How to Characterize Kaolinite's Chemical Composition
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Kaolinite Characterization Background and Objectives
Kaolinite, a clay mineral with the chemical formula Al₂Si₂O₅(OH)₄, has been extensively studied since its identification in the late 18th century. The characterization of kaolinite's chemical composition has evolved significantly over time, from basic wet chemical methods to advanced spectroscopic techniques. This evolution reflects broader trends in materials science and analytical chemistry, where increasingly sophisticated tools enable more precise understanding of mineral structures and compositions.
The primary objective of kaolinite characterization is to determine its elemental composition, structural arrangement, and surface properties with high accuracy and precision. This information is crucial for various industrial applications, including ceramics, paper coating, pharmaceuticals, and advanced materials development. Additionally, understanding kaolinite's composition helps in geological studies, environmental monitoring, and soil science research.
Historical approaches to kaolinite characterization began with simple gravimetric and volumetric analyses, which provided basic information about major elements but lacked precision for trace components. The mid-20th century saw the introduction of instrumental methods such as X-ray diffraction (XRD) and thermal analysis techniques, which significantly improved our understanding of kaolinite's structure and composition.
Recent technological advancements have led to the development of more sophisticated analytical methods, including X-ray fluorescence (XRF), inductively coupled plasma mass spectrometry (ICP-MS), nuclear magnetic resonance (NMR) spectroscopy, and various electron microscopy techniques. These methods offer unprecedented detail about kaolinite's chemical makeup, including trace elements and structural defects that influence its properties.
The current technical goal in kaolinite characterization focuses on developing integrated analytical approaches that combine multiple techniques to provide comprehensive compositional data. This includes not only identifying the elements present but also understanding their spatial distribution, oxidation states, and bonding environments within the mineral structure.
Another important objective is the standardization of characterization protocols to ensure consistency and comparability of results across different laboratories and research groups. This standardization is essential for quality control in industrial applications and for building reliable databases of kaolinite properties from different geological sources.
Future directions in kaolinite characterization aim to develop in-situ and real-time analytical methods that can monitor compositional changes during processing or environmental interactions. Additionally, there is growing interest in non-destructive techniques that preserve sample integrity while providing detailed compositional information, particularly for rare or historically significant specimens.
The primary objective of kaolinite characterization is to determine its elemental composition, structural arrangement, and surface properties with high accuracy and precision. This information is crucial for various industrial applications, including ceramics, paper coating, pharmaceuticals, and advanced materials development. Additionally, understanding kaolinite's composition helps in geological studies, environmental monitoring, and soil science research.
Historical approaches to kaolinite characterization began with simple gravimetric and volumetric analyses, which provided basic information about major elements but lacked precision for trace components. The mid-20th century saw the introduction of instrumental methods such as X-ray diffraction (XRD) and thermal analysis techniques, which significantly improved our understanding of kaolinite's structure and composition.
Recent technological advancements have led to the development of more sophisticated analytical methods, including X-ray fluorescence (XRF), inductively coupled plasma mass spectrometry (ICP-MS), nuclear magnetic resonance (NMR) spectroscopy, and various electron microscopy techniques. These methods offer unprecedented detail about kaolinite's chemical makeup, including trace elements and structural defects that influence its properties.
The current technical goal in kaolinite characterization focuses on developing integrated analytical approaches that combine multiple techniques to provide comprehensive compositional data. This includes not only identifying the elements present but also understanding their spatial distribution, oxidation states, and bonding environments within the mineral structure.
Another important objective is the standardization of characterization protocols to ensure consistency and comparability of results across different laboratories and research groups. This standardization is essential for quality control in industrial applications and for building reliable databases of kaolinite properties from different geological sources.
Future directions in kaolinite characterization aim to develop in-situ and real-time analytical methods that can monitor compositional changes during processing or environmental interactions. Additionally, there is growing interest in non-destructive techniques that preserve sample integrity while providing detailed compositional information, particularly for rare or historically significant specimens.
Market Applications and Demand Analysis for Kaolinite
The global market for kaolinite continues to expand significantly, driven by its versatile applications across multiple industries. The paper industry remains the largest consumer of kaolinite, accounting for approximately 40% of global demand, where it serves as a coating and filler material that enhances paper brightness, smoothness, and printability. Accurate characterization of kaolinite's chemical composition is essential for paper manufacturers to maintain consistent product quality and optimize production processes.
