How to Optimize Electrode Porosity for Power vs Energy in Supercapacitors — Modeling + Lab Data
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Supercapacitor Electrode Porosity Background and Objectives
Supercapacitors have emerged as critical energy storage devices in the landscape of modern power systems, offering unique advantages in power density, cycle life, and rapid charge-discharge capabilities. The evolution of supercapacitor technology can be traced back to the 1950s, with significant commercial development beginning in the 1970s. Over the past two decades, research has intensified dramatically, focusing on enhancing both energy and power densities to bridge the gap between conventional capacitors and batteries.
Electrode porosity represents one of the most fundamental parameters influencing supercapacitor performance. The porous structure of electrodes directly impacts the accessible surface area for ion adsorption, the diffusion pathways for electrolyte ions, and ultimately the balance between energy and power capabilities. Historically, carbon-based materials with high specific surface areas have dominated electrode development, with activated carbons, carbon nanotubes, and graphene being prominent examples.
The technical evolution in this field has progressed from simple high-surface-area carbons to precisely engineered hierarchical porous structures. This transition reflects growing understanding that mere surface area maximization is insufficient; the distribution, connectivity, and accessibility of pores are equally critical factors. Recent advances in material science have enabled increasingly sophisticated control over pore architecture at multiple scales.
Current research trends indicate a shift toward rational design of electrode porosity rather than empirical optimization. This includes developing multi-scale porous structures that combine macropores for ion transport, mesopores for ion buffering, and micropores for charge storage. The fundamental challenge lies in the inherent trade-off between energy density (favored by smaller pores that maximize surface area) and power density (enhanced by larger pores that facilitate rapid ion transport).
The primary objective of this technical investigation is to establish quantitative relationships between electrode porosity characteristics and supercapacitor performance metrics, particularly the power-energy balance. This includes developing predictive models that can accurately correlate pore size distribution, pore connectivity, and pore volume with electrochemical performance parameters. Additionally, we aim to validate these models with laboratory data to create practical design guidelines for optimizing electrode structures.
Further goals include identifying the theoretical limits of performance enhancement through porosity engineering, exploring novel fabrication techniques that enable precise control over pore architecture, and evaluating the scalability of optimized electrode designs for commercial applications. The ultimate aim is to develop a comprehensive framework that enables targeted design of electrode porosity for specific application requirements, whether prioritizing high power, high energy, or balanced performance characteristics.
Electrode porosity represents one of the most fundamental parameters influencing supercapacitor performance. The porous structure of electrodes directly impacts the accessible surface area for ion adsorption, the diffusion pathways for electrolyte ions, and ultimately the balance between energy and power capabilities. Historically, carbon-based materials with high specific surface areas have dominated electrode development, with activated carbons, carbon nanotubes, and graphene being prominent examples.
The technical evolution in this field has progressed from simple high-surface-area carbons to precisely engineered hierarchical porous structures. This transition reflects growing understanding that mere surface area maximization is insufficient; the distribution, connectivity, and accessibility of pores are equally critical factors. Recent advances in material science have enabled increasingly sophisticated control over pore architecture at multiple scales.
Current research trends indicate a shift toward rational design of electrode porosity rather than empirical optimization. This includes developing multi-scale porous structures that combine macropores for ion transport, mesopores for ion buffering, and micropores for charge storage. The fundamental challenge lies in the inherent trade-off between energy density (favored by smaller pores that maximize surface area) and power density (enhanced by larger pores that facilitate rapid ion transport).
The primary objective of this technical investigation is to establish quantitative relationships between electrode porosity characteristics and supercapacitor performance metrics, particularly the power-energy balance. This includes developing predictive models that can accurately correlate pore size distribution, pore connectivity, and pore volume with electrochemical performance parameters. Additionally, we aim to validate these models with laboratory data to create practical design guidelines for optimizing electrode structures.
Further goals include identifying the theoretical limits of performance enhancement through porosity engineering, exploring novel fabrication techniques that enable precise control over pore architecture, and evaluating the scalability of optimized electrode designs for commercial applications. The ultimate aim is to develop a comprehensive framework that enables targeted design of electrode porosity for specific application requirements, whether prioritizing high power, high energy, or balanced performance characteristics.
