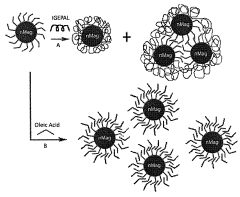
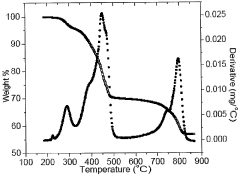
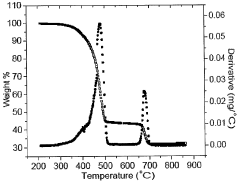
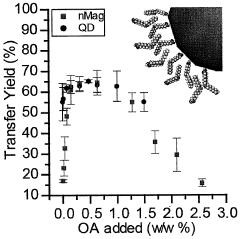
Innovations in Encapsulating Hydroxyapatite Nanoparticles with Lipid Bilayers
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydroxyapatite Nanoparticle Encapsulation Background
Hydroxyapatite (HAp) nanoparticles have emerged as a promising material in various biomedical applications due to their excellent biocompatibility, osteoconductivity, and similarity to natural bone mineral. The encapsulation of these nanoparticles with lipid bilayers represents a significant advancement in the field of nanomedicine and drug delivery systems.
The concept of encapsulating HAp nanoparticles with lipid bilayers originated from the need to enhance their stability, bioavailability, and targeted delivery capabilities. This innovative approach combines the beneficial properties of HAp with the versatility and biocompatibility of lipid-based systems, creating a hybrid nanostructure with improved functionality.
The development of this technology can be traced back to the early 2000s when researchers began exploring ways to modify the surface properties of HAp nanoparticles to improve their performance in biological environments. The idea of using lipid bilayers as a coating material was inspired by the structure of cell membranes, which naturally provide protection and selective permeability to cellular components.
Initial attempts to encapsulate HAp nanoparticles focused on simple lipid coatings, but as the field progressed, more sophisticated techniques were developed. These included the use of various lipid compositions, incorporation of functional molecules within the lipid bilayer, and optimization of the encapsulation process to achieve better control over particle size and uniformity.
The evolution of this technology has been driven by its potential applications in drug delivery, bone tissue engineering, and dental care. The lipid bilayer encapsulation allows for the incorporation of therapeutic agents, growth factors, or other bioactive molecules within the nanoparticle structure, enabling targeted and controlled release at specific sites in the body.
Recent advancements in the field have focused on improving the stability of the lipid-encapsulated HAp nanoparticles, enhancing their ability to cross biological barriers, and developing stimuli-responsive systems that can release their payload under specific conditions. These innovations have expanded the potential applications of the technology, opening up new possibilities in areas such as cancer treatment, gene therapy, and regenerative medicine.
The current state of the art in HAp nanoparticle encapsulation with lipid bilayers involves a multidisciplinary approach, combining principles from materials science, nanotechnology, and pharmaceutical sciences. Researchers are now exploring the use of advanced lipid formulations, such as liposomes and solid lipid nanoparticles, to further enhance the functionality and performance of these hybrid nanostructures.
As the field continues to evolve, there is growing interest in understanding the interactions between the HAp core and the lipid bilayer, as well as the behavior of these nanoparticles in complex biological environments. This knowledge is crucial for optimizing their design and improving their efficacy in various biomedical applications.
The concept of encapsulating HAp nanoparticles with lipid bilayers originated from the need to enhance their stability, bioavailability, and targeted delivery capabilities. This innovative approach combines the beneficial properties of HAp with the versatility and biocompatibility of lipid-based systems, creating a hybrid nanostructure with improved functionality.
The development of this technology can be traced back to the early 2000s when researchers began exploring ways to modify the surface properties of HAp nanoparticles to improve their performance in biological environments. The idea of using lipid bilayers as a coating material was inspired by the structure of cell membranes, which naturally provide protection and selective permeability to cellular components.
Initial attempts to encapsulate HAp nanoparticles focused on simple lipid coatings, but as the field progressed, more sophisticated techniques were developed. These included the use of various lipid compositions, incorporation of functional molecules within the lipid bilayer, and optimization of the encapsulation process to achieve better control over particle size and uniformity.