The ceramics industry represents the second-largest market segment, utilizing kaolinite as a primary raw material for porcelain, sanitaryware, and tiles. In this sector, precise understanding of chemical composition directly impacts product properties including whiteness, plasticity, and firing behavior. The growing construction industry in emerging economies has substantially increased demand for ceramic products, consequently driving the need for advanced kaolinite characterization methods.
Paint and coating manufacturers rely heavily on kaolinite as an extender and functional filler. Market analysis indicates this segment is growing at 5-7% annually, with particular emphasis on high-performance coatings that require precisely characterized kaolinite to ensure consistent rheological properties and opacity. Similarly, the rubber and plastics industries utilize kaolinite as a reinforcing filler where chemical composition directly affects mechanical properties and processing characteristics.
Emerging applications in pharmaceuticals and cosmetics represent rapidly growing market segments. Pharmaceutical-grade kaolinite serves as an excipient and active ingredient in various formulations, while cosmetic applications leverage its absorbent and texturizing properties. Both sectors demand exceptionally pure kaolinite with well-characterized chemical profiles to meet stringent regulatory requirements.
Environmental remediation represents a promising growth area, with kaolinite increasingly used in wastewater treatment, soil remediation, and as an adsorbent for pollutants. The effectiveness of kaolinite in these applications correlates directly with its chemical composition, particularly surface chemistry and cation exchange capacity.
Market forecasts indicate global kaolinite demand will continue growing steadily, with particularly strong expansion in Asia-Pacific regions where industrial development and urbanization drive consumption across multiple sectors. This geographic shift in demand is creating new requirements for characterization technologies that can be deployed in diverse manufacturing environments with varying levels of technical infrastructure.
Industry stakeholders consistently identify reliable chemical composition characterization as a critical factor in kaolinite valuation and application development. This underscores the economic importance of developing more accessible, accurate, and comprehensive characterization methodologies that can support both established and emerging market applications.
The ceramics industry represents the second-largest market segment, utilizing kaolinite as a primary raw material for porcelain, sanitaryware, and tiles. In this sector, precise understanding of chemical composition directly impacts product properties including whiteness, plasticity, and firing behavior. The growing construction industry in emerging economies has substantially increased demand for ceramic products, consequently driving the need for advanced kaolinite characterization methods.
Paint and coating manufacturers rely heavily on kaolinite as an extender and functional filler. Market analysis indicates this segment is growing at 5-7% annually, with particular emphasis on high-performance coatings that require precisely characterized kaolinite to ensure consistent rheological properties and opacity. Similarly, the rubber and plastics industries utilize kaolinite as a reinforcing filler where chemical composition directly affects mechanical properties and processing characteristics.
Emerging applications in pharmaceuticals and cosmetics represent rapidly growing market segments. Pharmaceutical-grade kaolinite serves as an excipient and active ingredient in various formulations, while cosmetic applications leverage its absorbent and texturizing properties. Both sectors demand exceptionally pure kaolinite with well-characterized chemical profiles to meet stringent regulatory requirements.
Environmental remediation represents a promising growth area, with kaolinite increasingly used in wastewater treatment, soil remediation, and as an adsorbent for pollutants. The effectiveness of kaolinite in these applications correlates directly with its chemical composition, particularly surface chemistry and cation exchange capacity.
Market forecasts indicate global kaolinite demand will continue growing steadily, with particularly strong expansion in Asia-Pacific regions where industrial development and urbanization drive consumption across multiple sectors. This geographic shift in demand is creating new requirements for characterization technologies that can be deployed in diverse manufacturing environments with varying levels of technical infrastructure.
Industry stakeholders consistently identify reliable chemical composition characterization as a critical factor in kaolinite valuation and application development. This underscores the economic importance of developing more accessible, accurate, and comprehensive characterization methodologies that can support both established and emerging market applications.
Current Analytical Techniques and Challenges
The characterization of kaolinite's chemical composition currently relies on a diverse array of analytical techniques, each with specific advantages and limitations. X-ray fluorescence (XRF) spectroscopy stands as one of the most widely employed methods, offering rapid elemental analysis with minimal sample preparation. This technique provides quantitative data on major elements like Si, Al, Fe, and trace elements, though it may struggle with light elements detection and requires careful calibration against standards.
