Supercapacitor Electrolyte Selection Guide: Aqueous, Organic and Ionic Liquids — Tradeoffs & Safety
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Supercapacitor Electrolyte Evolution and Research Objectives
Supercapacitors have evolved significantly since their inception in the late 1950s, with electrolyte development playing a crucial role in enhancing their performance characteristics. The journey began with simple aqueous electrolytes, primarily sulfuric acid and potassium hydroxide, which offered high ionic conductivity but limited voltage windows of approximately 1V. This constraint significantly restricted energy density capabilities of early devices.
The 1980s and 1990s marked a pivotal shift toward organic electrolytes, particularly acetonitrile and propylene carbonate-based solutions with tetraethylammonium tetrafluoroborate (TEABF4) as the conducting salt. This transition enabled voltage windows to expand to 2.7-2.8V, dramatically improving energy density by a factor of four to nine compared to aqueous systems, albeit with reduced power density due to lower ionic conductivity.
The early 2000s witnessed the emergence of ionic liquids as potential electrolytes, offering voltage windows exceeding 3.5V and enhanced thermal stability up to 300°C. Despite these advantages, their high viscosity and cost have limited widespread commercial adoption, relegating them primarily to specialized applications requiring extreme temperature operation.
Recent research has focused on hybrid and gel electrolytes that combine the benefits of different electrolyte types while mitigating their individual limitations. Water-in-salt electrolytes represent a particularly promising innovation, expanding the voltage window of aqueous systems to nearly 2.5V while maintaining safety advantages.
The primary research objectives in supercapacitor electrolyte development now center on several key parameters: maximizing the voltage window to enhance energy density; improving ionic conductivity for better power performance; ensuring thermal and chemical stability across wide temperature ranges (-40°C to 70°C for commercial applications); reducing environmental impact through less toxic formulations; and enhancing safety profiles by minimizing flammability and volatility.
Future research directions aim to develop electrolytes that can simultaneously achieve high voltage windows (>3V), excellent ionic conductivity (>20 mS/cm), wide temperature operation, and improved safety characteristics. Particular emphasis is being placed on novel ionic liquid mixtures, deep eutectic solvents, and solid-state electrolytes that could potentially revolutionize supercapacitor technology by enabling higher energy densities while maintaining the rapid charge-discharge capabilities that distinguish supercapacitors from batteries.
The 1980s and 1990s marked a pivotal shift toward organic electrolytes, particularly acetonitrile and propylene carbonate-based solutions with tetraethylammonium tetrafluoroborate (TEABF4) as the conducting salt. This transition enabled voltage windows to expand to 2.7-2.8V, dramatically improving energy density by a factor of four to nine compared to aqueous systems, albeit with reduced power density due to lower ionic conductivity.
The early 2000s witnessed the emergence of ionic liquids as potential electrolytes, offering voltage windows exceeding 3.5V and enhanced thermal stability up to 300°C. Despite these advantages, their high viscosity and cost have limited widespread commercial adoption, relegating them primarily to specialized applications requiring extreme temperature operation.
Recent research has focused on hybrid and gel electrolytes that combine the benefits of different electrolyte types while mitigating their individual limitations. Water-in-salt electrolytes represent a particularly promising innovation, expanding the voltage window of aqueous systems to nearly 2.5V while maintaining safety advantages.
The primary research objectives in supercapacitor electrolyte development now center on several key parameters: maximizing the voltage window to enhance energy density; improving ionic conductivity for better power performance; ensuring thermal and chemical stability across wide temperature ranges (-40°C to 70°C for commercial applications); reducing environmental impact through less toxic formulations; and enhancing safety profiles by minimizing flammability and volatility.
Future research directions aim to develop electrolytes that can simultaneously achieve high voltage windows (>3V), excellent ionic conductivity (>20 mS/cm), wide temperature operation, and improved safety characteristics. Particular emphasis is being placed on novel ionic liquid mixtures, deep eutectic solvents, and solid-state electrolytes that could potentially revolutionize supercapacitor technology by enabling higher energy densities while maintaining the rapid charge-discharge capabilities that distinguish supercapacitors from batteries.