The evolution of this technology has been driven by its potential applications in drug delivery, bone tissue engineering, and dental care. The lipid bilayer encapsulation allows for the incorporation of therapeutic agents, growth factors, or other bioactive molecules within the nanoparticle structure, enabling targeted and controlled release at specific sites in the body.
Recent advancements in the field have focused on improving the stability of the lipid-encapsulated HAp nanoparticles, enhancing their ability to cross biological barriers, and developing stimuli-responsive systems that can release their payload under specific conditions. These innovations have expanded the potential applications of the technology, opening up new possibilities in areas such as cancer treatment, gene therapy, and regenerative medicine.
The current state of the art in HAp nanoparticle encapsulation with lipid bilayers involves a multidisciplinary approach, combining principles from materials science, nanotechnology, and pharmaceutical sciences. Researchers are now exploring the use of advanced lipid formulations, such as liposomes and solid lipid nanoparticles, to further enhance the functionality and performance of these hybrid nanostructures.
As the field continues to evolve, there is growing interest in understanding the interactions between the HAp core and the lipid bilayer, as well as the behavior of these nanoparticles in complex biological environments. This knowledge is crucial for optimizing their design and improving their efficacy in various biomedical applications.
Market Demand Analysis
The market demand for innovations in encapsulating hydroxyapatite nanoparticles with lipid bilayers is driven by several key factors across multiple industries. In the biomedical field, there is a growing need for advanced drug delivery systems that can improve the efficacy and safety of therapeutic agents. Lipid-encapsulated hydroxyapatite nanoparticles offer a promising solution, as they can enhance drug targeting, control release rates, and reduce side effects.
The dental industry represents another significant market for this technology. With an increasing focus on preventive and restorative dental care, there is a rising demand for materials that can promote tooth remineralization and repair. Encapsulated hydroxyapatite nanoparticles have shown potential in enhancing the effectiveness of dental products, such as toothpaste and mouthwashes, by improving the delivery of active ingredients to tooth surfaces.
In the orthopedic sector, the aging population and the prevalence of bone-related disorders are driving the need for advanced bone graft substitutes and regenerative materials. Lipid-encapsulated hydroxyapatite nanoparticles can potentially enhance the biocompatibility and integration of bone implants, addressing a critical market need for improved patient outcomes in orthopedic surgeries.
The cosmetics industry is also showing interest in this technology, particularly for anti-aging and skin repair products. The ability of encapsulated hydroxyapatite nanoparticles to penetrate the skin barrier and deliver active ingredients more effectively aligns with consumer demands for high-performance skincare solutions.
Environmental applications represent an emerging market for this technology. The use of encapsulated hydroxyapatite nanoparticles in water treatment and soil remediation processes is gaining attention due to their potential to remove heavy metals and other contaminants more efficiently than traditional methods.
The global market for nanotechnology in drug delivery is expected to grow significantly in the coming years, with a substantial portion attributed to lipid-based nanocarriers. This growth is fueled by increasing research and development activities in nanomedicine and the rising prevalence of chronic diseases requiring targeted drug delivery solutions.
While the market potential is substantial, challenges such as regulatory hurdles, scalability of production, and concerns about long-term safety need to be addressed to fully realize the market opportunities. Nevertheless, the diverse applications and potential benefits of lipid-encapsulated hydroxyapatite nanoparticles indicate a strong and growing market demand across multiple sectors.
The dental industry represents another significant market for this technology. With an increasing focus on preventive and restorative dental care, there is a rising demand for materials that can promote tooth remineralization and repair. Encapsulated hydroxyapatite nanoparticles have shown potential in enhancing the effectiveness of dental products, such as toothpaste and mouthwashes, by improving the delivery of active ingredients to tooth surfaces.
In the orthopedic sector, the aging population and the prevalence of bone-related disorders are driving the need for advanced bone graft substitutes and regenerative materials. Lipid-encapsulated hydroxyapatite nanoparticles can potentially enhance the biocompatibility and integration of bone implants, addressing a critical market need for improved patient outcomes in orthopedic surgeries.
The cosmetics industry is also showing interest in this technology, particularly for anti-aging and skin repair products. The ability of encapsulated hydroxyapatite nanoparticles to penetrate the skin barrier and deliver active ingredients more effectively aligns with consumer demands for high-performance skincare solutions.
