Investigating Simplified Production Methods of Hydroxyapatite for Mass Applications
JUL 23, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydroxyapatite Background and Objectives
Hydroxyapatite (HAp) is a naturally occurring mineral form of calcium apatite, with the chemical formula Ca10(PO4)6(OH)2. It is the primary inorganic component of bone tissue and teeth enamel in vertebrates, comprising up to 70% of bone by weight. The unique properties of hydroxyapatite, including its biocompatibility, osteoconductivity, and ability to form strong bonds with surrounding tissues, have made it a subject of intense research and development in various fields, particularly in biomedicine and materials science.
The history of hydroxyapatite research can be traced back to the mid-20th century when scientists began to investigate the mineral composition of bones and teeth. As understanding of its structure and properties grew, so did the interest in its potential applications. In the 1970s and 1980s, researchers started exploring the use of synthetic hydroxyapatite in bone grafts and dental implants, marking the beginning of its widespread use in medical applications.
Over the past few decades, the applications of hydroxyapatite have expanded significantly. Beyond its initial use in orthopedics and dentistry, it has found applications in drug delivery systems, water purification, and even in certain industrial processes. This expansion of applications has driven the need for more efficient and cost-effective production methods, which is the focus of the current investigation into simplified production methods for mass applications.
The primary objective of this research is to develop and optimize production methods for hydroxyapatite that are suitable for large-scale manufacturing while maintaining the quality and purity required for various applications. This goal is driven by the increasing demand for hydroxyapatite across multiple industries and the need to make it more accessible and economically viable for widespread use.
Current production methods for high-quality hydroxyapatite often involve complex processes that are time-consuming and expensive, limiting its availability for mass applications. The challenge lies in simplifying these processes without compromising the critical properties of the material. This includes maintaining the correct stoichiometry, crystallinity, and particle size distribution, which are crucial for its performance in different applications.
The technological evolution in this field is expected to focus on several key areas. These include the development of novel synthesis routes that utilize more readily available precursors, the optimization of reaction conditions to reduce energy consumption and processing time, and the exploration of continuous production methods that can significantly increase output while ensuring consistent quality.
As research progresses, it is anticipated that breakthroughs in production methods will not only make hydroxyapatite more accessible for existing applications but also open up new possibilities for its use in emerging fields such as 3D printing of bone scaffolds, advanced water treatment technologies, and innovative composite materials. The success of this research has the potential to significantly impact various industries and contribute to advancements in healthcare, environmental protection, and materials engineering.
The history of hydroxyapatite research can be traced back to the mid-20th century when scientists began to investigate the mineral composition of bones and teeth. As understanding of its structure and properties grew, so did the interest in its potential applications. In the 1970s and 1980s, researchers started exploring the use of synthetic hydroxyapatite in bone grafts and dental implants, marking the beginning of its widespread use in medical applications.
Over the past few decades, the applications of hydroxyapatite have expanded significantly. Beyond its initial use in orthopedics and dentistry, it has found applications in drug delivery systems, water purification, and even in certain industrial processes. This expansion of applications has driven the need for more efficient and cost-effective production methods, which is the focus of the current investigation into simplified production methods for mass applications.
The primary objective of this research is to develop and optimize production methods for hydroxyapatite that are suitable for large-scale manufacturing while maintaining the quality and purity required for various applications. This goal is driven by the increasing demand for hydroxyapatite across multiple industries and the need to make it more accessible and economically viable for widespread use.
Current production methods for high-quality hydroxyapatite often involve complex processes that are time-consuming and expensive, limiting its availability for mass applications. The challenge lies in simplifying these processes without compromising the critical properties of the material. This includes maintaining the correct stoichiometry, crystallinity, and particle size distribution, which are crucial for its performance in different applications.
The technological evolution in this field is expected to focus on several key areas. These include the development of novel synthesis routes that utilize more readily available precursors, the optimization of reaction conditions to reduce energy consumption and processing time, and the exploration of continuous production methods that can significantly increase output while ensuring consistent quality.
As research progresses, it is anticipated that breakthroughs in production methods will not only make hydroxyapatite more accessible for existing applications but also open up new possibilities for its use in emerging fields such as 3D printing of bone scaffolds, advanced water treatment technologies, and innovative composite materials. The success of this research has the potential to significantly impact various industries and contribute to advancements in healthcare, environmental protection, and materials engineering.
Market Analysis for Hydroxyapatite Applications
The global hydroxyapatite market has been experiencing significant growth, driven by increasing applications in various industries. The market size was valued at approximately $2.5 billion in 2020 and is projected to reach $3.65 billion by 2027, growing at a CAGR of 5.8% during the forecast period. This growth is primarily attributed to the rising demand for hydroxyapatite in medical and dental applications, as well as its expanding use in water treatment and agriculture.