Market Analysis of Supercapacitor Electrolyte Applications
The global supercapacitor market has been experiencing robust growth, projected to reach $5.4 billion by 2025 with a CAGR of 28.7% from 2020. Within this expanding market, electrolytes represent a critical component, accounting for approximately 15-20% of the total supercapacitor value chain. The electrolyte selection significantly impacts device performance, safety, and cost-effectiveness, making it a key differentiator in commercial applications.
Aqueous electrolytes currently dominate in cost-sensitive applications, holding roughly 40% market share due to their environmental friendliness and safety advantages. These electrolytes are particularly prevalent in consumer electronics and backup power systems where moderate energy density is acceptable and safety concerns are paramount.
Organic electrolytes command approximately 45% of the market share, primarily in automotive applications, industrial power management, and high-performance electronics. Their wider voltage window (2.5-2.8V) enables higher energy densities, making them the preferred choice for electric vehicles and renewable energy storage systems despite their higher cost and safety concerns.
Ionic liquid electrolytes, though representing only about 15% of current market applications, are showing the fastest growth rate at 32% annually. Their exceptional thermal stability and wide electrochemical window position them ideally for extreme environment applications in aerospace, military, and advanced industrial systems.
Regional analysis reveals Asia-Pacific as the dominant market for supercapacitor electrolytes, accounting for 55% of global consumption. This is primarily driven by the massive electronics manufacturing base in China, Japan, and South Korea. North America follows with 25% market share, with particular strength in high-performance and specialty applications, while Europe represents 15% with a focus on automotive and renewable energy applications.
End-user segmentation shows transportation as the largest application sector (35%), followed by consumer electronics (25%), industrial applications (20%), energy (15%), and others (5%). The transportation sector's dominance is expected to strengthen further with the accelerating adoption of electric and hybrid vehicles, where supercapacitors complement battery systems for regenerative braking and power stabilization.
Market trends indicate a growing preference for hybrid electrolyte systems that combine the advantages of different electrolyte types. Additionally, there is increasing demand for environmentally sustainable electrolyte formulations with reduced toxicity and improved end-of-life recyclability, particularly in European markets where regulatory pressures are intensifying.
Aqueous electrolytes currently dominate in cost-sensitive applications, holding roughly 40% market share due to their environmental friendliness and safety advantages. These electrolytes are particularly prevalent in consumer electronics and backup power systems where moderate energy density is acceptable and safety concerns are paramount.
Organic electrolytes command approximately 45% of the market share, primarily in automotive applications, industrial power management, and high-performance electronics. Their wider voltage window (2.5-2.8V) enables higher energy densities, making them the preferred choice for electric vehicles and renewable energy storage systems despite their higher cost and safety concerns.
Ionic liquid electrolytes, though representing only about 15% of current market applications, are showing the fastest growth rate at 32% annually. Their exceptional thermal stability and wide electrochemical window position them ideally for extreme environment applications in aerospace, military, and advanced industrial systems.
Regional analysis reveals Asia-Pacific as the dominant market for supercapacitor electrolytes, accounting for 55% of global consumption. This is primarily driven by the massive electronics manufacturing base in China, Japan, and South Korea. North America follows with 25% market share, with particular strength in high-performance and specialty applications, while Europe represents 15% with a focus on automotive and renewable energy applications.
End-user segmentation shows transportation as the largest application sector (35%), followed by consumer electronics (25%), industrial applications (20%), energy (15%), and others (5%). The transportation sector's dominance is expected to strengthen further with the accelerating adoption of electric and hybrid vehicles, where supercapacitors complement battery systems for regenerative braking and power stabilization.
Market trends indicate a growing preference for hybrid electrolyte systems that combine the advantages of different electrolyte types. Additionally, there is increasing demand for environmentally sustainable electrolyte formulations with reduced toxicity and improved end-of-life recyclability, particularly in European markets where regulatory pressures are intensifying.
Current Electrolyte Technologies and Technical Barriers
Supercapacitor electrolyte technologies have evolved significantly over the past decades, with three primary categories dominating the market: aqueous, organic, and ionic liquid electrolytes. Each type presents distinct advantages and limitations that influence supercapacitor performance metrics including energy density, power density, operational temperature range, and cycle life.
Aqueous electrolytes, primarily composed of acids (H2SO4, H3PO4), bases (KOH, NaOH), or neutral salts (Na2SO4, Li2SO4), offer excellent ionic conductivity (up to 1 S/cm) and low cost. However, they face a critical limitation in their narrow electrochemical stability window (approximately 1.23V), which significantly constrains energy density according to the equation E = ½CV². Despite this limitation, aqueous systems remain attractive for applications where safety and cost outweigh energy density requirements.
