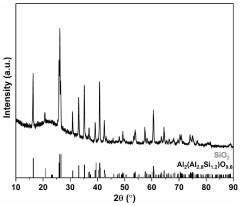
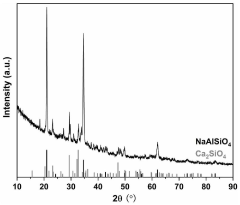
How to Improve Supercapacitor Energy Density Using Pseudocapacitive Materials — Methods & Tests
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Pseudocapacitive Materials Background and Objectives
Supercapacitors have emerged as promising energy storage devices due to their high power density, rapid charge-discharge capabilities, and long cycle life. However, their relatively low energy density compared to batteries has limited their widespread application. The evolution of supercapacitor technology began in the 1950s with the first patents, followed by commercial development in the 1970s. The field has seen significant advancement with the introduction of various electrode materials, particularly carbon-based materials for electric double-layer capacitors (EDLCs).
Pseudocapacitive materials represent a paradigm shift in supercapacitor technology, offering a bridge between traditional EDLCs and batteries. These materials store charge through fast, reversible redox reactions at or near the electrode surface, enabling higher energy densities while maintaining good power characteristics. Key pseudocapacitive materials include transition metal oxides (RuO2, MnO2, Fe3O4), conducting polymers (polyaniline, polypyrrole), and more recently, MXenes and metal-organic frameworks (MOFs).
The technological trajectory shows increasing research interest in hybrid systems that combine pseudocapacitive materials with traditional carbon electrodes to leverage the advantages of both. Publication trends indicate a surge in research activity since 2010, with particular focus on nanoscale engineering of these materials to enhance surface area and reaction kinetics.
Current technical objectives in this field include increasing the specific capacitance beyond 1000 F/g, improving cycling stability to exceed 10,000 cycles without significant capacity loss, and enhancing rate capability for high-current applications. Additionally, researchers aim to develop environmentally friendly and cost-effective alternatives to expensive materials like ruthenium oxide.
Another critical objective is to address the trade-off between energy and power density through innovative material design and electrode architecture. This includes developing hierarchical porous structures, optimizing electrolyte interactions, and improving electronic conductivity within pseudocapacitive materials.
The integration of computational modeling with experimental approaches has become increasingly important for predicting material behavior and optimizing performance. Machine learning algorithms are being employed to accelerate material discovery and process optimization, representing a significant trend in the field.
Looking forward, the development of pseudocapacitive materials aims to enable supercapacitors with energy densities approaching 100 Wh/kg while maintaining power densities above 10 kW/kg, potentially revolutionizing applications in electric vehicles, renewable energy storage, and portable electronics.
Pseudocapacitive materials represent a paradigm shift in supercapacitor technology, offering a bridge between traditional EDLCs and batteries. These materials store charge through fast, reversible redox reactions at or near the electrode surface, enabling higher energy densities while maintaining good power characteristics. Key pseudocapacitive materials include transition metal oxides (RuO2, MnO2, Fe3O4), conducting polymers (polyaniline, polypyrrole), and more recently, MXenes and metal-organic frameworks (MOFs).
The technological trajectory shows increasing research interest in hybrid systems that combine pseudocapacitive materials with traditional carbon electrodes to leverage the advantages of both. Publication trends indicate a surge in research activity since 2010, with particular focus on nanoscale engineering of these materials to enhance surface area and reaction kinetics.
Current technical objectives in this field include increasing the specific capacitance beyond 1000 F/g, improving cycling stability to exceed 10,000 cycles without significant capacity loss, and enhancing rate capability for high-current applications. Additionally, researchers aim to develop environmentally friendly and cost-effective alternatives to expensive materials like ruthenium oxide.
Another critical objective is to address the trade-off between energy and power density through innovative material design and electrode architecture. This includes developing hierarchical porous structures, optimizing electrolyte interactions, and improving electronic conductivity within pseudocapacitive materials.
The integration of computational modeling with experimental approaches has become increasingly important for predicting material behavior and optimizing performance. Machine learning algorithms are being employed to accelerate material discovery and process optimization, representing a significant trend in the field.