In the medical and dental sectors, hydroxyapatite is widely used for bone grafts, dental implants, and tissue engineering. The growing aging population and increasing prevalence of bone-related disorders are driving the demand for hydroxyapatite-based products. Additionally, the rise in dental procedures and cosmetic dentistry is further boosting market growth in this segment.
The water treatment industry is another significant market for hydroxyapatite, where it is used as an adsorbent for removing heavy metals and other contaminants from water. With increasing concerns about water pollution and stringent environmental regulations, the demand for hydroxyapatite in water treatment applications is expected to grow substantially in the coming years.
Agriculture is an emerging market for hydroxyapatite, where it is used as a slow-release fertilizer and soil amendment. The growing focus on sustainable agriculture practices and the need for improved crop yields are driving the adoption of hydroxyapatite-based products in this sector.
Geographically, North America and Europe currently dominate the hydroxyapatite market, owing to advanced healthcare infrastructure and high adoption rates of innovative medical technologies. However, the Asia-Pacific region is expected to witness the fastest growth during the forecast period, driven by rapid industrialization, increasing healthcare expenditure, and growing awareness about dental health in countries like China and India.
The market is characterized by the presence of several key players, including Berkeley Advanced Biomaterials, CAM Bioceramics, Zimmer Biomet, and SofSera Corporation. These companies are focusing on research and development activities to improve the quality and performance of hydroxyapatite products, as well as exploring new applications to expand their market share.
Despite the positive market outlook, challenges such as high production costs and stringent regulatory requirements for medical-grade hydroxyapatite products may hinder market growth to some extent. However, ongoing research into simplified production methods and the development of novel applications are expected to create new opportunities for market expansion in the coming years.
In the medical and dental sectors, hydroxyapatite is widely used for bone grafts, dental implants, and tissue engineering. The growing aging population and increasing prevalence of bone-related disorders are driving the demand for hydroxyapatite-based products. Additionally, the rise in dental procedures and cosmetic dentistry is further boosting market growth in this segment.
The water treatment industry is another significant market for hydroxyapatite, where it is used as an adsorbent for removing heavy metals and other contaminants from water. With increasing concerns about water pollution and stringent environmental regulations, the demand for hydroxyapatite in water treatment applications is expected to grow substantially in the coming years.
Agriculture is an emerging market for hydroxyapatite, where it is used as a slow-release fertilizer and soil amendment. The growing focus on sustainable agriculture practices and the need for improved crop yields are driving the adoption of hydroxyapatite-based products in this sector.
Geographically, North America and Europe currently dominate the hydroxyapatite market, owing to advanced healthcare infrastructure and high adoption rates of innovative medical technologies. However, the Asia-Pacific region is expected to witness the fastest growth during the forecast period, driven by rapid industrialization, increasing healthcare expenditure, and growing awareness about dental health in countries like China and India.
The market is characterized by the presence of several key players, including Berkeley Advanced Biomaterials, CAM Bioceramics, Zimmer Biomet, and SofSera Corporation. These companies are focusing on research and development activities to improve the quality and performance of hydroxyapatite products, as well as exploring new applications to expand their market share.
Despite the positive market outlook, challenges such as high production costs and stringent regulatory requirements for medical-grade hydroxyapatite products may hinder market growth to some extent. However, ongoing research into simplified production methods and the development of novel applications are expected to create new opportunities for market expansion in the coming years.
Current Challenges in Hydroxyapatite Production
The production of hydroxyapatite (HA) for mass applications faces several significant challenges that hinder its widespread adoption. One of the primary obstacles is the high cost associated with current production methods. Traditional synthesis techniques, such as wet chemical precipitation and sol-gel processes, often require expensive precursors and complex equipment, making large-scale production economically unfeasible for many applications.
Another major challenge is the difficulty in controlling the morphology and particle size of HA during synthesis. The properties of HA, including its biocompatibility and mechanical strength, are heavily influenced by its particle size and shape. Achieving consistent and reproducible results in terms of particle characteristics is crucial for ensuring the quality and performance of HA-based products. However, current production methods often struggle to maintain precise control over these parameters, leading to variability in the final product.
The energy-intensive nature of many HA production processes presents both economic and environmental challenges. High-temperature calcination steps, often required to achieve the desired crystallinity and purity, consume significant amounts of energy and contribute to increased production costs and carbon footprints. This aspect becomes particularly problematic when considering mass production scenarios.
Scalability is another critical issue facing HA production. Many laboratory-scale synthesis methods do not translate well to industrial-scale production. The challenges of maintaining reaction conditions, ensuring homogeneity, and managing heat transfer become more pronounced as production volumes increase. This scaling difficulty often results in compromised product quality or increased production costs when attempting to meet large-scale demand.