Organic electrolytes, typically acetonitrile or propylene carbonate-based with dissolved quaternary ammonium salts, currently dominate commercial supercapacitor applications. These systems provide a wider voltage window (2.5-2.8V), enabling higher energy densities. However, they present several technical barriers including lower ionic conductivity (10-60 mS/cm), flammability concerns, toxicity issues, and performance degradation at temperature extremes. The volatile and flammable nature of acetonitrile particularly raises safety concerns for large-scale energy storage applications.
Ionic liquid electrolytes represent the cutting edge in supercapacitor technology, offering exceptionally wide electrochemical windows (up to 4.5V) and excellent thermal stability (-50 to 300°C). Despite these advantages, widespread adoption faces significant barriers including high viscosity (resulting in low ionic conductivity at room temperature), prohibitive costs (5-20 times higher than organic alternatives), and manufacturing challenges related to moisture sensitivity during device assembly.
A critical technical barrier across all electrolyte types involves the electrolyte-electrode interface, where complex interactions determine capacitance, rate capability, and cycling stability. Interfacial resistance and chemical compatibility issues often lead to capacity fading and reduced cycle life. Additionally, electrolyte decomposition at high voltages generates gas evolution and pressure buildup within sealed devices, creating both performance and safety concerns.
Recent research has focused on hybrid and composite electrolyte systems that aim to combine the advantages of different electrolyte types while mitigating their individual limitations. These include water-in-salt electrolytes that expand the voltage window of aqueous systems, and organic-ionic liquid mixtures that balance conductivity with voltage stability. However, these approaches introduce new challenges in terms of long-term stability, temperature sensitivity, and manufacturing complexity.
Aqueous electrolytes, primarily composed of acids (H2SO4, H3PO4), bases (KOH, NaOH), or neutral salts (Na2SO4, Li2SO4), offer excellent ionic conductivity (up to 1 S/cm) and low cost. However, they face a critical limitation in their narrow electrochemical stability window (approximately 1.23V), which significantly constrains energy density according to the equation E = ½CV². Despite this limitation, aqueous systems remain attractive for applications where safety and cost outweigh energy density requirements.
Organic electrolytes, typically acetonitrile or propylene carbonate-based with dissolved quaternary ammonium salts, currently dominate commercial supercapacitor applications. These systems provide a wider voltage window (2.5-2.8V), enabling higher energy densities. However, they present several technical barriers including lower ionic conductivity (10-60 mS/cm), flammability concerns, toxicity issues, and performance degradation at temperature extremes. The volatile and flammable nature of acetonitrile particularly raises safety concerns for large-scale energy storage applications.
Ionic liquid electrolytes represent the cutting edge in supercapacitor technology, offering exceptionally wide electrochemical windows (up to 4.5V) and excellent thermal stability (-50 to 300°C). Despite these advantages, widespread adoption faces significant barriers including high viscosity (resulting in low ionic conductivity at room temperature), prohibitive costs (5-20 times higher than organic alternatives), and manufacturing challenges related to moisture sensitivity during device assembly.
A critical technical barrier across all electrolyte types involves the electrolyte-electrode interface, where complex interactions determine capacitance, rate capability, and cycling stability. Interfacial resistance and chemical compatibility issues often lead to capacity fading and reduced cycle life. Additionally, electrolyte decomposition at high voltages generates gas evolution and pressure buildup within sealed devices, creating both performance and safety concerns.
Recent research has focused on hybrid and composite electrolyte systems that aim to combine the advantages of different electrolyte types while mitigating their individual limitations. These include water-in-salt electrolytes that expand the voltage window of aqueous systems, and organic-ionic liquid mixtures that balance conductivity with voltage stability. However, these approaches introduce new challenges in terms of long-term stability, temperature sensitivity, and manufacturing complexity.
