologists, and electrophysiologists, creating an interdisciplinary approach that promises to accelerate progress in modeling ion transport phenomena in hydrated channels.
Market Applications for Ion Transport Simulation
Ion transport simulation technologies have found significant applications across multiple industries, driving innovation and efficiency improvements in various sectors. In the pharmaceutical industry, these simulations are revolutionizing drug discovery and development processes by enabling researchers to understand how potential drug molecules interact with ion channels in cell membranes. This capability has substantially reduced the time and cost associated with early-stage drug screening, allowing pharmaceutical companies to identify promising candidates more efficiently and accelerate the development pipeline.
The medical device sector has also embraced ion transport modeling, particularly in the development of implantable devices and biosensors. Companies are utilizing these simulations to design more biocompatible materials and predict how implanted devices will interact with surrounding tissues and bodily fluids. This application has led to improved device longevity and reduced rejection rates, addressing critical challenges in the medical device industry.
Water purification and desalination technologies represent another significant market application. By simulating ion transport through specialized membranes, engineers can design more efficient filtration systems with higher throughput and lower energy consumption. Several leading water technology companies have reported efficiency improvements of 15-30% in their newest generation systems that were developed with the assistance of advanced ion transport models.
The energy storage sector, particularly battery manufacturers, has become one of the largest adopters of ion transport simulation. These models are crucial for developing next-generation battery technologies with higher energy densities, faster charging capabilities, and improved safety profiles. The electric vehicle industry specifically relies on these simulations to overcome current limitations in battery performance and accelerate the transition to sustainable transportation.
Environmental remediation represents an emerging application area where ion transport models are being used to develop more effective methods for removing contaminants from soil and groundwater. These simulations help predict how pollutants move through different geological formations and how various remediation strategies might perform under specific conditions.
Semiconductor manufacturing has also begun incorporating ion transport simulations into process development, particularly for advanced etching and deposition techniques where precise control of ion behavior is critical for achieving nanometer-scale features. This application has become increasingly important as semiconductor devices continue to shrink in size while growing in complexity.
Agricultural technology companies are utilizing these models to develop improved fertilizer formulations and delivery systems that maximize nutrient uptake while minimizing environmental runoff. This application addresses growing concerns about agricultural sustainability and the environmental impact of conventional farming practices.
The medical device sector has also embraced ion transport modeling, particularly in the development of implantable devices and biosensors. Companies are utilizing these simulations to design more biocompatible materials and predict how implanted devices will interact with surrounding tissues and bodily fluids. This application has led to improved device longevity and reduced rejection rates, addressing critical challenges in the medical device industry.
Water purification and desalination technologies represent another significant market application. By simulating ion transport through specialized membranes, engineers can design more efficient filtration systems with higher throughput and lower energy consumption. Several leading water technology companies have reported efficiency improvements of 15-30% in their newest generation systems that were developed with the assistance of advanced ion transport models.
The energy storage sector, particularly battery manufacturers, has become one of the largest adopters of ion transport simulation. These models are crucial for developing next-generation battery technologies with higher energy densities, faster charging capabilities, and improved safety profiles. The electric vehicle industry specifically relies on these simulations to overcome current limitations in battery performance and accelerate the transition to sustainable transportation.
Environmental remediation represents an emerging application area where ion transport models are being used to develop more effective methods for removing contaminants from soil and groundwater. These simulations help predict how pollutants move through different geological formations and how various remediation strategies might perform under specific conditions.
Semiconductor manufacturing has also begun incorporating ion transport simulations into process development, particularly for advanced etching and deposition techniques where precise control of ion behavior is critical for achieving nanometer-scale features. This application has become increasingly important as semiconductor devices continue to shrink in size while growing in complexity.
Agricultural technology companies are utilizing these models to develop improved fertilizer formulations and delivery systems that maximize nutrient uptake while minimizing environmental runoff. This application addresses growing concerns about agricultural sustainability and the environmental impact of conventional farming practices.