Looking forward, the development of pseudocapacitive materials aims to enable supercapacitors with energy densities approaching 100 Wh/kg while maintaining power densities above 10 kW/kg, potentially revolutionizing applications in electric vehicles, renewable energy storage, and portable electronics.
Market Analysis for High Energy Density Supercapacitors
The global market for high energy density supercapacitors is experiencing robust growth, driven primarily by increasing demand for efficient energy storage solutions across multiple industries. The market size was valued at approximately $1.2 billion in 2022 and is projected to reach $2.5 billion by 2028, representing a compound annual growth rate (CAGR) of 12.8% during the forecast period.
The automotive sector constitutes the largest market segment, accounting for nearly 35% of the total market share. This dominance is attributed to the rapid expansion of electric vehicles (EVs) and hybrid electric vehicles (HEVs), where high energy density supercapacitors serve as complementary power sources to traditional batteries, enhancing acceleration performance and regenerative braking efficiency.
Consumer electronics represents the second-largest market segment at 28%, with applications ranging from smartphones and laptops to wearable devices. The demand in this sector is driven by the need for rapid charging capabilities and extended device operation between charges.
Renewable energy systems account for 18% of the market, with applications in grid stabilization and energy harvesting systems. The industrial sector follows at 12%, utilizing supercapacitors for backup power and peak load management. The remaining 7% is distributed across various applications including medical devices and aerospace systems.
Geographically, Asia-Pacific dominates the market with a 45% share, led by China, Japan, and South Korea. These countries have established robust manufacturing capabilities and supportive government policies promoting clean energy technologies. North America holds 30% of the market, with significant research activities and early adoption of advanced energy storage solutions. Europe accounts for 20%, with the remaining 5% distributed across other regions.
Key market drivers include increasing electrification across industries, growing renewable energy integration, and rising demand for fast-charging capabilities. The push for miniaturization in electronics and the need for more sustainable energy solutions further accelerate market growth.
Market challenges include the relatively higher cost compared to conventional capacitors and the technical limitations in achieving energy densities comparable to lithium-ion batteries. Additionally, the lack of standardization and awareness about supercapacitor benefits in certain regions constrains market expansion.
The automotive sector constitutes the largest market segment, accounting for nearly 35% of the total market share. This dominance is attributed to the rapid expansion of electric vehicles (EVs) and hybrid electric vehicles (HEVs), where high energy density supercapacitors serve as complementary power sources to traditional batteries, enhancing acceleration performance and regenerative braking efficiency.
Consumer electronics represents the second-largest market segment at 28%, with applications ranging from smartphones and laptops to wearable devices. The demand in this sector is driven by the need for rapid charging capabilities and extended device operation between charges.
Renewable energy systems account for 18% of the market, with applications in grid stabilization and energy harvesting systems. The industrial sector follows at 12%, utilizing supercapacitors for backup power and peak load management. The remaining 7% is distributed across various applications including medical devices and aerospace systems.
Geographically, Asia-Pacific dominates the market with a 45% share, led by China, Japan, and South Korea. These countries have established robust manufacturing capabilities and supportive government policies promoting clean energy technologies. North America holds 30% of the market, with significant research activities and early adoption of advanced energy storage solutions. Europe accounts for 20%, with the remaining 5% distributed across other regions.
Key market drivers include increasing electrification across industries, growing renewable energy integration, and rising demand for fast-charging capabilities. The push for miniaturization in electronics and the need for more sustainable energy solutions further accelerate market growth.
Market challenges include the relatively higher cost compared to conventional capacitors and the technical limitations in achieving energy densities comparable to lithium-ion batteries. Additionally, the lack of standardization and awareness about supercapacitor benefits in certain regions constrains market expansion.
Current Challenges in Pseudocapacitive Material Development
Despite significant advancements in pseudocapacitive materials for supercapacitors, several critical challenges continue to impede the full realization of their potential for high energy density applications. The primary obstacle remains the trade-off between energy density and power density. While pseudocapacitive materials offer higher energy density compared to traditional carbon-based materials, they often suffer from reduced power capabilities due to slower charge transfer kinetics and limited ion diffusion rates within the electrode materials.