Comparative Analysis of Aqueous, Organic and Ionic Liquid Electrolytes
01 Ionic liquid electrolytes for enhanced performance
Ionic liquids serve as effective electrolytes in supercapacitors due to their wide electrochemical window, high thermal stability, and negligible vapor pressure. These properties allow for higher operating voltages, which directly increases energy density while maintaining safety. The non-flammable nature of many ionic liquids also addresses safety concerns in high-power applications. Their customizable structure enables optimization of conductivity and viscosity for specific performance requirements.- Ionic liquid electrolytes for enhanced safety: Ionic liquids are being used as electrolytes in supercapacitors due to their non-flammability and high thermal stability, which significantly improves safety compared to conventional organic electrolytes. These materials offer wider electrochemical windows, allowing for higher operating voltages and consequently higher energy densities. However, they typically have higher viscosity which can limit ionic conductivity and power performance, creating a trade-off between safety and power capability.
- Solid-state and gel electrolytes for safety improvement: Solid-state and gel electrolytes eliminate leakage risks and improve the mechanical stability of supercapacitors. These electrolytes contain polymers or other materials that immobilize the conductive ions while maintaining adequate ionic conductivity. The trade-off is typically reduced ionic mobility compared to liquid electrolytes, which can impact the power density and rate capability of the device. However, the enhanced safety profile makes these electrolytes particularly suitable for applications where safety is paramount.
- Aqueous electrolytes balancing safety and performance: Aqueous electrolytes offer excellent safety characteristics due to their non-flammability and environmental friendliness. They provide high ionic conductivity, enabling high power performance in supercapacitors. The main limitation is their restricted voltage window (typically below 1.23V) due to water decomposition, which constrains energy density. Research focuses on additives and pH adjustments to extend the operating voltage while maintaining the inherent safety advantages of water-based systems.
- Hybrid and composite electrolyte systems: Hybrid electrolyte systems combine different types of electrolytes to achieve an optimal balance between safety and performance. These may include mixtures of organic solvents with ionic liquids, or composite systems incorporating solid components in liquid electrolytes. Such approaches aim to mitigate the limitations of individual electrolyte types while preserving their advantages. The design challenge lies in ensuring compatibility between components and maintaining long-term stability of the hybrid system.
- Electrolyte additives for performance enhancement and safety: Various additives are incorporated into supercapacitor electrolytes to enhance specific properties while maintaining safety. These include flame retardants to reduce flammability of organic electrolytes, redox-active species to increase energy density through faradaic reactions, and stabilizing agents to extend operational temperature ranges and cycle life. While additives can significantly improve certain aspects of performance, they may introduce complexity to the electrolyte system and potentially create new trade-offs in terms of cost, long-term stability, or environmental impact.
02 Solid-state and gel electrolytes for improved safety
Solid-state and gel electrolytes offer significant safety advantages over liquid electrolytes by eliminating leakage risks and reducing flammability. These electrolytes incorporate polymers or other binding materials that provide mechanical stability while maintaining ion conductivity. Though they typically have lower ionic conductivity than liquid systems, resulting in higher internal resistance and lower power density, they enable the development of flexible, compact, and safer supercapacitor designs for applications where safety is paramount.Expand Specific Solutions03 Aqueous electrolytes balancing safety and performance
Aqueous electrolytes offer excellent safety profiles with non-flammability and environmental friendliness while providing high ionic conductivity for good power performance. Their main limitation is the restricted voltage window (typically <1.23V) due to water decomposition, which constrains energy density. Research focuses on pH-optimized systems and additives to expand the operating voltage while maintaining the inherent safety advantages. These electrolytes are particularly suitable for applications where safety and cost considerations outweigh maximum energy density requirements.Expand Specific Solutions04 Organic electrolytes for high energy density
Organic electrolytes enable higher operating voltages (2.5-3.0V) compared to aqueous systems, significantly increasing energy density. Typically based on organic solvents like acetonitrile or propylene carbonate with dissolved salts, these electrolytes offer a balance between conductivity and voltage window. However, they present safety challenges due to flammability and potential toxicity. Performance trade-offs include temperature sensitivity and aging effects that impact long-term stability. Safety enhancements focus on flame-retardant additives and improved packaging to mitigate risks.Expand Specific Solutions05 Hybrid and composite electrolyte systems
Hybrid electrolyte systems combine different types of electrolytes to leverage complementary advantages while minimizing individual limitations. These may include ionic liquid/organic solvent mixtures, polymer-reinforced liquid electrolytes, or layered electrolyte structures. Such combinations can simultaneously address multiple performance parameters like operating voltage, temperature range, and safety. Advanced composite electrolytes incorporating nanomaterials or phase-change materials offer innovative approaches to overcome traditional performance trade-offs between energy density, power capability, and safety.Expand Specific Solutions
Leading Manufacturers and Research Institutions
The supercapacitor electrolyte market is currently in a growth phase, with increasing demand driven by renewable energy storage and electric vehicle applications. The market is expected to reach $2.5 billion by 2027, growing at a CAGR of approximately 12%. Technologically, the field shows varying maturity levels across different electrolyte types. Research institutions like Nanyang Technological University and Central South University are advancing fundamental research, while commercial players demonstrate different specialization patterns: Capchem Technology and Shanghai Aowei focus on electrolyte manufacturing, Inmatech and InnoCELL develop proprietary capacitor technologies, and established corporations like Robert Bosch integrate supercapacitors into broader energy solutions. The competition between aqueous, organic, and ionic liquid electrolytes continues to evolve as safety requirements and performance demands reshape market dynamics.