Environmental applications represent an emerging market for this technology. The use of encapsulated hydroxyapatite nanoparticles in water treatment and soil remediation processes is gaining attention due to their potential to remove heavy metals and other contaminants more efficiently than traditional methods.
The global market for nanotechnology in drug delivery is expected to grow significantly in the coming years, with a substantial portion attributed to lipid-based nanocarriers. This growth is fueled by increasing research and development activities in nanomedicine and the rising prevalence of chronic diseases requiring targeted drug delivery solutions.
While the market potential is substantial, challenges such as regulatory hurdles, scalability of production, and concerns about long-term safety need to be addressed to fully realize the market opportunities. Nevertheless, the diverse applications and potential benefits of lipid-encapsulated hydroxyapatite nanoparticles indicate a strong and growing market demand across multiple sectors.
Current Challenges in Lipid Bilayer Encapsulation
The encapsulation of hydroxyapatite nanoparticles with lipid bilayers presents several significant challenges that researchers and industry professionals are currently grappling with. One of the primary obstacles is achieving consistent and uniform encapsulation across a large population of nanoparticles. The inherent variability in nanoparticle size and shape can lead to inconsistencies in the lipid bilayer coating, potentially affecting the stability and functionality of the final product.
Another major challenge lies in maintaining the structural integrity of the lipid bilayer during and after the encapsulation process. The high surface energy of nanoparticles can disrupt the delicate organization of lipid molecules, leading to defects or incomplete coverage. This issue is particularly pronounced when dealing with hydroxyapatite nanoparticles, which have a complex surface chemistry that can interact unpredictably with lipid molecules.
The scalability of the encapsulation process also poses a significant hurdle. While laboratory-scale production may yield satisfactory results, translating these methods to industrial-scale manufacturing while maintaining quality and consistency remains a formidable challenge. The need for specialized equipment and precise control over environmental conditions further complicates large-scale production efforts.
Stability of the encapsulated nanoparticles during storage and application is another critical concern. The lipid bilayer must provide adequate protection to the hydroxyapatite core while also allowing for controlled release or interaction with the target environment. Achieving this delicate balance requires careful optimization of the lipid composition and encapsulation techniques.
Furthermore, the characterization and quality control of lipid bilayer-encapsulated hydroxyapatite nanoparticles present unique challenges. Traditional analytical methods may not be sufficient to fully assess the uniformity and integrity of the lipid coating, necessitating the development of new, more sophisticated characterization techniques.
Lastly, the biocompatibility and biodegradability of the encapsulated nanoparticles remain areas of ongoing research and concern. While lipid bilayers are generally considered biocompatible, the long-term effects of these hybrid nanostructures in biological systems are not yet fully understood. Ensuring that the encapsulated nanoparticles can be safely metabolized or excreted from the body without adverse effects is crucial for their potential use in biomedical applications.
Another major challenge lies in maintaining the structural integrity of the lipid bilayer during and after the encapsulation process. The high surface energy of nanoparticles can disrupt the delicate organization of lipid molecules, leading to defects or incomplete coverage. This issue is particularly pronounced when dealing with hydroxyapatite nanoparticles, which have a complex surface chemistry that can interact unpredictably with lipid molecules.
The scalability of the encapsulation process also poses a significant hurdle. While laboratory-scale production may yield satisfactory results, translating these methods to industrial-scale manufacturing while maintaining quality and consistency remains a formidable challenge. The need for specialized equipment and precise control over environmental conditions further complicates large-scale production efforts.
Stability of the encapsulated nanoparticles during storage and application is another critical concern. The lipid bilayer must provide adequate protection to the hydroxyapatite core while also allowing for controlled release or interaction with the target environment. Achieving this delicate balance requires careful optimization of the lipid composition and encapsulation techniques.
Furthermore, the characterization and quality control of lipid bilayer-encapsulated hydroxyapatite nanoparticles present unique challenges. Traditional analytical methods may not be sufficient to fully assess the uniformity and integrity of the lipid coating, necessitating the development of new, more sophisticated characterization techniques.