Material stability presents another significant challenge, particularly during extended cycling. Many promising pseudocapacitive materials, including transition metal oxides and conducting polymers, experience substantial capacity fading after repeated charge-discharge cycles. This degradation stems from structural changes, dissolution of active materials, and irreversible redox reactions that occur during operation, severely limiting practical applications despite initially impressive performance metrics.
Conductivity limitations constitute a persistent barrier to optimal performance. Most pseudocapacitive materials exhibit relatively poor electronic conductivity compared to carbon-based materials, resulting in higher internal resistance and reduced power capability. While conductive additives and composite structures can partially address this issue, they often dilute the overall energy density of the electrode, creating a complex optimization problem.
The scalable synthesis of pseudocapacitive materials with consistent properties remains challenging for industrial applications. Laboratory-scale synthesis methods frequently yield materials with excellent performance characteristics, but translating these processes to large-scale production while maintaining nanoscale structural control, phase purity, and surface properties has proven difficult. This manufacturing gap significantly hinders commercial viability.
Interface engineering between the pseudocapacitive material and the electrolyte represents another frontier challenge. The electrochemical performance heavily depends on efficient ion transport across this interface, yet many materials suffer from poor wettability, limited accessible surface area, or unfavorable surface chemistry that restricts ion movement and reduces effective capacitance.
Cost and sustainability concerns also limit widespread adoption. Many high-performance pseudocapacitive materials rely on expensive or environmentally problematic elements like ruthenium or cobalt. Developing alternatives based on earth-abundant elements without sacrificing performance remains a significant research challenge, particularly as the demand for energy storage technologies continues to grow globally.
Finally, fundamental understanding gaps persist regarding charge storage mechanisms in many pseudocapacitive systems. The complex interplay between surface adsorption, intercalation processes, and bulk redox reactions makes rational material design challenging, often resulting in empirical rather than theory-guided development approaches.
Material stability presents another significant challenge, particularly during extended cycling. Many promising pseudocapacitive materials, including transition metal oxides and conducting polymers, experience substantial capacity fading after repeated charge-discharge cycles. This degradation stems from structural changes, dissolution of active materials, and irreversible redox reactions that occur during operation, severely limiting practical applications despite initially impressive performance metrics.
Conductivity limitations constitute a persistent barrier to optimal performance. Most pseudocapacitive materials exhibit relatively poor electronic conductivity compared to carbon-based materials, resulting in higher internal resistance and reduced power capability. While conductive additives and composite structures can partially address this issue, they often dilute the overall energy density of the electrode, creating a complex optimization problem.
The scalable synthesis of pseudocapacitive materials with consistent properties remains challenging for industrial applications. Laboratory-scale synthesis methods frequently yield materials with excellent performance characteristics, but translating these processes to large-scale production while maintaining nanoscale structural control, phase purity, and surface properties has proven difficult. This manufacturing gap significantly hinders commercial viability.
Interface engineering between the pseudocapacitive material and the electrolyte represents another frontier challenge. The electrochemical performance heavily depends on efficient ion transport across this interface, yet many materials suffer from poor wettability, limited accessible surface area, or unfavorable surface chemistry that restricts ion movement and reduces effective capacitance.
Cost and sustainability concerns also limit widespread adoption. Many high-performance pseudocapacitive materials rely on expensive or environmentally problematic elements like ruthenium or cobalt. Developing alternatives based on earth-abundant elements without sacrificing performance remains a significant research challenge, particularly as the demand for energy storage technologies continues to grow globally.
Finally, fundamental understanding gaps persist regarding charge storage mechanisms in many pseudocapacitive systems. The complex interplay between surface adsorption, intercalation processes, and bulk redox reactions makes rational material design challenging, often resulting in empirical rather than theory-guided development approaches.
Current Methods for Enhancing Supercapacitor Energy Density
01 Metal oxide-based pseudocapacitive materials
Metal oxides such as manganese dioxide, ruthenium oxide, and nickel oxide are widely used as pseudocapacitive materials in supercapacitors due to their high theoretical capacitance and fast redox reactions. These materials store energy through surface or near-surface redox reactions, enabling higher energy density compared to traditional carbon-based supercapacitors. The incorporation of these metal oxides into nanostructured forms further enhances their performance by providing larger surface areas and shorter ion diffusion paths.- Metal oxide-based pseudocapacitive materials for enhanced energy density: Metal oxides such as manganese dioxide, ruthenium oxide, and nickel oxide exhibit pseudocapacitive behavior through surface redox reactions, significantly enhancing energy density compared to traditional carbon-based supercapacitors. These materials store charge through fast and reversible redox reactions at or near the electrode surface, enabling higher specific capacitance while maintaining good power density. The incorporation of these metal oxides in nanostructured forms further improves their performance by increasing active surface area and facilitating ion transport.