Shenzhen Capchem Technology Co., Ltd.
Technical Solution: Capchem has developed advanced organic electrolytes for supercapacitors featuring acetonitrile (ACN) and propylene carbonate (PC) as primary solvents with quaternary ammonium salts. Their proprietary formulations achieve 2.7-3.0V operating windows while maintaining cycle stability over 500,000 cycles. The company has pioneered additives that form stable solid electrolyte interphase (SEI) layers on electrode surfaces, reducing self-discharge rates by approximately 15% compared to standard formulations. Capchem's electrolytes demonstrate conductivity values of 50-60 mS/cm for ACN-based systems and 20-30 mS/cm for PC-based alternatives, with temperature stability ranging from -40°C to 70°C. Their manufacturing process includes molecular sieve purification to achieve water content below 20 ppm, critical for preventing voltage degradation and hydrogen evolution.
Strengths: Superior voltage stability allowing higher energy density; excellent temperature range performance; established manufacturing scale with high purity control. Weaknesses: ACN-based formulations present flammability concerns; higher cost compared to aqueous alternatives; environmental concerns regarding organic solvent disposal.
Inmatech, Inc.
Technical Solution: Inmatech has pioneered aqueous electrolyte formulations for supercapacitors that achieve expanded voltage windows through pH-optimized systems. Their patented technology utilizes neutral aqueous electrolytes with carefully selected salts (Li2SO4, Na2SO4) at high concentrations (>1M) to achieve operating voltages up to 2.2V, significantly higher than conventional aqueous systems limited to 1.23V. The company has developed proprietary additives that form protective films on carbon electrodes, preventing hydrogen and oxygen evolution at higher voltages. Their electrolyte systems demonstrate conductivity values exceeding 100 mS/cm, superior to organic alternatives, while maintaining operational stability from -5°C to 70°C. Inmatech's aqueous formulations have shown remarkable cycle life exceeding 100,000 cycles with less than 10% capacitance degradation. The company has also developed manufacturing processes that eliminate the need for dry rooms and reduce production costs by approximately 40% compared to organic electrolyte manufacturing, while simultaneously improving environmental and safety profiles.
Strengths: Environmentally friendly and non-flammable composition; higher ionic conductivity enabling better power performance; significantly lower production costs and simplified manufacturing. Weaknesses: Lower voltage window compared to organic and ionic liquid systems; limited low-temperature performance; higher self-discharge rates than organic alternatives.
Key Patents and Scientific Breakthroughs in Electrolyte Design
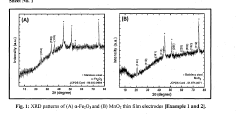
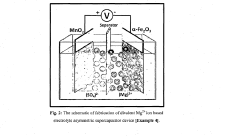
Divalent magnesium (mg2+) ion electrolyte based asymmetric supercapacitor device using Α-fe2o3 as anode and mno2 as cathode
PatentPendingIN202421023825A
Innovation
- The fabrication of a divalent magnesium (Mg2+) ion electrolyte-based asymmetric supercapacitor device using α-Fe2O3 as the anode and MnO2 as the cathode, with magnesium sulfate (MgSO4) as the electrolyte, synthesized through the successive ionic layer adsorption and reaction (SILAR) method, optimizing the electrolyte concentration and temperature for improved specific capacitance and stability.