Lastly, the biocompatibility and biodegradability of the encapsulated nanoparticles remain areas of ongoing research and concern. While lipid bilayers are generally considered biocompatible, the long-term effects of these hybrid nanostructures in biological systems are not yet fully understood. Ensuring that the encapsulated nanoparticles can be safely metabolized or excreted from the body without adverse effects is crucial for their potential use in biomedical applications.
Existing Encapsulation Techniques
01 Encapsulation methods for hydroxyapatite nanoparticles
Various methods are employed to encapsulate hydroxyapatite nanoparticles, including emulsion techniques, sol-gel processes, and layer-by-layer assembly. These methods aim to improve the stability, biocompatibility, and controlled release properties of the nanoparticles for applications in drug delivery, tissue engineering, and dental care.- Encapsulation methods for hydroxyapatite nanoparticles: Various methods are employed to encapsulate hydroxyapatite nanoparticles, including emulsion techniques, sol-gel processes, and spray drying. These methods aim to protect the nanoparticles, control their release, and enhance their stability. The encapsulation process often involves using polymers or lipids as coating materials.
- Applications of encapsulated hydroxyapatite nanoparticles: Encapsulated hydroxyapatite nanoparticles find applications in various fields, including drug delivery systems, bone tissue engineering, dental materials, and cosmetics. The encapsulation enhances the biocompatibility and controlled release properties of the nanoparticles, making them suitable for diverse biomedical applications.
- Functionalization of encapsulated hydroxyapatite nanoparticles: Encapsulated hydroxyapatite nanoparticles can be functionalized with various molecules or compounds to enhance their properties or add new functionalities. This may include attaching targeting ligands, incorporating growth factors, or modifying surface properties to improve their interaction with biological systems.
- Characterization techniques for encapsulated hydroxyapatite nanoparticles: Various analytical techniques are used to characterize encapsulated hydroxyapatite nanoparticles, including electron microscopy, X-ray diffraction, dynamic light scattering, and spectroscopic methods. These techniques help evaluate the size, morphology, encapsulation efficiency, and release kinetics of the nanoparticles.
- Composite materials incorporating encapsulated hydroxyapatite nanoparticles: Encapsulated hydroxyapatite nanoparticles are used to develop composite materials with enhanced properties. These composites find applications in bone substitutes, dental implants, and drug delivery systems. The encapsulation helps in improving the dispersion of nanoparticles within the matrix and controlling their release behavior.
02 Polymer-based encapsulation of hydroxyapatite nanoparticles
Hydroxyapatite nanoparticles are encapsulated within various polymeric materials to enhance their properties and functionality. Biodegradable polymers, such as chitosan, alginate, and PLGA, are commonly used for this purpose. The polymer coating can improve the dispersion, biocompatibility, and controlled release characteristics of the nanoparticles.Expand Specific Solutions03 Applications of encapsulated hydroxyapatite nanoparticles in biomedicine
Encapsulated hydroxyapatite nanoparticles find numerous applications in biomedicine, including drug delivery systems, bone tissue engineering, dental materials, and cancer therapy. The encapsulation process allows for better control over the release of bioactive agents and improves the overall performance of the nanoparticles in various medical applications.Expand Specific Solutions04 Functionalization of encapsulated hydroxyapatite nanoparticles
Encapsulated hydroxyapatite nanoparticles can be further functionalized with various molecules or compounds to enhance their properties or add new functionalities. This includes the incorporation of growth factors, antibiotics, or targeting ligands to improve their therapeutic efficacy or specificity in biomedical applications.Expand Specific Solutions05 Characterization and analysis of encapsulated hydroxyapatite nanoparticles
Various analytical techniques are employed to characterize the properties of encapsulated hydroxyapatite nanoparticles, including particle size analysis, zeta potential measurements, electron microscopy, and spectroscopic methods. These techniques help in assessing the success of the encapsulation process and the resulting properties of the nanoparticles.Expand Specific Solutions
Key Players in Nanoparticle Encapsulation
The field of encapsulating hydroxyapatite nanoparticles with lipid bilayers is in a nascent stage of development, characterized by ongoing research and emerging applications. The market size is relatively small but growing, driven by potential applications in drug delivery and bone tissue engineering. Technological maturity varies among key players, with companies like Promimic AB and GlaxoSmithKline Biologicals SA leading in commercialization efforts. Academic institutions such as Sichuan University and Shandong University are contributing significantly to fundamental research. The competitive landscape is diverse, with a mix of established pharmaceutical companies, specialized biotech firms, and research-intensive universities collaborating and competing to advance this promising technology.