The purity of the final HA product is a persistent concern in current production methods. Impurities and unwanted phases can significantly affect the performance and biocompatibility of HA, especially in medical applications. Achieving high purity levels while maintaining cost-effectiveness and scalability remains a delicate balance that many production processes struggle to achieve.
Lastly, the environmental impact of HA production is an emerging challenge that needs addressing. Many current methods involve the use of hazardous chemicals or generate significant waste streams. As sustainability becomes an increasingly important consideration in industrial processes, developing greener production methods for HA that minimize environmental impact while maintaining product quality and economic viability is becoming a critical focus area for researchers and manufacturers alike.
Another major challenge is the difficulty in controlling the morphology and particle size of HA during synthesis. The properties of HA, including its biocompatibility and mechanical strength, are heavily influenced by its particle size and shape. Achieving consistent and reproducible results in terms of particle characteristics is crucial for ensuring the quality and performance of HA-based products. However, current production methods often struggle to maintain precise control over these parameters, leading to variability in the final product.
The energy-intensive nature of many HA production processes presents both economic and environmental challenges. High-temperature calcination steps, often required to achieve the desired crystallinity and purity, consume significant amounts of energy and contribute to increased production costs and carbon footprints. This aspect becomes particularly problematic when considering mass production scenarios.
Scalability is another critical issue facing HA production. Many laboratory-scale synthesis methods do not translate well to industrial-scale production. The challenges of maintaining reaction conditions, ensuring homogeneity, and managing heat transfer become more pronounced as production volumes increase. This scaling difficulty often results in compromised product quality or increased production costs when attempting to meet large-scale demand.
The purity of the final HA product is a persistent concern in current production methods. Impurities and unwanted phases can significantly affect the performance and biocompatibility of HA, especially in medical applications. Achieving high purity levels while maintaining cost-effectiveness and scalability remains a delicate balance that many production processes struggle to achieve.
Lastly, the environmental impact of HA production is an emerging challenge that needs addressing. Many current methods involve the use of hazardous chemicals or generate significant waste streams. As sustainability becomes an increasingly important consideration in industrial processes, developing greener production methods for HA that minimize environmental impact while maintaining product quality and economic viability is becoming a critical focus area for researchers and manufacturers alike.
Existing Simplified Production Techniques
01 Wet chemical precipitation method
This method involves the reaction of calcium and phosphate ions in an aqueous solution under controlled pH and temperature conditions. It is a widely used technique for producing hydroxyapatite due to its simplicity and ability to produce nanoparticles with high purity and controlled morphology.- Wet chemical precipitation method: This method involves the reaction of calcium and phosphate ions in an aqueous solution under controlled pH and temperature conditions. It is a widely used technique for producing hydroxyapatite due to its simplicity and cost-effectiveness. The process typically involves mixing calcium-containing and phosphate-containing solutions, followed by aging, filtration, and drying steps to obtain the final product.
- Sol-gel synthesis: The sol-gel method is a versatile technique for producing hydroxyapatite with high purity and controlled morphology. It involves the hydrolysis and condensation of precursors to form a gel, which is then dried and calcined to obtain the final product. This method allows for better control over particle size, shape, and composition compared to traditional precipitation methods.
- Hydrothermal synthesis: Hydrothermal synthesis is a method that uses high temperature and pressure to produce hydroxyapatite crystals. This technique allows for the formation of well-crystallized hydroxyapatite particles with controlled size and morphology. The process typically involves the reaction of calcium and phosphate precursors in an autoclave under specific temperature and pressure conditions.
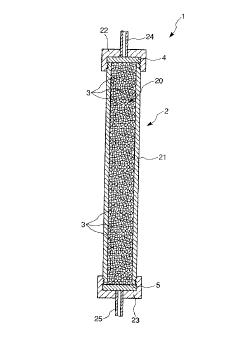
- Electrochemical deposition: This method involves the electrochemical deposition of hydroxyapatite onto a conductive substrate. It is particularly useful for creating hydroxyapatite coatings on metallic implants or other surfaces. The process typically involves the use of an electrolyte solution containing calcium and phosphate ions, with the substrate acting as the cathode in an electrochemical cell.
- Biomimetic synthesis: Biomimetic synthesis aims to produce hydroxyapatite under conditions that mimic the natural formation process in biological systems. This method often involves the use of simulated body fluids or organic templates to guide the growth of hydroxyapatite crystals. The resulting material typically has properties similar to natural bone mineral, making it particularly suitable for biomedical applications.