Thixotropic organic electrolyte composition for supercapacitor and preparation method thereof
PatentInactiveUS20110108754A1
Innovation
- A thixotropic organic electrolyte composition is developed, comprising an organic solvent, a salt, and hydrophilic oxide particles, which transforms into a gel or solid state at normal temperatures, maintaining high ionic conductivity and reducing volatility, allowing for enhanced safety and service life while enabling flexible design and shape production.
Safety Standards and Risk Mitigation Strategies
The safety of supercapacitor electrolytes is governed by comprehensive standards that vary across regions and applications. In the United States, UL 810A specifically addresses electrochemical capacitors, while IEC 62576 provides international guidelines for electrical double-layer capacitors. These standards establish critical parameters for thermal stability, pressure tolerance, and chemical containment that manufacturers must adhere to when developing electrolyte systems.
Risk assessment frameworks for supercapacitor electrolytes typically evaluate three primary hazard categories: flammability, toxicity, and environmental impact. Aqueous electrolytes generally present lower safety risks but require careful consideration of hydrogen evolution potential and corrosion effects. Organic electrolytes, particularly those containing acetonitrile, demand stringent handling protocols due to their flammability and potential to release hydrogen cyanide under thermal stress.
Ionic liquid electrolytes, while offering improved thermal stability, still require careful evaluation for potential decomposition products under extreme conditions. The EUCAR hazard classification system, originally developed for batteries, has been adapted for supercapacitor applications to provide standardized risk assessment metrics across different electrolyte chemistries.
Effective risk mitigation strategies begin at the design phase with inherent safety principles. These include substituting hazardous components with safer alternatives where possible, implementing redundant protection mechanisms, and designing for fail-safe operation. For manufacturing environments, specialized ventilation systems, spark-proof equipment, and rigorous material segregation protocols are essential when handling volatile organic electrolytes.
End-user safety is enhanced through multiple protection layers including thermal fuses, pressure relief mechanisms, and electronic management systems that prevent operation outside safe voltage and temperature windows. For large-scale installations, particularly in transportation or grid applications, isolation strategies and fire suppression systems specifically designed for electrical fires are critical components of the safety infrastructure.
Emerging approaches to risk mitigation include the development of solid-state and gel electrolytes that inherently reduce leakage and flammability concerns. Advanced monitoring technologies utilizing impedance spectroscopy can detect early signs of electrolyte degradation before safety-critical failures occur. These proactive measures, combined with rigorous adherence to established safety protocols, form a comprehensive approach to managing the inherent risks associated with different electrolyte chemistries.
Risk assessment frameworks for supercapacitor electrolytes typically evaluate three primary hazard categories: flammability, toxicity, and environmental impact. Aqueous electrolytes generally present lower safety risks but require careful consideration of hydrogen evolution potential and corrosion effects. Organic electrolytes, particularly those containing acetonitrile, demand stringent handling protocols due to their flammability and potential to release hydrogen cyanide under thermal stress.
Ionic liquid electrolytes, while offering improved thermal stability, still require careful evaluation for potential decomposition products under extreme conditions. The EUCAR hazard classification system, originally developed for batteries, has been adapted for supercapacitor applications to provide standardized risk assessment metrics across different electrolyte chemistries.
Effective risk mitigation strategies begin at the design phase with inherent safety principles. These include substituting hazardous components with safer alternatives where possible, implementing redundant protection mechanisms, and designing for fail-safe operation. For manufacturing environments, specialized ventilation systems, spark-proof equipment, and rigorous material segregation protocols are essential when handling volatile organic electrolytes.
End-user safety is enhanced through multiple protection layers including thermal fuses, pressure relief mechanisms, and electronic management systems that prevent operation outside safe voltage and temperature windows. For large-scale installations, particularly in transportation or grid applications, isolation strategies and fire suppression systems specifically designed for electrical fires are critical components of the safety infrastructure.
Emerging approaches to risk mitigation include the development of solid-state and gel electrolytes that inherently reduce leakage and flammability concerns. Advanced monitoring technologies utilizing impedance spectroscopy can detect early signs of electrolyte degradation before safety-critical failures occur. These proactive measures, combined with rigorous adherence to established safety protocols, form a comprehensive approach to managing the inherent risks associated with different electrolyte chemistries.