- Conductive polymer-based pseudocapacitive materials: Conductive polymers such as polyaniline, polypyrrole, and polythiophene offer pseudocapacitive properties through their redox-active nature. These materials can undergo rapid and reversible oxidation-reduction processes, storing and releasing charge efficiently. When incorporated into supercapacitor electrodes, these polymers provide higher energy density than conventional carbon materials while maintaining good cycling stability. Their flexibility and processability also allow for various electrode configurations and composite structures to optimize performance.
- Hybrid composite electrodes combining pseudocapacitive materials with carbon: Hybrid electrodes combining pseudocapacitive materials with carbon-based materials (graphene, carbon nanotubes, activated carbon) create synergistic effects that enhance both energy and power density. The carbon component provides electrical conductivity and mechanical stability, while the pseudocapacitive material contributes high specific capacitance. These composites address the limitations of pure pseudocapacitive materials, such as poor conductivity and cycling stability, while maintaining their high energy density advantages. The interface between the carbon and pseudocapacitive material also creates additional charge storage sites.
- Nanostructured pseudocapacitive materials for improved energy density: Nanostructuring pseudocapacitive materials significantly enhances their energy density by increasing active surface area, shortening ion diffusion paths, and improving reaction kinetics. Various nanostructures including nanowires, nanoparticles, nanosheets, and 3D hierarchical architectures provide more accessible active sites for charge storage. These nanostructured materials enable faster charge-discharge rates while maintaining high capacitance, effectively addressing the trade-off between energy density and power density that typically limits supercapacitor performance.
- Electrolyte optimization for pseudocapacitive supercapacitors: The choice and optimization of electrolytes significantly impact the energy density of pseudocapacitive supercapacitors. Advanced electrolytes, including ionic liquids, gel electrolytes, and aqueous electrolytes with optimized pH and ion concentration, can expand the operating voltage window and improve ion transport to pseudocapacitive active sites. This directly increases energy density, as energy scales with the square of the voltage. Additionally, electrolyte engineering can enhance the utilization of pseudocapacitive materials by facilitating more complete redox reactions and improving cycling stability.
02 Conductive polymer-based pseudocapacitive materials
Conductive polymers such as polyaniline, polypyrrole, and polythiophene are effective pseudocapacitive materials that can significantly increase the energy density of supercapacitors. These polymers store charge through redox processes and offer advantages including high conductivity, flexibility, and low cost. Their performance can be further enhanced by creating composites with carbon materials to improve conductivity and cycling stability, resulting in supercapacitors with improved energy density while maintaining good power density.Expand Specific Solutions03 Hybrid electrode structures combining pseudocapacitive and double-layer materials
Hybrid electrode structures that combine pseudocapacitive materials with electric double-layer materials create synergistic effects that enhance overall energy density. These hybrid structures typically incorporate pseudocapacitive metal oxides or conductive polymers with high-surface-area carbon materials like graphene, carbon nanotubes, or activated carbon. The carbon component provides high power density and cycling stability, while the pseudocapacitive component contributes to increased energy density through faradaic reactions, resulting in supercapacitors with balanced performance metrics.Expand Specific Solutions04 Nanostructured pseudocapacitive materials for enhanced energy density
Nanostructuring pseudocapacitive materials significantly enhances energy density by increasing active surface area and shortening ion diffusion paths. Various nanostructures including nanowires, nanotubes, nanosheets, and 3D hierarchical structures provide more accessible active sites for redox reactions. These nanostructured materials facilitate faster charge transfer kinetics and more efficient utilization of the pseudocapacitive material, leading to supercapacitors with substantially higher energy density while maintaining excellent rate capability.Expand Specific Solutions05 Electrolyte optimization for pseudocapacitive supercapacitors