Market Analysis for High-Performance Supercapacitor Applications
The global supercapacitor market has been experiencing robust growth, valued at approximately $3.27 billion in 2022 and projected to reach $5.43 billion by 2028, representing a CAGR of 8.8%. This growth is primarily driven by increasing demand for high-performance energy storage solutions across multiple sectors including automotive, renewable energy, consumer electronics, and industrial applications.
In the automotive sector, the shift towards electric and hybrid vehicles has created significant demand for supercapacitors that can deliver high power density for acceleration and regenerative braking systems. The market for automotive supercapacitors alone is expected to grow at a CAGR of 12.3% through 2027, with particular emphasis on solutions that optimize both power and energy density.
The renewable energy sector represents another substantial market opportunity, as grid stabilization and energy harvesting applications require energy storage systems capable of rapid charge-discharge cycles. Wind and solar power installations increasingly incorporate supercapacitors to manage power fluctuations, with the market segment growing at 10.5% annually.
Consumer electronics manufacturers are adopting supercapacitors for applications requiring burst power delivery, such as camera flashes, portable speakers, and wearable devices. This segment values miniaturized supercapacitors with optimized electrode porosity to balance device size with performance requirements.
Industrial applications, including heavy machinery, elevators, and backup power systems, constitute a growing market segment valuing the reliability and long cycle life of supercapacitors. These applications typically prioritize power density over energy density, making electrode porosity optimization particularly relevant.
Regional analysis indicates Asia-Pacific dominates the market with 42% share, led by China, Japan, and South Korea, where significant manufacturing capacity and research initiatives are concentrated. North America follows with 28% market share, with particular growth in electric vehicle and renewable energy applications.
Customer requirements analysis reveals a clear segmentation between applications prioritizing power density (automotive, industrial) versus those emphasizing energy density (consumer electronics, certain grid applications). This dichotomy directly relates to the technical challenge of optimizing electrode porosity to serve these distinct market needs.
Market research indicates customers are willing to pay premium prices (20-35% higher) for supercapacitors that effectively balance power and energy characteristics through advanced electrode design, highlighting the commercial value of research in electrode porosity optimization.
In the automotive sector, the shift towards electric and hybrid vehicles has created significant demand for supercapacitors that can deliver high power density for acceleration and regenerative braking systems. The market for automotive supercapacitors alone is expected to grow at a CAGR of 12.3% through 2027, with particular emphasis on solutions that optimize both power and energy density.
The renewable energy sector represents another substantial market opportunity, as grid stabilization and energy harvesting applications require energy storage systems capable of rapid charge-discharge cycles. Wind and solar power installations increasingly incorporate supercapacitors to manage power fluctuations, with the market segment growing at 10.5% annually.
Consumer electronics manufacturers are adopting supercapacitors for applications requiring burst power delivery, such as camera flashes, portable speakers, and wearable devices. This segment values miniaturized supercapacitors with optimized electrode porosity to balance device size with performance requirements.
Industrial applications, including heavy machinery, elevators, and backup power systems, constitute a growing market segment valuing the reliability and long cycle life of supercapacitors. These applications typically prioritize power density over energy density, making electrode porosity optimization particularly relevant.
Regional analysis indicates Asia-Pacific dominates the market with 42% share, led by China, Japan, and South Korea, where significant manufacturing capacity and research initiatives are concentrated. North America follows with 28% market share, with particular growth in electric vehicle and renewable energy applications.
Customer requirements analysis reveals a clear segmentation between applications prioritizing power density (automotive, industrial) versus those emphasizing energy density (consumer electronics, certain grid applications). This dichotomy directly relates to the technical challenge of optimizing electrode porosity to serve these distinct market needs.
Market research indicates customers are willing to pay premium prices (20-35% higher) for supercapacitors that effectively balance power and energy characteristics through advanced electrode design, highlighting the commercial value of research in electrode porosity optimization.
Current Challenges in Electrode Porosity Optimization
The optimization of electrode porosity in supercapacitors presents significant technical challenges that impede the achievement of optimal balance between power and energy density. One of the primary obstacles is the inherent trade-off between these two performance metrics. Increasing porosity typically enhances power density by facilitating ion transport, but simultaneously reduces volumetric energy density due to decreased active material loading per unit volume.