Current Challenges in Hydrated Channel Modeling
Despite significant advancements in computational methods, modeling ion transport in hydrated channels remains fraught with complex challenges. The multiscale nature of these systems presents a fundamental difficulty, as processes span from quantum-level electron interactions to macroscopic fluid dynamics. Current molecular dynamics simulations struggle to bridge these disparate scales effectively, often sacrificing accuracy at one level to gain insights at another.
Computational resource limitations continue to constrain simulation capabilities. Even with modern supercomputing facilities, achieving biologically relevant timescales (milliseconds to seconds) while maintaining atomic resolution remains prohibitively expensive. Most simulations are limited to nanosecond or microsecond timescales, missing critical rare events and slow conformational changes that influence ion permeation.
Force field accuracy represents another significant hurdle. Existing parameterizations inadequately capture the complex polarization effects and quantum mechanical interactions that occur in confined water environments. The highly polarizable nature of water molecules in narrow channels and the quantum effects of proton transport via Grotthuss mechanisms are particularly challenging to model with classical force fields.
The treatment of water molecules in confined spaces poses unique difficulties. Bulk water models fail to accurately represent the behavior of water in nanoscale confinement, where properties differ dramatically from bulk solutions. The discrete nature of water molecules becomes crucial in narrow channels, yet continuum approaches that work well for bulk solutions break down in these environments.
Boundary condition treatments remain problematic, particularly at the interface between the channel and bulk solution. Current methods often introduce artificial effects at these boundaries, distorting ion concentration profiles and transport kinetics. The challenge of properly accounting for membrane potentials and concentration gradients across these boundaries further complicates accurate modeling.
Validation of computational models against experimental data presents ongoing challenges. The limited spatial and temporal resolution of experimental techniques makes direct comparison difficult, creating a validation gap. Additionally, experimental conditions often differ significantly from simulation setups, complicating meaningful comparisons.
Integration of machine learning approaches with physics-based models offers promising directions but faces challenges in training data availability and transferability across different channel systems. The black-box nature of many machine learning methods also limits mechanistic understanding, a crucial aspect for rational channel design and drug development targeting ion channels.
Computational resource limitations continue to constrain simulation capabilities. Even with modern supercomputing facilities, achieving biologically relevant timescales (milliseconds to seconds) while maintaining atomic resolution remains prohibitively expensive. Most simulations are limited to nanosecond or microsecond timescales, missing critical rare events and slow conformational changes that influence ion permeation.
Force field accuracy represents another significant hurdle. Existing parameterizations inadequately capture the complex polarization effects and quantum mechanical interactions that occur in confined water environments. The highly polarizable nature of water molecules in narrow channels and the quantum effects of proton transport via Grotthuss mechanisms are particularly challenging to model with classical force fields.
The treatment of water molecules in confined spaces poses unique difficulties. Bulk water models fail to accurately represent the behavior of water in nanoscale confinement, where properties differ dramatically from bulk solutions. The discrete nature of water molecules becomes crucial in narrow channels, yet continuum approaches that work well for bulk solutions break down in these environments.
Boundary condition treatments remain problematic, particularly at the interface between the channel and bulk solution. Current methods often introduce artificial effects at these boundaries, distorting ion concentration profiles and transport kinetics. The challenge of properly accounting for membrane potentials and concentration gradients across these boundaries further complicates accurate modeling.
Validation of computational models against experimental data presents ongoing challenges. The limited spatial and temporal resolution of experimental techniques makes direct comparison difficult, creating a validation gap. Additionally, experimental conditions often differ significantly from simulation setups, complicating meaningful comparisons.
Integration of machine learning approaches with physics-based models offers promising directions but faces challenges in training data availability and transferability across different channel systems. The black-box nature of many machine learning methods also limits mechanistic understanding, a crucial aspect for rational channel design and drug development targeting ion channels.