X-ray diffraction (XRD) complements XRF by providing crystallographic information, allowing researchers to identify kaolinite and distinguish it from other clay minerals based on characteristic diffraction patterns. However, XRD has limitations in quantifying amorphous phases and requires relatively pure samples for optimal results.
Scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDX) offers high-resolution imaging combined with elemental mapping capabilities, providing spatial distribution of elements within kaolinite samples. This technique is particularly valuable for heterogeneous samples but is semi-quantitative and requires careful sample preparation to avoid artifacts.
Infrared spectroscopy (FTIR) has emerged as a powerful tool for identifying functional groups in kaolinite, particularly hydroxyl groups and Si-O-Al bonds. While offering rapid analysis with minimal sample preparation, FTIR faces challenges in quantitative analysis and may require complementary techniques for comprehensive characterization.
Thermal analysis techniques, including thermogravimetric analysis (TGA) and differential thermal analysis (DTA), provide insights into dehydroxylation processes and phase transformations in kaolinite. These methods are valuable for determining hydroxyl content but offer limited information about other compositional aspects.
Despite these advanced techniques, significant challenges persist in kaolinite characterization. Sample heterogeneity represents a major obstacle, as natural kaolinite deposits often contain impurities and interstratified minerals that complicate analysis. The presence of amorphous phases and structural water further complicates quantitative determination of composition.
Standardization issues also plague the field, with variations in sample preparation, instrument calibration, and data interpretation leading to inconsistencies across laboratories. The development of universal reference materials specifically for kaolinite characterization remains an ongoing challenge.
Additionally, most current techniques provide bulk analysis, offering limited insights into compositional variations at micro and nanoscales. This limitation becomes particularly problematic when studying kaolinite's surface properties and reactivity, which often depend on localized compositional features.
X-ray diffraction (XRD) complements XRF by providing crystallographic information, allowing researchers to identify kaolinite and distinguish it from other clay minerals based on characteristic diffraction patterns. However, XRD has limitations in quantifying amorphous phases and requires relatively pure samples for optimal results.
Scanning electron microscopy coupled with energy-dispersive X-ray spectroscopy (SEM-EDX) offers high-resolution imaging combined with elemental mapping capabilities, providing spatial distribution of elements within kaolinite samples. This technique is particularly valuable for heterogeneous samples but is semi-quantitative and requires careful sample preparation to avoid artifacts.
Infrared spectroscopy (FTIR) has emerged as a powerful tool for identifying functional groups in kaolinite, particularly hydroxyl groups and Si-O-Al bonds. While offering rapid analysis with minimal sample preparation, FTIR faces challenges in quantitative analysis and may require complementary techniques for comprehensive characterization.
Thermal analysis techniques, including thermogravimetric analysis (TGA) and differential thermal analysis (DTA), provide insights into dehydroxylation processes and phase transformations in kaolinite. These methods are valuable for determining hydroxyl content but offer limited information about other compositional aspects.
Despite these advanced techniques, significant challenges persist in kaolinite characterization. Sample heterogeneity represents a major obstacle, as natural kaolinite deposits often contain impurities and interstratified minerals that complicate analysis. The presence of amorphous phases and structural water further complicates quantitative determination of composition.
Standardization issues also plague the field, with variations in sample preparation, instrument calibration, and data interpretation leading to inconsistencies across laboratories. The development of universal reference materials specifically for kaolinite characterization remains an ongoing challenge.
Additionally, most current techniques provide bulk analysis, offering limited insights into compositional variations at micro and nanoscales. This limitation becomes particularly problematic when studying kaolinite's surface properties and reactivity, which often depend on localized compositional features.