Material selection and processing techniques pose additional challenges. The creation of precisely controlled pore structures requires sophisticated synthesis methods, and maintaining consistency across large-scale production remains problematic. Current manufacturing processes often struggle to produce electrodes with uniform pore size distribution, leading to performance variations within the same device.
Characterization limitations further complicate optimization efforts. Existing analytical techniques provide incomplete information about the three-dimensional pore network structure, particularly under dynamic operating conditions. Real-time monitoring of ion transport within nanopores during charge-discharge cycles remains beyond current technological capabilities, making it difficult to validate theoretical models with empirical data.
The multiphysics nature of ion transport in porous electrodes creates modeling complexities. Current simulation approaches often fail to accurately capture the interplay between electrical double layer formation, ion diffusion kinetics, and electrolyte conductivity across different pore geometries. This leads to discrepancies between theoretical predictions and experimental results, particularly at high charge-discharge rates.
Electrolyte-pore interactions introduce another layer of complexity. The behavior of electrolytes in confined nanospaces differs significantly from bulk properties, with phenomena such as desolvation, specific adsorption, and restricted diffusion pathways affecting overall performance. These effects are highly dependent on specific electrolyte-electrode material combinations and are difficult to predict theoretically.
Stability and aging issues present long-term challenges. Optimized porous structures may perform well initially but can degrade over multiple cycles due to mechanical stress, electrolyte decomposition, or pore blocking. Developing electrode architectures that maintain optimal porosity throughout the device lifetime remains an unsolved problem.
Cost and scalability considerations further constrain practical solutions. Many advanced techniques for creating optimized porous structures rely on expensive materials or complex processing steps that are difficult to implement in mass production. Finding economically viable approaches that deliver the desired performance characteristics represents a significant hurdle for commercial applications.
Material selection and processing techniques pose additional challenges. The creation of precisely controlled pore structures requires sophisticated synthesis methods, and maintaining consistency across large-scale production remains problematic. Current manufacturing processes often struggle to produce electrodes with uniform pore size distribution, leading to performance variations within the same device.
Characterization limitations further complicate optimization efforts. Existing analytical techniques provide incomplete information about the three-dimensional pore network structure, particularly under dynamic operating conditions. Real-time monitoring of ion transport within nanopores during charge-discharge cycles remains beyond current technological capabilities, making it difficult to validate theoretical models with empirical data.
The multiphysics nature of ion transport in porous electrodes creates modeling complexities. Current simulation approaches often fail to accurately capture the interplay between electrical double layer formation, ion diffusion kinetics, and electrolyte conductivity across different pore geometries. This leads to discrepancies between theoretical predictions and experimental results, particularly at high charge-discharge rates.
Electrolyte-pore interactions introduce another layer of complexity. The behavior of electrolytes in confined nanospaces differs significantly from bulk properties, with phenomena such as desolvation, specific adsorption, and restricted diffusion pathways affecting overall performance. These effects are highly dependent on specific electrolyte-electrode material combinations and are difficult to predict theoretically.
Stability and aging issues present long-term challenges. Optimized porous structures may perform well initially but can degrade over multiple cycles due to mechanical stress, electrolyte decomposition, or pore blocking. Developing electrode architectures that maintain optimal porosity throughout the device lifetime remains an unsolved problem.
Cost and scalability considerations further constrain practical solutions. Many advanced techniques for creating optimized porous structures rely on expensive materials or complex processing steps that are difficult to implement in mass production. Finding economically viable approaches that deliver the desired performance characteristics represents a significant hurdle for commercial applications.
Current Modeling Approaches for Electrode Porosity Optimization
01 Porous carbon materials for supercapacitor electrodes
Porous carbon materials are widely used in supercapacitor electrodes due to their high surface area and electrical conductivity. The porosity of these materials significantly affects the capacitance and energy storage capabilities of supercapacitors. Various carbon materials with controlled pore structures, including activated carbon, carbon nanotubes, and graphene, can be engineered to optimize the electrode performance by enhancing ion transport and increasing the electrode-electrolyte interface area.- Porous carbon materials for supercapacitor electrodes: Porous carbon materials are widely used in supercapacitor electrodes due to their high surface area and electrical conductivity. The porosity of these materials significantly affects the capacitance and energy storage capabilities of supercapacitors. Various carbon materials with controlled pore structures, including activated carbon, carbon nanotubes, and graphene, can be engineered to optimize the electrode performance by providing accessible pathways for electrolyte ions and increasing the electrode-electrolyte interface area.