State-of-the-Art Computational Approaches
01 Molecular dynamics simulation of ion transport
Computational methods using molecular dynamics simulations are employed to model ion transport through hydrated channels. These simulations account for the interactions between ions, water molecules, and channel structures to predict transport phenomena at the molecular level. The models incorporate parameters such as diffusion coefficients, electrostatic interactions, and channel geometry to accurately represent ion movement in confined hydrated environments.- Computational modeling of ion transport in hydrated channels: Advanced computational methods are used to model and simulate ion transport phenomena in hydrated channels. These models incorporate molecular dynamics, quantum mechanics, and statistical mechanics to predict ion behavior in confined spaces. The simulations account for factors such as channel geometry, hydration levels, and electrostatic interactions to accurately represent ion movement through nanoscale channels. These computational approaches help researchers understand fundamental transport mechanisms and optimize channel designs for specific applications.
- Biological ion channels and membrane transport systems: Research on biological ion channels focuses on understanding how ions are transported across cell membranes through specialized protein structures. These studies examine the mechanisms of selective ion permeation, gating, and regulation in natural systems. The hydration of ions plays a crucial role in determining transport rates and selectivity. Biomimetic approaches aim to replicate the efficiency and selectivity of biological channels in synthetic systems for applications in sensing, separation, and drug delivery.
- Synthetic nanochannels for controlled ion transport: Engineered nanochannels with precisely controlled dimensions and surface properties are developed for selective ion transport applications. These synthetic channels can be fabricated from various materials including polymers, ceramics, and composite structures. The hydration environment within these channels can be tuned to control ion selectivity, flux rates, and energy barriers. Applications include water purification, energy harvesting, sensing technologies, and separation processes where specific ion transport characteristics are required.
- Influence of hydration on ion transport mechanisms: The hydration structure surrounding ions significantly affects their transport behavior in confined channels. Research examines how water molecules organize around different ions, creating hydration shells that determine mobility and selectivity. Factors such as channel hydrophobicity, confinement effects, and hydrogen bonding networks influence the hydration environment. Understanding these hydration effects is crucial for predicting ion transport rates and designing channels with specific transport properties for applications in energy storage, desalination, and sensing.
- Experimental techniques for measuring ion transport in hydrated channels: Various experimental methods are employed to measure and characterize ion transport through hydrated channels. These include electrochemical impedance spectroscopy, patch-clamp techniques, fluorescence-based assays, and advanced microscopy approaches. These measurements provide data on ion conductance, selectivity, and gating behavior under different conditions. The experimental results are essential for validating computational models and advancing the understanding of transport phenomena in both biological and synthetic channel systems.
02 Nanopore and membrane channel transport mechanisms
Ion transport through biological and synthetic nanopores involves complex mechanisms influenced by channel hydration, surface charges, and ion selectivity. Research focuses on understanding how ions move through confined hydrated spaces in cell membranes and artificial nanochannels. These transport mechanisms are critical for applications in biosensing, drug delivery, and mimicking biological processes, with models accounting for factors such as channel diameter, surface chemistry, and hydration layers.Expand Specific Solutions03 Electrochemical transport modeling in energy applications
Ion transport modeling in hydrated channels is crucial for energy storage and conversion technologies. These models describe how ions move through hydrated pathways in materials used in batteries, fuel cells, and supercapacitors. The transport phenomena account for factors such as hydration shells around ions, interfacial effects, and the influence of electric fields on ion mobility, which are essential for improving energy device performance and efficiency.Expand Specific Solutions04 Microfluidic and lab-on-chip ion transport systems
Microfluidic platforms incorporate hydrated channels for controlled ion transport in analytical and diagnostic applications. These systems model and manipulate ion movement through precisely designed hydrated pathways at the microscale. The transport modeling accounts for fluid dynamics, surface interactions, and electrokinetic phenomena to enable applications such as chemical analysis, medical diagnostics, and environmental monitoring in miniaturized devices.Expand Specific Solutions05 Hydrogel and polymer-based ion transport materials