State-of-the-Art Chemical Analysis Solutions
01 Basic chemical composition of kaolinite
Kaolinite is a clay mineral with the chemical formula Al₂Si₂O₅(OH)₄. It is a layered silicate mineral, with one tetrahedral sheet of silica linked through oxygen atoms to one octahedral sheet of alumina. This structure gives kaolinite its characteristic properties, including its white color, fine particle size, and plasticity when wet. The chemical composition typically consists of about 46% SiO₂, 39% Al₂O₃, and 14% H₂O.- Basic chemical composition of kaolinite: Kaolinite is a clay mineral with the chemical formula Al₂Si₂O₅(OH)₄. It is a layered silicate mineral, with one tetrahedral sheet of silica linked through oxygen atoms to one octahedral sheet of alumina. This structure gives kaolinite its characteristic properties, including its white color, fine particle size, and plasticity when wet. The chemical composition typically consists of about 46% SiO₂, 39.5% Al₂O₃, and 14% H₂O, though natural samples may contain impurities.
- Modification of kaolinite composition: The chemical composition of kaolinite can be modified through various processes to enhance its properties for specific applications. These modifications may involve intercalation with organic compounds, surface treatment with chemicals, or thermal treatment to alter the hydroxyl groups. Modified kaolinite may exhibit improved dispersion, increased surface area, enhanced adsorption capacity, or better compatibility with polymers and other materials.
- Impurities and variations in natural kaolinite: Natural kaolinite deposits contain various impurities that affect its chemical composition. Common impurities include iron oxides, titanium dioxide, quartz, mica, and other clay minerals. The presence and concentration of these impurities can significantly influence the properties of kaolinite, such as its color, plasticity, and reactivity. Purification processes are often employed to remove these impurities and obtain high-grade kaolinite with a more consistent chemical composition.
- Kaolinite in composite materials: Kaolinite is frequently incorporated into composite materials, where its chemical composition plays a crucial role in determining the properties of the final product. When combined with polymers, resins, or other materials, kaolinite can enhance mechanical strength, thermal stability, and fire resistance. The interaction between kaolinite's chemical structure and the matrix material is essential for achieving desired properties in applications such as paper coating, ceramics, and polymer composites.
- Analysis methods for kaolinite composition: Various analytical techniques are employed to determine the chemical composition of kaolinite. These include X-ray fluorescence (XRF), X-ray diffraction (XRD), infrared spectroscopy, thermal analysis, and electron microscopy. These methods provide information about the elemental composition, crystal structure, and surface properties of kaolinite. Advanced characterization techniques help in understanding the structural features and chemical bonding in kaolinite, which are crucial for its applications in different industries.
02 Modified kaolinite compositions
Kaolinite can be modified through various chemical treatments to enhance its properties for specific applications. These modifications may include intercalation with organic compounds, surface treatment with silanes or other coupling agents, or ion exchange processes. Modified kaolinite often exhibits improved dispersion characteristics, increased hydrophobicity, or enhanced compatibility with polymers and other materials. These modifications alter the surface chemistry while maintaining the basic alumino-silicate structure.Expand Specific Solutions03 Kaolinite impurities and purification
Natural kaolinite often contains impurities such as quartz, mica, feldspar, iron oxides, titanium dioxide, and organic matter. These impurities can affect the properties and performance of kaolinite in various applications. Purification processes may include physical separation methods like centrifugation, flotation, and magnetic separation, as well as chemical treatments such as bleaching, acid leaching, and selective flocculation. The goal is to obtain high-purity kaolinite with controlled particle size distribution and minimal contaminants.Expand Specific Solutions04 Kaolinite structural characterization
The structural characterization of kaolinite involves various analytical techniques to determine its crystalline structure, particle morphology, and surface properties. X-ray diffraction (XRD) is commonly used to identify the crystalline phases and assess the degree of crystallinity. Scanning electron microscopy (SEM) and transmission electron microscopy (TEM) provide information about particle shape and size. Infrared spectroscopy helps identify the characteristic Al-O, Si-O, and OH bonds in the kaolinite structure. These analyses are crucial for understanding kaolinite behavior in different applications.Expand Specific Solutions05 Kaolinite in composite materials
Kaolinite is widely used as a component in composite materials due to its unique chemical composition and physical properties. When incorporated into polymers, ceramics, or other matrices, kaolinite can improve mechanical strength, thermal stability, fire resistance, and barrier properties. The interface between kaolinite and the matrix material is critical for achieving desired performance characteristics. Surface modification of kaolinite is often employed to enhance compatibility with the matrix and improve dispersion. These composites find applications in various industries including plastics, rubber, paper, and construction materials.Expand Specific Solutions
Leading Research Institutions and Industry Players
The kaolinite chemical composition characterization field is currently in a mature development stage, with a global market size estimated at over $5 billion. The technology landscape shows varying degrees of sophistication across research institutions and industrial players. Leading academic entities like China University of Geosciences and Central South University have established advanced analytical frameworks, while major corporations including BASF SE, China Petroleum & Chemical Corp., and Imerys Pigments demonstrate commercial-scale applications. The industry exhibits a balanced ecosystem where specialized mining companies like China Kaolin Co. and Yunnan Tianhong Gaoling collaborate with research organizations such as CNRS and IFP Energies Nouvelles to continuously refine characterization techniques, particularly focusing on high-precision elemental analysis and structural determination methodologies.