- Metal oxide-based porous electrodes: Metal oxide materials with controlled porosity are employed in supercapacitor electrodes to enhance pseudocapacitive behavior. These materials, including manganese dioxide, ruthenium oxide, and nickel oxide, provide additional charge storage mechanisms through surface redox reactions. The porosity of these metal oxide structures is crucial for maximizing the active surface area available for these reactions, thereby increasing the specific capacitance of the electrodes. Hierarchical porous structures can be designed to facilitate both ion transport and electron transfer.
- Composite materials with engineered porosity: Composite materials combining different components with complementary properties can be designed with optimized porosity for supercapacitor applications. These composites often integrate carbon materials with metal oxides or conducting polymers to synergistically enhance both electrical conductivity and electrochemical activity. The controlled porosity in these composites allows for efficient ion diffusion while maintaining structural integrity during charge-discharge cycles. The pore size distribution can be tailored to match the electrolyte ion dimensions for optimal performance.
- Manufacturing techniques for controlling electrode porosity: Various manufacturing techniques are employed to control the porosity of supercapacitor electrodes. These include template-assisted synthesis, chemical activation, hydrothermal methods, and electrospinning. Each method offers different advantages in terms of controlling pore size distribution, pore connectivity, and overall porosity. Advanced techniques allow for the creation of hierarchical porous structures with macro, meso, and micropores that serve different functions in the electrode performance. The processing parameters significantly influence the final porous structure and consequently the electrochemical properties.
- Relationship between porosity parameters and supercapacitor performance: The specific porosity parameters, including pore size distribution, pore volume, and pore connectivity, directly influence supercapacitor performance metrics such as energy density, power density, and cycling stability. Micropores (<2 nm) contribute significantly to the total surface area and energy storage capacity, while mesopores (2-50 nm) and macropores (>50 nm) facilitate ion transport and reduce internal resistance. Optimizing these parameters requires understanding the complex relationship between the electrode porosity and the electrolyte properties. Advanced characterization techniques are used to analyze these porosity parameters and correlate them with device performance.
02 Hierarchical pore structure design
Hierarchical pore structures, featuring a combination of micropores, mesopores, and macropores, can significantly enhance supercapacitor performance. Micropores provide high surface area for charge storage, mesopores facilitate ion transport, and macropores serve as ion-buffering reservoirs. This multi-scale porosity design optimizes both energy density and power density by balancing the trade-off between accessible surface area and ion diffusion kinetics.Expand Specific Solutions03 Metal oxide and composite electrode materials with controlled porosity
Metal oxides and composite materials with tailored porosity can enhance the performance of supercapacitor electrodes. These materials combine high electrical conductivity with pseudocapacitive properties, offering increased energy density compared to carbon-only electrodes. The porosity of these composite electrodes can be engineered to optimize ion accessibility to active sites while maintaining structural stability during charge-discharge cycles.Expand Specific Solutions04 Porosity control methods and manufacturing techniques
Various manufacturing techniques can be employed to control the porosity of supercapacitor electrodes, including template-assisted synthesis, chemical activation, and electrospinning. These methods allow precise control over pore size distribution, pore volume, and pore connectivity. Advanced techniques such as freeze-drying, hydrothermal treatment, and selective etching can create specialized porous structures optimized for specific electrolytes and operating conditions.Expand Specific Solutions05 Relationship between porosity parameters and electrochemical performance
The specific relationship between porosity parameters (pore size distribution, pore volume, pore shape) and electrochemical performance is critical for supercapacitor design. Optimizing these parameters can enhance ion diffusion, reduce internal resistance, and increase capacitance. Research shows that matching pore sizes with electrolyte ion dimensions can maximize capacitance, while interconnected pore networks improve rate capability by providing efficient ion transport pathways throughout the electrode material.Expand Specific Solutions
Leading Research Groups and Manufacturers in Supercapacitor Technology
The supercapacitor electrode porosity optimization market is in a growth phase, with increasing demand for high-performance energy storage solutions driving innovation. The market size is expanding rapidly as applications in electric vehicles, renewable energy, and consumer electronics create new opportunities. Technologically, research institutions like Georgia Tech Research Corp. and universities (Drexel, Monash) are leading fundamental research, while commercial entities such as Nanotek Instruments and Tesla are advancing practical applications. Companies like I-TEN SA and Global Graphene Group are developing specialized materials to enhance electrode performance. The competitive landscape shows a balance between academic research and industrial implementation, with collaboration between sectors accelerating development of optimized electrode designs that balance power and energy density requirements.