Hydrogels and specialized polymers create hydrated networks that facilitate controlled ion transport. These materials contain water-filled channels where ion movement can be precisely modeled and manipulated. The transport properties depend on polymer composition, crosslinking density, and environmental conditions such as pH and temperature. Applications include drug delivery systems, sensors, artificial tissues, and smart materials where controlled ion movement through hydrated pathways is essential for functionality.Expand Specific Solutions
Leading Research Groups and Companies in the Field
Ion transport modeling in hydrated channels is currently in a growth phase, with the market expanding due to increasing applications in drug discovery, medical diagnostics, and materials science. The global market size for related technologies is projected to reach several billion dollars by 2025. Technologically, the field shows moderate maturity with significant recent advancements. Leading players include Thermo Fisher Scientific, which dominates analytical instrumentation, and academic powerhouses like California Institute of Technology and Nanjing University driving fundamental research. Pharmaceutical companies such as Incyte Corp. and Pfizer (via Pharmacia & Upjohn) are leveraging these models for drug development, while specialized firms like Owlstone Medical and Hydra Biosciences focus on novel ion channel applications in diagnostics and therapeutics.
The Regents of the University of California
Technical Solution: The University of California has developed sophisticated multi-scale modeling approaches for ion transport in hydrated channels. Their methodology combines molecular dynamics (MD) simulations with continuum theories to bridge atomic and macroscopic scales. They employ enhanced sampling techniques like metadynamics and umbrella sampling to overcome energy barriers and calculate free energy profiles for ion permeation. Their models incorporate explicit water molecules and account for ion-water-channel interactions, allowing for accurate representation of hydration effects on ion selectivity and gating mechanisms. Recent advances include the development of polarizable force fields that better capture the electronic redistribution during ion transport, and machine learning approaches to parameterize these models from quantum mechanical calculations. They've successfully applied these methods to biological channels like potassium channels and synthetic nanopores, revealing how water structuring within confined spaces affects ion mobility and selectivity.
Strengths: Comprehensive multi-scale approach that bridges atomic and continuum descriptions; advanced sampling techniques that access rare events in ion permeation; integration of quantum effects through polarizable force fields. Weaknesses: Computational expense limits simulation timescales; parameterization challenges for complex biological systems; difficulty in directly validating atomic-level predictions experimentally.
California Institute of Technology
Technical Solution: Caltech has pioneered quantum mechanics/molecular mechanics (QM/MM) hybrid approaches for modeling ion transport in hydrated channels. Their methodology treats the ion and its immediate hydration shell quantum mechanically while representing the channel protein and bulk water with classical force fields. This allows accurate representation of electronic polarization effects critical for ion-water interactions in confined spaces. They've developed specialized algorithms to handle the QM/MM boundary and to efficiently sample configurations relevant to ion permeation. Their models incorporate the dynamic coupling between ion movement, water reorientation, and protein conformational changes. Recent innovations include path integral molecular dynamics to account for nuclear quantum effects in hydrogen bonding networks, which are particularly important for proton transport through water wires. They've applied these methods to understand selectivity mechanisms in ion channels and to design biomimetic nanopores with tailored transport properties.
Strengths: Accurate treatment of electronic polarization effects critical for ion-water interactions; ability to model chemical reactions during transport processes; sophisticated handling of quantum effects in hydrogen bonding networks. Weaknesses: Extremely computationally intensive, limiting system size and simulation duration; challenges in parameterizing QM/MM interfaces; requires specialized expertise spanning quantum chemistry and molecular simulation.
Key Theoretical Frameworks and Algorithms
Ion transfer tube with spatially alternating DC fields
PatentActiveUS7982183B2
Innovation
- An ion transfer arrangement utilizing a DC electrode assembly with alternating electrode widths and voltages to create spatially alternating electric fields, focusing ions away from the channel wall and toward the centerline, thereby reducing ion loss and improving transmission efficiency across a broader range of pressures.