Promimic AB
Technical Solution: Promimic AB has developed a unique HAnano Surface technology for encapsulating hydroxyapatite nanoparticles with lipid bilayers. Their approach involves creating a nanometer-thin layer of hydroxyapatite on implant surfaces, which mimics the natural composition of human bone. This technology enhances osseointegration and accelerates bone formation around medical implants[1]. The company's method utilizes a wet chemical process to deposit hydroxyapatite nanocrystals onto various substrate materials, including titanium, stainless steel, and polymers. The lipid bilayer encapsulation helps in maintaining the nanoparticle stability and improves their biocompatibility[2].
Strengths: Enhances implant integration, accelerates bone formation, and applicable to various materials. Weaknesses: May have limited applications beyond bone-related implants, potential long-term stability issues in vivo.
GlaxoSmithKline Biologicals SA
Technical Solution: GlaxoSmithKline Biologicals SA has developed an innovative approach to encapsulating hydroxyapatite nanoparticles with lipid bilayers for vaccine adjuvant applications. Their technology involves creating calcium phosphate-based nanoparticles coated with lipid bilayers, which can effectively deliver antigens and immunostimulatory molecules. This system enhances the stability of the nanoparticles and improves their interaction with immune cells[3]. The company's method utilizes a controlled precipitation process to form the hydroxyapatite core, followed by a lipid coating step using specific phospholipid compositions. This approach allows for the incorporation of various antigens and adjuvants within the nanoparticle structure, enabling tailored immune responses[4].
Strengths: Versatile platform for vaccine development, enhanced antigen delivery, and improved immune response. Weaknesses: Potential complexity in large-scale manufacturing, possible regulatory challenges for novel adjuvant systems.
Core Innovations in Lipid Bilayer Technology
Nanoparticle compositions comprising a lipid bilayer and associated methods
PatentWO2011005916A1
Innovation
- A nanoparticle composition comprising a lipid bilayer formed using sparingly water-soluble fatty acids, such as oleic acid, around the nanoparticle core, allowing for efficient phase transfer into aqueous solutions while maintaining stability and preserving size-dependent properties.
Nanocapsules and method for manufacturing thereof
PatentActiveUS20190365660A1
Innovation
- A method involving the formation of a supported lipid bilayer on porous silica nanoparticles with specific ζ-potential, incorporating DOTAP and other lipids through ultra-sonication, followed by the addition of cross-linked sodium alginate to create a stable and biodegradable outer layer that controls the release of active moieties.
Regulatory Considerations for Nanomaterials
The regulatory landscape for nanomaterials, including hydroxyapatite nanoparticles encapsulated with lipid bilayers, is complex and evolving. Regulatory bodies worldwide are grappling with the unique challenges posed by nanomaterials, which often exhibit properties distinct from their bulk counterparts.
In the United States, the Food and Drug Administration (FDA) has developed a regulatory framework for nanomaterials used in food, drugs, and medical devices. The FDA's approach emphasizes the importance of characterizing nanomaterials' physicochemical properties and their potential biological effects. For hydroxyapatite nanoparticles with lipid bilayers, this would include assessing particle size, surface properties, and potential interactions with biological systems.
The European Union has implemented the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which applies to nanomaterials. Under REACH, manufacturers must provide detailed information on the safety and potential risks of nanomaterials. The European Medicines Agency (EMA) has also issued guidelines specific to nanomedicines, which would be relevant for lipid-encapsulated hydroxyapatite nanoparticles intended for medical applications.
Internationally, the Organization for Economic Co-operation and Development (OECD) has established working groups to address the safety and regulatory challenges of nanomaterials. Their efforts focus on developing standardized testing methods and risk assessment frameworks applicable to various nanomaterial types.