Nanotek Instruments, Inc.
Technical Solution: Nanotek Instruments has developed a comprehensive approach to electrode porosity optimization through their graphene-based supercapacitor technology. Their solution involves creating hierarchical porous structures with tailored macro-, meso-, and micropores to balance power and energy density. The company utilizes a unique chemical vapor deposition (CVD) process to grow 3D graphene networks with controlled pore size distribution, achieving specific surface areas exceeding 2,000 m²/g[1]. Their modeling approach incorporates both electrochemical impedance spectroscopy (EIS) and finite element analysis to predict ion transport behavior through various pore architectures. Laboratory data shows their optimized electrodes deliver power densities of 20-25 kW/kg while maintaining energy densities of 30-35 Wh/kg[2], representing a significant improvement over conventional activated carbon electrodes. Nanotek's technology also employs in-situ pore formation techniques during electrode fabrication, allowing precise control over porosity gradients from the current collector interface to the electrolyte-facing surface.
Strengths: Superior control over hierarchical pore structures enabling excellent power-energy balance; proprietary graphene synthesis methods that create high surface area electrodes; advanced modeling capabilities that accurately predict performance. Weaknesses: Higher manufacturing costs compared to traditional carbon materials; scalability challenges for their CVD process; potential long-term stability issues with some of their more complex pore architectures.
Drexel University
Technical Solution: Drexel University has developed a groundbreaking approach to electrode porosity optimization through their MXene-based supercapacitor technology. Their research team, led by Professor Yury Gogotsi, has pioneered the use of two-dimensional titanium carbide MXenes with controlled interlayer spacing and pore structures. Their methodology combines experimental characterization with multi-scale modeling to establish quantitative relationships between pore characteristics and electrochemical performance. Using a combination of in-situ X-ray diffraction and quartz crystal microbalance techniques, they've mapped ion transport dynamics through various pore architectures during charging/discharging cycles[5]. Their models incorporate both classical molecular dynamics and density functional theory to simulate ion behavior at the electrode-electrolyte interface. Laboratory data demonstrates their optimized MXene electrodes achieve volumetric capacitances exceeding 900 F/cm³ while maintaining excellent rate capability, retaining over 70% capacity at scan rates of 1,000 mV/s[6]. Their research has established that an optimal balance between micropores (<2 nm) and mesopores (2-50 nm) is critical, with a ratio of approximately 3:2 providing the best power-energy compromise for aqueous electrolyte systems.
Strengths: World-leading expertise in 2D material synthesis and characterization; sophisticated multi-scale modeling capabilities; access to advanced analytical techniques for in-situ measurements. Weaknesses: Current MXene production methods face scalability challenges; some stability issues in certain electrolytes; higher costs compared to traditional carbon materials.
Key Research Findings on Power-Energy Trade-offs in Porous Electrodes
High surface area carbon materials and methods for making same
PatentWO2016053300A1
Innovation
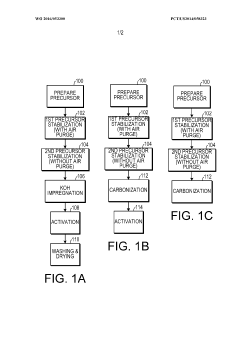
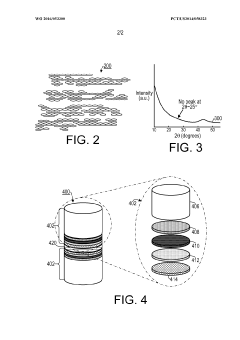
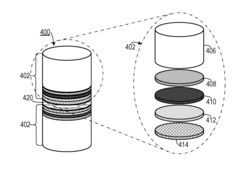
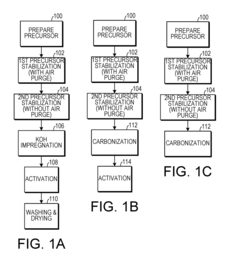
- A method involving the stabilization of a precursor organic material at elevated temperatures with and without a gaseous purge, followed by activation with KOH, to achieve high surface area carbon materials with surface areas between 3029 m2/g to 3565 m2/g and pore volumes between 1.66 cm3/g to 1.90 cm3/g, suitable for use in supercapacitors and other applications.