Environmental Impact and Sustainability Considerations
The environmental impact of supercapacitor electrolytes represents a critical consideration in the sustainable development of energy storage technologies. Aqueous electrolytes generally demonstrate the most favorable environmental profile, utilizing water as the primary solvent and avoiding the volatile organic compounds (VOCs) associated with organic alternatives. These water-based solutions typically contain dissolved salts such as H2SO4, KOH, or Na2SO4, which present lower toxicity levels and reduced environmental persistence compared to organic counterparts.
Organic electrolytes, while offering enhanced voltage windows and energy densities, introduce significant environmental concerns. Solvents like acetonitrile and propylene carbonate require careful handling throughout their lifecycle due to their toxicity profiles. Acetonitrile, in particular, presents acute environmental hazards if released untreated, with potential for groundwater contamination and aquatic ecosystem disruption. The manufacturing processes for these organic compounds also typically involve higher carbon footprints and energy-intensive purification steps.
Ionic liquids represent an emerging middle ground in the sustainability spectrum. Their negligible vapor pressure significantly reduces air pollution risks and fire hazards compared to volatile organic electrolytes. However, the environmental persistence of many ionic liquids remains inadequately studied, with concerns regarding their potential bioaccumulation and long-term ecological impacts. The synthesis of ionic liquids also currently involves complex, multi-step processes with considerable energy requirements.
End-of-life management varies substantially across electrolyte categories. Aqueous systems generally permit simpler recycling protocols and waste treatment processes, while organic electrolytes require specialized handling to prevent environmental contamination. The recovery of valuable materials from spent electrolytes represents an evolving field with significant potential for reducing the overall environmental footprint of supercapacitor technologies.
Manufacturing sustainability metrics indicate that aqueous electrolytes typically require 40-60% less energy input during production compared to organic alternatives. Carbon footprint assessments similarly favor aqueous systems, though recent advances in green chemistry approaches for organic electrolyte synthesis have narrowed this gap. Ionic liquid production currently demonstrates the highest environmental burden per unit volume, though economies of scale may improve this profile as adoption increases.
Regulatory frameworks increasingly influence electrolyte selection decisions, with restrictions on certain organic solvents tightening in regions with stringent environmental protection policies. The European Union's REACH regulations and similar frameworks in other jurisdictions have accelerated research into environmentally benign alternatives across all electrolyte categories, driving innovation in green chemistry approaches to electrolyte formulation.
Organic electrolytes, while offering enhanced voltage windows and energy densities, introduce significant environmental concerns. Solvents like acetonitrile and propylene carbonate require careful handling throughout their lifecycle due to their toxicity profiles. Acetonitrile, in particular, presents acute environmental hazards if released untreated, with potential for groundwater contamination and aquatic ecosystem disruption. The manufacturing processes for these organic compounds also typically involve higher carbon footprints and energy-intensive purification steps.
Ionic liquids represent an emerging middle ground in the sustainability spectrum. Their negligible vapor pressure significantly reduces air pollution risks and fire hazards compared to volatile organic electrolytes. However, the environmental persistence of many ionic liquids remains inadequately studied, with concerns regarding their potential bioaccumulation and long-term ecological impacts. The synthesis of ionic liquids also currently involves complex, multi-step processes with considerable energy requirements.
End-of-life management varies substantially across electrolyte categories. Aqueous systems generally permit simpler recycling protocols and waste treatment processes, while organic electrolytes require specialized handling to prevent environmental contamination. The recovery of valuable materials from spent electrolytes represents an evolving field with significant potential for reducing the overall environmental footprint of supercapacitor technologies.
Manufacturing sustainability metrics indicate that aqueous electrolytes typically require 40-60% less energy input during production compared to organic alternatives. Carbon footprint assessments similarly favor aqueous systems, though recent advances in green chemistry approaches for organic electrolyte synthesis have narrowed this gap. Ionic liquid production currently demonstrates the highest environmental burden per unit volume, though economies of scale may improve this profile as adoption increases.
Regulatory frameworks increasingly influence electrolyte selection decisions, with restrictions on certain organic solvents tightening in regions with stringent environmental protection policies. The European Union's REACH regulations and similar frameworks in other jurisdictions have accelerated research into environmentally benign alternatives across all electrolyte categories, driving innovation in green chemistry approaches to electrolyte formulation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!