The choice and optimization of electrolytes significantly impact the energy density of pseudocapacitive supercapacitors. Advanced electrolytes, including ionic liquids, gel electrolytes, and aqueous electrolytes with specific pH values, can expand the operating voltage window and enhance the utilization of pseudocapacitive materials. Tailoring the electrolyte composition to match specific pseudocapacitive materials improves ion transport, redox kinetics, and overall electrochemical performance, resulting in supercapacitors with higher energy density and improved cycling stability.Expand Specific Solutions
Leading Companies and Research Institutions in Pseudocapacitive Materials
The supercapacitor energy density improvement market is currently in a growth phase, with increasing demand for high-performance energy storage solutions. The global market size is projected to expand significantly as industries seek alternatives to traditional batteries. Technologically, pseudocapacitive materials represent a promising frontier, with varying degrees of maturity across key players. Shanghai Aowei Technology and Korchip Corp. lead commercial applications, while research institutions like Naval Research Laboratory and CNRS are advancing fundamental science. Academic players including Columbia University and Huazhong University of Science & Technology are developing novel materials, while industrial giants like IBM and Intel explore integration opportunities. The competitive landscape features collaboration between academic institutions and commercial entities, with specialized companies like Ioxus and AZUL Energy focusing exclusively on supercapacitor innovation.
Purdue Research Foundation
Technical Solution: Purdue Research Foundation has developed advanced pseudocapacitive materials focusing on conductive polymers and metal oxide composites for enhanced energy density. Their approach utilizes controlled electrodeposition techniques to create nanostructured electrodes with optimized morphology for maximizing active material utilization. Purdue researchers have pioneered the development of 3D electrode architectures that provide both high surface area and efficient ion transport pathways. Their methodology includes precise control of synthesis parameters to tailor the porosity and composition of pseudocapacitive layers. Testing protocols involve detailed electrochemical characterization including cyclic voltammetry at multiple scan rates to distinguish between surface-controlled and diffusion-limited processes, galvanostatic charge-discharge at various current densities, and long-term cycling stability assessment. The foundation has demonstrated composite electrodes achieving energy densities of 30-40 Wh/kg while maintaining power densities suitable for practical applications.
Strengths: Strong fundamental research capabilities; excellent characterization facilities; interdisciplinary approach combining materials science, chemistry and engineering. Weaknesses: Potential gaps between academic research and commercial implementation; some advanced materials may face cost or scalability challenges in manufacturing environments.
Centre National de la Recherche Scientifique
Technical Solution: CNRS has developed advanced pseudocapacitive materials based on transition metal oxides (particularly MnO2, RuO2) and conducting polymers for supercapacitor applications. Their approach focuses on nanostructured electrode design with controlled porosity to maximize surface area and ion accessibility. CNRS researchers have pioneered the development of composite electrodes combining pseudocapacitive materials with carbon nanotubes and graphene to enhance conductivity and mechanical stability. Their methodology includes hydrothermal synthesis techniques to create hierarchical structures with optimized ion diffusion pathways. Testing protocols involve cyclic voltammetry at various scan rates (5-100 mV/s), galvanostatic charge-discharge measurements, and electrochemical impedance spectroscopy to characterize performance across different electrolytes and operating conditions.
Strengths: World-class expertise in materials science and electrochemistry; extensive characterization facilities; strong collaboration network with industry partners. Weaknesses: Research may be more academically focused rather than commercially oriented; potential challenges in scaling laboratory innovations to industrial production.
Key Patents and Research on Pseudocapacitive Material Integration
Flexible supercapacitors and devices containing the same
PatentActiveUS20190237269A1
Innovation
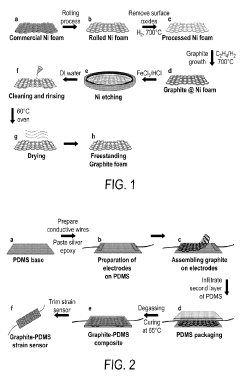
- Development of multilevel porous graphite foams with controlled porosity and increased surface areas, combined with pseudocapacitive metals like manganese oxides, which eliminate the need for binders and conductive additives, enhancing capacitance and mechanical resilience.