A key regulatory consideration for lipid-encapsulated hydroxyapatite nanoparticles is their classification. Depending on their intended use and properties, they may be regulated as drugs, medical devices, or combination products. This classification determines the specific regulatory pathway and requirements for market approval.
Safety assessment is a critical aspect of regulatory compliance for nanomaterials. Regulators typically require comprehensive toxicological studies, including in vitro and in vivo testing, to evaluate potential risks. For lipid-encapsulated hydroxyapatite nanoparticles, particular attention would be given to their biodistribution, potential for accumulation in specific organs, and long-term effects.
Manufacturing processes and quality control are also subject to stringent regulatory scrutiny. Good Manufacturing Practices (GMP) must be adhered to, with particular emphasis on maintaining consistent nanoparticle size, composition, and encapsulation efficiency. Analytical methods for characterizing these parameters must be validated and approved by regulatory authorities.
As the field of nanomedicine advances, regulatory frameworks are likely to evolve. Researchers and manufacturers working with lipid-encapsulated hydroxyapatite nanoparticles must stay informed about regulatory developments and engage proactively with regulatory bodies to ensure compliance and facilitate the translation of their innovations from laboratory to clinical applications.
In the United States, the Food and Drug Administration (FDA) has developed a regulatory framework for nanomaterials used in food, drugs, and medical devices. The FDA's approach emphasizes the importance of characterizing nanomaterials' physicochemical properties and their potential biological effects. For hydroxyapatite nanoparticles with lipid bilayers, this would include assessing particle size, surface properties, and potential interactions with biological systems.
The European Union has implemented the REACH (Registration, Evaluation, Authorization, and Restriction of Chemicals) regulation, which applies to nanomaterials. Under REACH, manufacturers must provide detailed information on the safety and potential risks of nanomaterials. The European Medicines Agency (EMA) has also issued guidelines specific to nanomedicines, which would be relevant for lipid-encapsulated hydroxyapatite nanoparticles intended for medical applications.
Internationally, the Organization for Economic Co-operation and Development (OECD) has established working groups to address the safety and regulatory challenges of nanomaterials. Their efforts focus on developing standardized testing methods and risk assessment frameworks applicable to various nanomaterial types.
A key regulatory consideration for lipid-encapsulated hydroxyapatite nanoparticles is their classification. Depending on their intended use and properties, they may be regulated as drugs, medical devices, or combination products. This classification determines the specific regulatory pathway and requirements for market approval.
Safety assessment is a critical aspect of regulatory compliance for nanomaterials. Regulators typically require comprehensive toxicological studies, including in vitro and in vivo testing, to evaluate potential risks. For lipid-encapsulated hydroxyapatite nanoparticles, particular attention would be given to their biodistribution, potential for accumulation in specific organs, and long-term effects.
Manufacturing processes and quality control are also subject to stringent regulatory scrutiny. Good Manufacturing Practices (GMP) must be adhered to, with particular emphasis on maintaining consistent nanoparticle size, composition, and encapsulation efficiency. Analytical methods for characterizing these parameters must be validated and approved by regulatory authorities.
As the field of nanomedicine advances, regulatory frameworks are likely to evolve. Researchers and manufacturers working with lipid-encapsulated hydroxyapatite nanoparticles must stay informed about regulatory developments and engage proactively with regulatory bodies to ensure compliance and facilitate the translation of their innovations from laboratory to clinical applications.
Biocompatibility and Safety Assessment
The biocompatibility and safety assessment of lipid bilayer-encapsulated hydroxyapatite nanoparticles (HAp-LBs) is crucial for their potential applications in biomedicine. These nanostructures combine the biocompatibility of hydroxyapatite with the versatility of lipid bilayers, offering promising prospects for drug delivery, bone regeneration, and other therapeutic applications.
In vitro studies have demonstrated the generally low cytotoxicity of HAp-LBs across various cell lines, including osteoblasts, fibroblasts, and endothelial cells. The lipid bilayer coating appears to mitigate potential cytotoxic effects of bare hydroxyapatite nanoparticles, likely due to improved cellular interactions and reduced particle agglomeration. However, the exact mechanisms of cellular uptake and intracellular processing of HAp-LBs require further elucidation.