China University of Geosciences
Technical Solution: China University of Geosciences has developed a comprehensive multi-analytical approach for kaolinite characterization that integrates traditional and advanced techniques. Their methodology begins with X-ray diffraction (XRD) for phase identification and crystallinity assessment, followed by X-ray fluorescence (XRF) for bulk elemental composition. The university has pioneered the application of laser-induced breakdown spectroscopy (LIBS) for rapid elemental mapping of kaolinite samples, allowing visualization of elemental distribution patterns at the microscale. Their approach also incorporates electron probe microanalysis (EPMA) for high-precision quantitative analysis of major and minor elements at the micrometer scale. A distinctive aspect of their methodology is the integration of Mössbauer spectroscopy for detailed characterization of iron species in kaolinite, differentiating between structural iron and iron-bearing impurities. The university has also developed protocols for sequential chemical extraction procedures to differentiate between exchangeable, acid-soluble, reducible, and residual fractions of trace elements in kaolinite samples, providing insights into element mobility and bioavailability.
Strengths: Comprehensive integration of multiple analytical techniques; strong expertise in geological context and genesis of kaolinite deposits; advanced facilities for microanalysis. Weaknesses: Some techniques require specialized equipment and expertise; methodology is more oriented toward research applications than routine industrial analysis.
BASF SE
Technical Solution: BASF has developed an integrated analytical framework for kaolinite characterization that emphasizes both chemical composition and functional properties relevant to industrial applications. Their approach combines traditional elemental analysis techniques with advanced surface characterization methods. BASF employs X-ray photoelectron spectroscopy (XPS) to analyze the surface chemistry of kaolinite particles, providing information about elemental composition and chemical states in the top few nanometers of the material. This is complemented by solid-state nuclear magnetic resonance (NMR) spectroscopy, particularly 27Al and 29Si NMR, to elucidate the local atomic environments and structural features. For comprehensive elemental analysis, BASF utilizes inductively coupled plasma optical emission spectrometry (ICP-OES) following complete digestion of samples using a proprietary microwave-assisted acid digestion protocol. Their methodology also incorporates zeta potential measurements to characterize surface charge properties, which are critical for understanding kaolinite behavior in various industrial formulations.
Strengths: Comprehensive approach linking chemical composition to functional properties; advanced surface characterization capabilities; strong focus on industrially relevant parameters. Weaknesses: Methods are primarily developed for processed kaolinite used in chemical applications rather than geological samples; high equipment costs for full implementation.
Key Spectroscopic and Diffraction Technologies
Kaolin pigments and methods of making the same
PatentInactiveUS7494541B2
Innovation
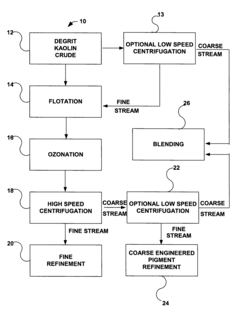
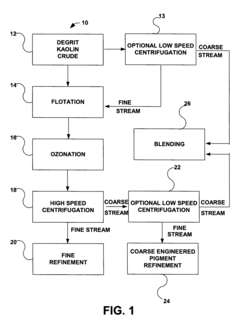
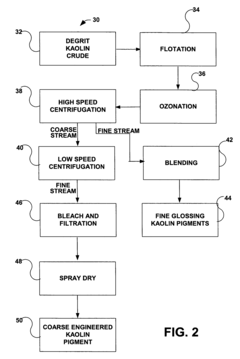
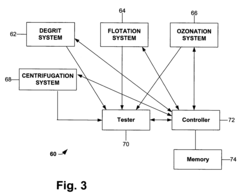
- A simplified process involving degritting, flotation, ozonation, and high-speed centrifugation of kaolin crude to separate it into coarse and fine streams, which are then refined into coarse engineered and fine glossing kaolin pigments, respectively, without the need for separate starting crudes or additional dispersants.