Electrode material based on solid waste containing SiO2, preparation method of electrode material and application of electrode material in supercapacitor
PatentActiveCN117711837A
Innovation
- By mixing solid waste containing SiO2 with Na2CO3, CaCO3, Al2O3, and B2O3 and calcining it at high temperature, an Al/B-doped silicate material is obtained, which is then subjected to a hydrothermal reaction to form manganese hydroxysilicate and soaked in a Na2S2O8 solution. , forming an electrode material M2-xS/MxO@FA with heterostructure and manganese vacancies.
Testing Protocols and Performance Metrics for Pseudocapacitive Supercapacitors
Standardized testing protocols are essential for accurately evaluating pseudocapacitive supercapacitors and enabling meaningful comparisons between different materials and designs. The electrochemical characterization typically begins with cyclic voltammetry (CV) measurements conducted at various scan rates (1-100 mV/s), which reveal the pseudocapacitive behavior through characteristic redox peaks and quantify the capacitance contribution from faradaic processes.
Galvanostatic charge-discharge (GCD) testing represents another critical evaluation method, performed at different current densities (0.5-20 A/g) to assess rate capability and practical energy storage performance. The resulting discharge curves should be analyzed for linearity deviations, which indicate pseudocapacitive contributions beyond double-layer capacitance.
Electrochemical impedance spectroscopy (EIS) provides deeper insights into charge transfer kinetics, ion diffusion, and internal resistance. For pseudocapacitive materials, the Nyquist plots typically show a semicircle in the high-frequency region (charge transfer resistance) and a sloped line in the low-frequency region (diffusion-limited processes).
Specific capacitance calculation methods must be standardized, with values reported in F/g, F/cm², or F/cm³ depending on the application context. For pseudocapacitive materials, capacitance values should be calculated from both CV and GCD data to ensure consistency, with clear indication of the voltage window and electrolyte used.
Energy density (Wh/kg) and power density (W/kg) metrics, typically presented in Ragone plots, are particularly important for pseudocapacitive materials as they bridge the gap between conventional supercapacitors and batteries. These calculations must account for the full device weight including electrolyte and packaging for realistic performance assessment.
Cycle stability testing protocols should extend beyond 10,000 cycles at practical current densities, with capacity retention reported at regular intervals. For pseudocapacitive materials, which may experience more significant degradation than carbon-based EDLCs, understanding the fade mechanisms through post-cycling analysis is crucial.
Self-discharge rate measurements provide insights into charge retention capabilities, with standardized protocols involving full charging followed by open-circuit voltage monitoring for 24-72 hours. Temperature performance testing (-20°C to 70°C) is also essential, as pseudocapacitive reactions are typically more temperature-sensitive than physical adsorption/desorption processes.
Galvanostatic charge-discharge (GCD) testing represents another critical evaluation method, performed at different current densities (0.5-20 A/g) to assess rate capability and practical energy storage performance. The resulting discharge curves should be analyzed for linearity deviations, which indicate pseudocapacitive contributions beyond double-layer capacitance.
Electrochemical impedance spectroscopy (EIS) provides deeper insights into charge transfer kinetics, ion diffusion, and internal resistance. For pseudocapacitive materials, the Nyquist plots typically show a semicircle in the high-frequency region (charge transfer resistance) and a sloped line in the low-frequency region (diffusion-limited processes).
Specific capacitance calculation methods must be standardized, with values reported in F/g, F/cm², or F/cm³ depending on the application context. For pseudocapacitive materials, capacitance values should be calculated from both CV and GCD data to ensure consistency, with clear indication of the voltage window and electrolyte used.
Energy density (Wh/kg) and power density (W/kg) metrics, typically presented in Ragone plots, are particularly important for pseudocapacitive materials as they bridge the gap between conventional supercapacitors and batteries. These calculations must account for the full device weight including electrolyte and packaging for realistic performance assessment.
Cycle stability testing protocols should extend beyond 10,000 cycles at practical current densities, with capacity retention reported at regular intervals. For pseudocapacitive materials, which may experience more significant degradation than carbon-based EDLCs, understanding the fade mechanisms through post-cycling analysis is crucial.