Hemocompatibility assessments have shown minimal hemolytic activity and negligible effects on blood coagulation parameters, suggesting the potential for intravenous administration. Nevertheless, long-term circulation studies and detailed pharmacokinetic profiles are necessary to fully understand their behavior in the bloodstream.
In vivo biocompatibility studies in animal models have yielded promising results, with no significant adverse effects observed in major organs following systemic administration of HAp-LBs. Histopathological analyses have shown minimal inflammatory responses and no apparent signs of tissue damage or necrosis. However, more comprehensive long-term studies are needed to assess potential chronic toxicity and evaluate the fate of these nanoparticles in the body.
The biodegradability of HAp-LBs is an important aspect of their safety profile. While hydroxyapatite is known to be biodegradable and resorbable, the rate and mechanisms of degradation for the composite HAp-LB structures require further investigation. Understanding the degradation kinetics and metabolic pathways of both the inorganic and organic components is crucial for predicting long-term safety and efficacy.
Immunogenicity studies have shown low to moderate immune responses to HAp-LBs, with no significant production of anti-HAp antibodies observed in animal models. However, the potential for complement activation and interactions with the innate immune system warrant closer examination, particularly for repeated administration scenarios.
Genotoxicity and carcinogenicity assessments have thus far not revealed any significant mutagenic or carcinogenic potential of HAp-LBs. However, more extensive studies, including long-term carcinogenicity tests and detailed evaluations of potential DNA damage or chromosomal aberrations, are necessary to conclusively establish their safety in this regard.
As research in this field progresses, standardization of safety assessment protocols specifically tailored for HAp-LBs will be crucial. This should include guidelines for characterizing physicochemical properties, evaluating biodistribution and clearance, and assessing potential long-term effects on organ systems and cellular functions. Additionally, investigating potential interactions with other biomolecules and drugs will be essential for their safe application in complex biological environments.
In vitro studies have demonstrated the generally low cytotoxicity of HAp-LBs across various cell lines, including osteoblasts, fibroblasts, and endothelial cells. The lipid bilayer coating appears to mitigate potential cytotoxic effects of bare hydroxyapatite nanoparticles, likely due to improved cellular interactions and reduced particle agglomeration. However, the exact mechanisms of cellular uptake and intracellular processing of HAp-LBs require further elucidation.
Hemocompatibility assessments have shown minimal hemolytic activity and negligible effects on blood coagulation parameters, suggesting the potential for intravenous administration. Nevertheless, long-term circulation studies and detailed pharmacokinetic profiles are necessary to fully understand their behavior in the bloodstream.
In vivo biocompatibility studies in animal models have yielded promising results, with no significant adverse effects observed in major organs following systemic administration of HAp-LBs. Histopathological analyses have shown minimal inflammatory responses and no apparent signs of tissue damage or necrosis. However, more comprehensive long-term studies are needed to assess potential chronic toxicity and evaluate the fate of these nanoparticles in the body.
The biodegradability of HAp-LBs is an important aspect of their safety profile. While hydroxyapatite is known to be biodegradable and resorbable, the rate and mechanisms of degradation for the composite HAp-LB structures require further investigation. Understanding the degradation kinetics and metabolic pathways of both the inorganic and organic components is crucial for predicting long-term safety and efficacy.
Immunogenicity studies have shown low to moderate immune responses to HAp-LBs, with no significant production of anti-HAp antibodies observed in animal models. However, the potential for complement activation and interactions with the innate immune system warrant closer examination, particularly for repeated administration scenarios.
Genotoxicity and carcinogenicity assessments have thus far not revealed any significant mutagenic or carcinogenic potential of HAp-LBs. However, more extensive studies, including long-term carcinogenicity tests and detailed evaluations of potential DNA damage or chromosomal aberrations, are necessary to conclusively establish their safety in this regard.
As research in this field progresses, standardization of safety assessment protocols specifically tailored for HAp-LBs will be crucial. This should include guidelines for characterizing physicochemical properties, evaluating biodistribution and clearance, and assessing potential long-term effects on organ systems and cellular functions. Additionally, investigating potential interactions with other biomolecules and drugs will be essential for their safe application in complex biological environments.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!