Environmental Impact and Sustainability Considerations
The characterization of kaolinite's chemical composition carries significant environmental implications that must be considered within sustainable mining and industrial practices. Traditional extraction and processing methods often involve extensive land disturbance, habitat destruction, and the generation of waste materials that can lead to soil and water contamination. Chemical characterization techniques themselves may employ hazardous reagents or generate toxic waste streams that require proper management and disposal.
Modern sustainable approaches to kaolinite characterization increasingly emphasize non-destructive and environmentally friendly analytical methods. X-ray fluorescence (XRF) and near-infrared spectroscopy represent greener alternatives to conventional wet chemical analyses, as they minimize chemical waste generation while providing accurate compositional data. These techniques allow for rapid on-site analysis, reducing the carbon footprint associated with sample transportation to laboratories.
The life cycle assessment of kaolinite characterization processes reveals opportunities for environmental optimization. Energy consumption during sample preparation and analysis constitutes a significant environmental burden, particularly when high-temperature treatments are required. Implementing energy-efficient instrumentation and renewable energy sources can substantially reduce the carbon footprint of characterization laboratories.
Water usage presents another critical environmental consideration. Traditional wet chemical methods for kaolinite characterization can consume substantial volumes of water and generate contaminated effluents. Developing closed-loop water systems and water-efficient characterization protocols helps preserve this valuable resource while preventing pollution of natural water bodies with chemical reagents and suspended clay particles.
Regulatory frameworks increasingly mandate environmental impact assessments for mining operations, including the characterization processes. Companies must demonstrate compliance with stringent environmental standards regarding emissions, waste management, and resource consumption. This regulatory pressure drives innovation toward greener characterization technologies that minimize environmental harm while maintaining analytical precision.
The sustainability of kaolinite characterization extends to social dimensions as well. Mining communities often face health risks from airborne particulates and water contamination. Advanced characterization techniques that enable precise identification of potentially harmful impurities in kaolinite deposits allow for better risk assessment and mitigation strategies, protecting both workers and surrounding communities.
Looking forward, the integration of artificial intelligence with characterization technologies promises to optimize resource efficiency by predicting kaolinite properties from minimal sample quantities, thereby reducing the environmental footprint of both sampling and analysis activities. This technological evolution represents a crucial step toward truly sustainable practices in kaolinite utilization across industries.
Modern sustainable approaches to kaolinite characterization increasingly emphasize non-destructive and environmentally friendly analytical methods. X-ray fluorescence (XRF) and near-infrared spectroscopy represent greener alternatives to conventional wet chemical analyses, as they minimize chemical waste generation while providing accurate compositional data. These techniques allow for rapid on-site analysis, reducing the carbon footprint associated with sample transportation to laboratories.
The life cycle assessment of kaolinite characterization processes reveals opportunities for environmental optimization. Energy consumption during sample preparation and analysis constitutes a significant environmental burden, particularly when high-temperature treatments are required. Implementing energy-efficient instrumentation and renewable energy sources can substantially reduce the carbon footprint of characterization laboratories.
Water usage presents another critical environmental consideration. Traditional wet chemical methods for kaolinite characterization can consume substantial volumes of water and generate contaminated effluents. Developing closed-loop water systems and water-efficient characterization protocols helps preserve this valuable resource while preventing pollution of natural water bodies with chemical reagents and suspended clay particles.
Regulatory frameworks increasingly mandate environmental impact assessments for mining operations, including the characterization processes. Companies must demonstrate compliance with stringent environmental standards regarding emissions, waste management, and resource consumption. This regulatory pressure drives innovation toward greener characterization technologies that minimize environmental harm while maintaining analytical precision.
The sustainability of kaolinite characterization extends to social dimensions as well. Mining communities often face health risks from airborne particulates and water contamination. Advanced characterization techniques that enable precise identification of potentially harmful impurities in kaolinite deposits allow for better risk assessment and mitigation strategies, protecting both workers and surrounding communities.