Ion Mobility Spectrometer
PatentActiveUS20080054174A1
Innovation
- A micro machined field asymmetric ion mobility spectrometer that uses electric fields to move ions between conducting electrodes without a drift gas flow, employing a tunable ion gate to selectively admit ions to the detector, allowing for a solid-state construction with reduced size and voltage requirements.
Experimental Validation Techniques
Experimental validation is critical for confirming the accuracy and reliability of ion transport models in hydrated channels. Researchers employ various sophisticated techniques to measure and characterize ion movement through biological and synthetic channels under controlled hydration conditions.
Electrophysiology remains the gold standard for validating ion transport models, with patch-clamp techniques offering single-channel resolution of ion currents. These measurements provide direct evidence of ion conductance, selectivity, and gating kinetics that can be compared against computational predictions. Recent advances in automated patch-clamp systems have significantly increased throughput, enabling more comprehensive validation of model parameters across multiple experimental conditions.
Fluorescence-based methods offer complementary approaches for model validation. Techniques such as Förster Resonance Energy Transfer (FRET) can track conformational changes in channel proteins during ion transport, while fluorescent ion indicators provide real-time visualization of ion concentration gradients. These optical methods are particularly valuable for validating spatiotemporal aspects of ion transport models that may be difficult to capture with electrophysiological approaches alone.
Structural validation techniques, including X-ray crystallography, cryo-electron microscopy, and nuclear magnetic resonance spectroscopy, provide atomic-resolution data on channel architecture and hydration patterns. These structural insights are essential for validating the physical dimensions and water distribution patterns used in computational models. Time-resolved structural studies are emerging as powerful tools for capturing intermediate states during ion permeation events.
Isotope tracking experiments using radioactive or stable isotopes offer another validation approach. By monitoring the movement of labeled ions through channels, researchers can directly measure transport rates and selectivity under various hydration conditions. These experiments are particularly valuable for validating predictions about ion competition and cooperative transport phenomena.
Microfluidic platforms represent an innovative validation approach, allowing precise control over channel environments while enabling high-throughput measurements. These systems can recreate complex physiological conditions and are increasingly being coupled with advanced imaging techniques to provide multi-parameter validation of ion transport models.
Statistical validation frameworks are essential for quantitatively assessing model performance against experimental data. Techniques such as Bayesian inference and sensitivity analysis help identify which model parameters are most critical for accurate predictions and quantify uncertainty in model outputs. These statistical approaches guide iterative refinement of ion transport models to improve their predictive power.
Electrophysiology remains the gold standard for validating ion transport models, with patch-clamp techniques offering single-channel resolution of ion currents. These measurements provide direct evidence of ion conductance, selectivity, and gating kinetics that can be compared against computational predictions. Recent advances in automated patch-clamp systems have significantly increased throughput, enabling more comprehensive validation of model parameters across multiple experimental conditions.
Fluorescence-based methods offer complementary approaches for model validation. Techniques such as Förster Resonance Energy Transfer (FRET) can track conformational changes in channel proteins during ion transport, while fluorescent ion indicators provide real-time visualization of ion concentration gradients. These optical methods are particularly valuable for validating spatiotemporal aspects of ion transport models that may be difficult to capture with electrophysiological approaches alone.
Structural validation techniques, including X-ray crystallography, cryo-electron microscopy, and nuclear magnetic resonance spectroscopy, provide atomic-resolution data on channel architecture and hydration patterns. These structural insights are essential for validating the physical dimensions and water distribution patterns used in computational models. Time-resolved structural studies are emerging as powerful tools for capturing intermediate states during ion permeation events.
Isotope tracking experiments using radioactive or stable isotopes offer another validation approach. By monitoring the movement of labeled ions through channels, researchers can directly measure transport rates and selectivity under various hydration conditions. These experiments are particularly valuable for validating predictions about ion competition and cooperative transport phenomena.