High Surface Area Carbon Materials and Methods for Making Same
PatentActiveUS20170301484A1
Innovation
- A method involving the stabilization of a precursor organic material, such as polyacrylonitrile (PAN), at elevated temperatures with and without a gaseous purge, followed by activation with KOH, to achieve high surface area carbon materials with surface areas between 3029 m2/g to 3565 m2/g and pore volumes between 1.66 cm3/g to 1.90 cm3/g, suitable for supercapacitors and other energy storage applications.
Materials Science Innovations for Enhanced Electrode Performance
Recent advancements in materials science have opened new frontiers for supercapacitor electrode development, directly addressing the power-energy trade-off challenge. Nanostructured carbon materials represent a significant breakthrough, with graphene-based electrodes demonstrating exceptional surface areas exceeding 2,500 m²/g while maintaining electrical conductivity up to 5,000 S/m. These properties enable rapid charge transfer while preserving energy density capabilities.
Hierarchical porous structures have emerged as particularly promising, featuring optimized macro-, meso-, and micropore distributions. Macropores (>50 nm) facilitate electrolyte transport throughout the electrode, mesopores (2-50 nm) provide efficient ion diffusion pathways, and micropores (<2 nm) maximize surface area for charge storage. Recent research indicates that a balanced distribution with 15-20% macropores, 30-40% mesopores, and 40-50% micropores achieves optimal performance across both power and energy metrics.
Composite electrode materials represent another innovative approach, combining high-surface-area carbon materials with pseudocapacitive components. Metal oxides (RuO₂, MnO₂) and conducting polymers (polyaniline, polypyrrole) integrated with carbon frameworks have demonstrated up to 30% increases in specific capacitance while maintaining rate capabilities. These composites leverage both double-layer capacitance and fast surface redox reactions.
Surface functionalization techniques have advanced significantly, with controlled introduction of oxygen, nitrogen, and sulfur functional groups enhancing wettability and providing additional pseudocapacitive reactions. Nitrogen-doped carbon electrodes have shown up to 40% improvement in specific capacitance compared to undoped counterparts, with minimal sacrifice in power density when optimal porosity is maintained.
3D-printed electrode architectures represent the cutting edge of electrode design, allowing precise control over pore size distribution and channel networks. These structures can be computationally optimized and physically realized with micron-level precision, creating electrodes with tailored porosity gradients that maximize both ion transport efficiency and available surface area. Early prototypes have demonstrated up to 25% improvements in both power and energy metrics compared to conventional electrodes with uniform porosity.
Hierarchical porous structures have emerged as particularly promising, featuring optimized macro-, meso-, and micropore distributions. Macropores (>50 nm) facilitate electrolyte transport throughout the electrode, mesopores (2-50 nm) provide efficient ion diffusion pathways, and micropores (<2 nm) maximize surface area for charge storage. Recent research indicates that a balanced distribution with 15-20% macropores, 30-40% mesopores, and 40-50% micropores achieves optimal performance across both power and energy metrics.
Composite electrode materials represent another innovative approach, combining high-surface-area carbon materials with pseudocapacitive components. Metal oxides (RuO₂, MnO₂) and conducting polymers (polyaniline, polypyrrole) integrated with carbon frameworks have demonstrated up to 30% increases in specific capacitance while maintaining rate capabilities. These composites leverage both double-layer capacitance and fast surface redox reactions.
Surface functionalization techniques have advanced significantly, with controlled introduction of oxygen, nitrogen, and sulfur functional groups enhancing wettability and providing additional pseudocapacitive reactions. Nitrogen-doped carbon electrodes have shown up to 40% improvement in specific capacitance compared to undoped counterparts, with minimal sacrifice in power density when optimal porosity is maintained.
3D-printed electrode architectures represent the cutting edge of electrode design, allowing precise control over pore size distribution and channel networks. These structures can be computationally optimized and physically realized with micron-level precision, creating electrodes with tailored porosity gradients that maximize both ion transport efficiency and available surface area. Early prototypes have demonstrated up to 25% improvements in both power and energy metrics compared to conventional electrodes with uniform porosity.