Looking forward, the integration of artificial intelligence with characterization technologies promises to optimize resource efficiency by predicting kaolinite properties from minimal sample quantities, thereby reducing the environmental footprint of both sampling and analysis activities. This technological evolution represents a crucial step toward truly sustainable practices in kaolinite utilization across industries.
Quality Control Standards and Certification Requirements
The standardization of kaolinite characterization is governed by several international and regional quality control frameworks. ISO 9001 serves as the foundational quality management system standard, providing a structured approach for organizations to ensure consistent quality in kaolinite analysis. More specifically, ASTM D4318 and D7928 standards outline procedures for determining the physical properties of kaolinite, while ASTM C323 addresses the chemical composition analysis of ceramic materials including kaolinite.
For precise chemical characterization, laboratories must adhere to ISO/IEC 17025, which specifies general requirements for testing competence. This certification ensures that analytical methods for determining Al2O3, SiO2, Fe2O3, TiO2, and other trace elements in kaolinite follow validated protocols with appropriate quality controls. The certification process typically requires regular proficiency testing and inter-laboratory comparisons to maintain accreditation status.
Industry-specific standards also play crucial roles in kaolinite quality control. The European Committee for Standardization (CEN) has developed EN 725 specifically for advanced technical ceramics, providing detailed methodologies for chemical analysis of ceramic powders including kaolinite. Similarly, the China National Standard GB/T 14563 outlines requirements for kaolinite used in various industrial applications, with specific thresholds for chemical composition.
Certification requirements vary by application sector. For pharmaceutical-grade kaolinite, compliance with USP (United States Pharmacopeia) or EP (European Pharmacopoeia) standards is mandatory, imposing strict limits on heavy metal content and microbial contamination. In the food industry, kaolinite must meet FDA regulations in the US or EFSA guidelines in Europe, with certification under FSSC 22000 or similar food safety management systems.
Environmental considerations have introduced additional certification requirements. The EU's REACH regulation requires registration and safety assessment of kaolinite when used in quantities exceeding one ton annually. Similarly, RoHS compliance certification ensures kaolinite products are free from restricted hazardous substances when used in electronic applications.
Modern certification trends are moving toward more comprehensive sustainability assessments. The Cradle to Cradle Certified Product Standard evaluates kaolinite products across five quality categories: material health, material reutilization, renewable energy use, water stewardship, and social fairness. This holistic approach reflects the growing importance of environmental and social responsibility in quality control frameworks for mineral resources like kaolinite.
For precise chemical characterization, laboratories must adhere to ISO/IEC 17025, which specifies general requirements for testing competence. This certification ensures that analytical methods for determining Al2O3, SiO2, Fe2O3, TiO2, and other trace elements in kaolinite follow validated protocols with appropriate quality controls. The certification process typically requires regular proficiency testing and inter-laboratory comparisons to maintain accreditation status.
Industry-specific standards also play crucial roles in kaolinite quality control. The European Committee for Standardization (CEN) has developed EN 725 specifically for advanced technical ceramics, providing detailed methodologies for chemical analysis of ceramic powders including kaolinite. Similarly, the China National Standard GB/T 14563 outlines requirements for kaolinite used in various industrial applications, with specific thresholds for chemical composition.
Certification requirements vary by application sector. For pharmaceutical-grade kaolinite, compliance with USP (United States Pharmacopeia) or EP (European Pharmacopoeia) standards is mandatory, imposing strict limits on heavy metal content and microbial contamination. In the food industry, kaolinite must meet FDA regulations in the US or EFSA guidelines in Europe, with certification under FSSC 22000 or similar food safety management systems.
Environmental considerations have introduced additional certification requirements. The EU's REACH regulation requires registration and safety assessment of kaolinite when used in quantities exceeding one ton annually. Similarly, RoHS compliance certification ensures kaolinite products are free from restricted hazardous substances when used in electronic applications.
Modern certification trends are moving toward more comprehensive sustainability assessments. The Cradle to Cradle Certified Product Standard evaluates kaolinite products across five quality categories: material health, material reutilization, renewable energy use, water stewardship, and social fairness. This holistic approach reflects the growing importance of environmental and social responsibility in quality control frameworks for mineral resources like kaolinite.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!