Self-discharge rate measurements provide insights into charge retention capabilities, with standardized protocols involving full charging followed by open-circuit voltage monitoring for 24-72 hours. Temperature performance testing (-20°C to 70°C) is also essential, as pseudocapacitive reactions are typically more temperature-sensitive than physical adsorption/desorption processes.
Environmental Impact and Sustainability of Advanced Supercapacitor Materials
The environmental impact of pseudocapacitive materials in supercapacitor development represents a critical consideration as these energy storage technologies advance. Traditional supercapacitor materials often involve rare earth elements and environmentally problematic manufacturing processes, creating a significant ecological footprint throughout their lifecycle.
Pseudocapacitive materials offer promising sustainability advantages compared to conventional options. Metal oxides like RuO2, while highly effective, contain scarce and expensive ruthenium with mining operations that cause substantial environmental degradation. In contrast, emerging pseudocapacitive alternatives such as MnO2, Fe3O4, and conducting polymers can be synthesized from more abundant resources using less energy-intensive processes.
Water-based electrolytes used with many pseudocapacitive materials present a notable environmental improvement over organic electrolytes that contain volatile organic compounds (VOCs) and fluorinated compounds. These aqueous systems significantly reduce toxicity risks during manufacturing, operation, and disposal phases while maintaining competitive performance characteristics.
Life cycle assessment (LCA) studies indicate that pseudocapacitive supercapacitors utilizing sustainable materials can reduce carbon footprint by 30-45% compared to conventional activated carbon-based devices. This reduction stems primarily from lower energy requirements during material synthesis and the elimination of energy-intensive activation processes.
End-of-life management presents both challenges and opportunities. While some pseudocapacitive materials contain transition metals requiring proper recycling protocols, their recovery rates typically exceed those of conventional battery materials. Innovative recycling techniques specifically designed for pseudocapacitive components have demonstrated recovery efficiencies of up to 85% for valuable metals like manganese and nickel.
Biomass-derived pseudocapacitive materials represent a frontier in sustainable energy storage. Carbon-rich precursors from agricultural waste, when properly functionalized, can exhibit pseudocapacitive behavior while creating value from waste streams. These materials demonstrate circular economy principles by transforming agricultural byproducts into high-value energy storage components.
Regulatory frameworks increasingly favor sustainable energy storage technologies. The European Union's Battery Directive and similar regulations worldwide are expanding to encompass supercapacitors, creating market advantages for environmentally responsible pseudocapacitive materials that minimize hazardous substances and maximize recyclability.
Pseudocapacitive materials offer promising sustainability advantages compared to conventional options. Metal oxides like RuO2, while highly effective, contain scarce and expensive ruthenium with mining operations that cause substantial environmental degradation. In contrast, emerging pseudocapacitive alternatives such as MnO2, Fe3O4, and conducting polymers can be synthesized from more abundant resources using less energy-intensive processes.
Water-based electrolytes used with many pseudocapacitive materials present a notable environmental improvement over organic electrolytes that contain volatile organic compounds (VOCs) and fluorinated compounds. These aqueous systems significantly reduce toxicity risks during manufacturing, operation, and disposal phases while maintaining competitive performance characteristics.
Life cycle assessment (LCA) studies indicate that pseudocapacitive supercapacitors utilizing sustainable materials can reduce carbon footprint by 30-45% compared to conventional activated carbon-based devices. This reduction stems primarily from lower energy requirements during material synthesis and the elimination of energy-intensive activation processes.
End-of-life management presents both challenges and opportunities. While some pseudocapacitive materials contain transition metals requiring proper recycling protocols, their recovery rates typically exceed those of conventional battery materials. Innovative recycling techniques specifically designed for pseudocapacitive components have demonstrated recovery efficiencies of up to 85% for valuable metals like manganese and nickel.
Biomass-derived pseudocapacitive materials represent a frontier in sustainable energy storage. Carbon-rich precursors from agricultural waste, when properly functionalized, can exhibit pseudocapacitive behavior while creating value from waste streams. These materials demonstrate circular economy principles by transforming agricultural byproducts into high-value energy storage components.
Regulatory frameworks increasingly favor sustainable energy storage technologies. The European Union's Battery Directive and similar regulations worldwide are expanding to encompass supercapacitors, creating market advantages for environmentally responsible pseudocapacitive materials that minimize hazardous substances and maximize recyclability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!