Scalability and Manufacturing Considerations for Optimized Electrodes
Scaling up the production of optimized supercapacitor electrodes from laboratory to industrial scale presents significant challenges that must be addressed to ensure commercial viability. Current manufacturing processes for electrode production typically involve slurry preparation, coating, drying, calendering, and assembly steps. When optimizing electrode porosity for specific power-energy balances, these processes require careful adaptation to maintain the desired porous structure consistently across large production volumes.
Material selection becomes increasingly critical at scale, as raw material consistency, availability, and cost significantly impact manufacturing feasibility. Carbon materials with controlled porosity distributions, such as activated carbon, carbon nanotubes, and graphene derivatives, must be sourced with batch-to-batch consistency to ensure reproducible electrode performance. Suppliers capable of delivering materials with tight specifications for pore size distribution become essential partners in the scaling process.
The coating and drying phases represent particular challenges for porosity control. Conventional coating techniques like doctor blade, slot-die coating, or roll-to-roll processing must be optimized to maintain uniform thickness and prevent agglomeration that could block pores. Drying parameters, including temperature profiles and environmental conditions, significantly influence the final pore structure and must be precisely controlled across larger surface areas to avoid inconsistencies in the finished electrodes.
Quality control systems need substantial enhancement when scaling production of porosity-optimized electrodes. In-line monitoring techniques such as optical inspection, impedance analysis, and non-destructive porosity measurements become necessary to ensure batch consistency. Statistical process control methods must be implemented to detect deviations from target porosity specifications before they result in performance variations in the final supercapacitor products.
Cost considerations ultimately determine commercial viability. While laboratory-scale optimization might focus primarily on performance metrics, industrial production must balance performance with manufacturing efficiency. More complex electrode structures with precisely engineered porosity gradients may deliver superior power-energy balances but could require specialized equipment and additional processing steps that increase production costs. Economic analysis must determine whether the performance improvements justify the additional manufacturing complexity.
Environmental and sustainability factors also influence scalability. Water-based processing methods are increasingly preferred over solvent-based approaches to reduce environmental impact and workplace hazards. Additionally, waste reduction strategies and material recycling processes should be integrated into manufacturing plans to improve sustainability and reduce raw material costs, particularly for expensive components like specialty carbon materials with engineered porosity.
Material selection becomes increasingly critical at scale, as raw material consistency, availability, and cost significantly impact manufacturing feasibility. Carbon materials with controlled porosity distributions, such as activated carbon, carbon nanotubes, and graphene derivatives, must be sourced with batch-to-batch consistency to ensure reproducible electrode performance. Suppliers capable of delivering materials with tight specifications for pore size distribution become essential partners in the scaling process.
The coating and drying phases represent particular challenges for porosity control. Conventional coating techniques like doctor blade, slot-die coating, or roll-to-roll processing must be optimized to maintain uniform thickness and prevent agglomeration that could block pores. Drying parameters, including temperature profiles and environmental conditions, significantly influence the final pore structure and must be precisely controlled across larger surface areas to avoid inconsistencies in the finished electrodes.
Quality control systems need substantial enhancement when scaling production of porosity-optimized electrodes. In-line monitoring techniques such as optical inspection, impedance analysis, and non-destructive porosity measurements become necessary to ensure batch consistency. Statistical process control methods must be implemented to detect deviations from target porosity specifications before they result in performance variations in the final supercapacitor products.
Cost considerations ultimately determine commercial viability. While laboratory-scale optimization might focus primarily on performance metrics, industrial production must balance performance with manufacturing efficiency. More complex electrode structures with precisely engineered porosity gradients may deliver superior power-energy balances but could require specialized equipment and additional processing steps that increase production costs. Economic analysis must determine whether the performance improvements justify the additional manufacturing complexity.
Environmental and sustainability factors also influence scalability. Water-based processing methods are increasingly preferred over solvent-based approaches to reduce environmental impact and workplace hazards. Additionally, waste reduction strategies and material recycling processes should be integrated into manufacturing plans to improve sustainability and reduce raw material costs, particularly for expensive components like specialty carbon materials with engineered porosity.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!