Microfluidic platforms represent an innovative validation approach, allowing precise control over channel environments while enabling high-throughput measurements. These systems can recreate complex physiological conditions and are increasingly being coupled with advanced imaging techniques to provide multi-parameter validation of ion transport models.
Statistical validation frameworks are essential for quantitatively assessing model performance against experimental data. Techniques such as Bayesian inference and sensitivity analysis help identify which model parameters are most critical for accurate predictions and quantify uncertainty in model outputs. These statistical approaches guide iterative refinement of ion transport models to improve their predictive power.
Computational Resources and Infrastructure Requirements
Modeling ion transport in hydrated channels requires substantial computational resources due to the complex nature of molecular dynamics simulations and quantum mechanical calculations. High-performance computing (HPC) clusters are essential for running these simulations efficiently, with minimum requirements typically including multi-core processors (32+ cores), high-speed interconnects, and at least 128GB RAM per node. GPU acceleration has become increasingly important, with NVIDIA Tesla or AMD Instinct GPUs significantly reducing computation time for molecular dynamics simulations by up to 10x compared to CPU-only calculations.
Storage infrastructure presents another critical consideration, as these simulations generate massive datasets. A typical ion channel simulation can produce 1-5TB of trajectory data over a microsecond timescale. High-performance parallel file systems like Lustre or BeeGFS are recommended to handle the I/O demands, with a minimum of 10TB storage capacity and data transfer rates of at least 10GB/s to prevent bottlenecks during analysis.
Software requirements include specialized molecular dynamics packages such as NAMD, GROMACS, or AMBER, which must be properly optimized and compiled for the specific hardware architecture. Quantum chemistry software like Gaussian, ORCA, or CP2K may also be necessary for electronic structure calculations at channel binding sites. These applications require specific library dependencies and compiler optimizations to achieve maximum performance.
Cloud computing has emerged as a viable alternative to on-premises infrastructure, with providers like AWS, Google Cloud, and Microsoft Azure offering specialized HPC instances. These services provide flexibility in scaling resources according to computational demands, though careful cost analysis is essential as extended simulations can incur significant expenses. Hybrid approaches combining on-premises resources with cloud bursting capabilities during peak demand periods often represent the most cost-effective solution.
Data management systems must be implemented to handle the large volumes of simulation data, including automated backup solutions and data lifecycle management. Tools like JupyterHub or RStudio Server enable collaborative analysis environments, while workflow management systems such as Nextflow or Snakemake help orchestrate complex simulation pipelines across distributed computing resources.
Storage infrastructure presents another critical consideration, as these simulations generate massive datasets. A typical ion channel simulation can produce 1-5TB of trajectory data over a microsecond timescale. High-performance parallel file systems like Lustre or BeeGFS are recommended to handle the I/O demands, with a minimum of 10TB storage capacity and data transfer rates of at least 10GB/s to prevent bottlenecks during analysis.
Software requirements include specialized molecular dynamics packages such as NAMD, GROMACS, or AMBER, which must be properly optimized and compiled for the specific hardware architecture. Quantum chemistry software like Gaussian, ORCA, or CP2K may also be necessary for electronic structure calculations at channel binding sites. These applications require specific library dependencies and compiler optimizations to achieve maximum performance.
Cloud computing has emerged as a viable alternative to on-premises infrastructure, with providers like AWS, Google Cloud, and Microsoft Azure offering specialized HPC instances. These services provide flexibility in scaling resources according to computational demands, though careful cost analysis is essential as extended simulations can incur significant expenses. Hybrid approaches combining on-premises resources with cloud bursting capabilities during peak demand periods often represent the most cost-effective solution.
Data management systems must be implemented to handle the large volumes of simulation data, including automated backup solutions and data lifecycle management. Tools like JupyterHub or RStudio Server enable collaborative analysis environments, while workflow management systems such as Nextflow or Snakemake help orchestrate complex simulation pipelines across distributed computing resources.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!