02 Sol-gel synthesis
The sol-gel method is a versatile technique for producing hydroxyapatite with high surface area and controlled porosity. It involves the hydrolysis and condensation of precursors to form a gel, which is then dried and calcined to obtain the final product. This method allows for better control over particle size and composition.Expand Specific Solutions03 Hydrothermal synthesis
Hydrothermal synthesis is a method that uses high temperature and pressure to produce hydroxyapatite crystals. This technique allows for the production of well-crystallized and highly pure hydroxyapatite particles with various morphologies. It is particularly useful for producing nanostructured materials.Expand Specific Solutions04 Electrochemical deposition
This method involves the electrochemical deposition of calcium and phosphate ions onto a substrate to form hydroxyapatite coatings or particles. It allows for precise control over the thickness and composition of the deposited layer and is often used for producing bioactive coatings on implants.Expand Specific Solutions05 Biomimetic synthesis
Biomimetic synthesis aims to mimic the natural process of hydroxyapatite formation in biological systems. This method often involves the use of simulated body fluids or organic templates to guide the growth of hydroxyapatite crystals. It can produce materials with properties similar to natural bone mineral.Expand Specific Solutions
Key Players in Hydroxyapatite Industry
The hydroxyapatite production market is in a growth phase, driven by increasing demand in medical and dental applications. The global market size is projected to expand significantly, with a compound annual growth rate exceeding 6% through 2027. Technologically, the field is advancing rapidly, with companies like Bio-Rad Laboratories and Mitsubishi Paper Mills leading innovations in simplified production methods. Academic institutions such as Rutgers University and Carnegie Mellon University are contributing to research advancements. The involvement of major players like Panasonic and HOYA Corp suggests a maturing industry, while the presence of specialized firms like Nara Machinery Co. indicates a diverse competitive landscape focused on process optimization and scalability.
National Institute for Materials Science IAI
Technical Solution: The National Institute for Materials Science (NIMS) in Japan has developed an innovative approach to synthesizing hydroxyapatite (HAp) using a mechanochemical method. This process involves the dry milling of calcium hydroxide and calcium hydrogen phosphate, followed by a low-temperature heat treatment. The method produces highly pure, nanocrystalline HAp with controlled stoichiometry and crystallinity[1]. NIMS researchers have also explored the use of various precursors and milling conditions to tailor the properties of the resulting HAp, such as particle size, surface area, and morphology. Additionally, they have investigated the incorporation of ions like strontium and magnesium into the HAp structure during synthesis to enhance its biological properties[2]. The institute has further developed a scalable continuous flow synthesis method for HAp production, which allows for better control over particle characteristics and increased production rates[3].
Strengths: High purity, controllable properties, and scalable production. Weaknesses: Potential for contamination from milling media and energy-intensive process for large-scale production.
Beijing University of Chemical Technology
Technical Solution: Researchers at Beijing University of Chemical Technology have developed a simplified sol-gel method for the synthesis of hydroxyapatite. This approach utilizes calcium nitrate and phosphoric acid as precursors, with careful control of pH and reaction conditions to produce high-quality HAp nanoparticles[1]. The process has been optimized to reduce the number of steps and minimize the use of organic solvents, making it more environmentally friendly and cost-effective. The university's team has also explored the incorporation of various dopants, such as strontium and zinc, into the HAp structure during synthesis to enhance its biological properties[2]. Furthermore, they have developed a spray-drying technique to convert the HAp sol into a fine powder, which is suitable for various applications, including bone tissue engineering and drug delivery[3]. The process has been successfully scaled up to produce kilogram-scale batches of HAp, demonstrating its potential for industrial production[4].
Strengths: Simple and cost-effective process, ability to incorporate various dopants, and potential for large-scale production. Weaknesses: Potential for agglomeration of nanoparticles and challenges in controlling particle size distribution in large-scale production.
Innovative Approaches in Hydroxyapatite Synthesis
Method for producing hydroxyapatite
PatentActiveJP2020007190A
Innovation
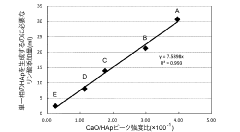
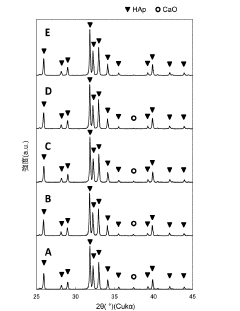
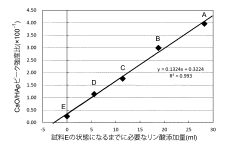
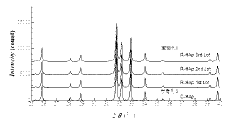
- A method involving the addition of phosphoric acid to a calcium hydroxide slurry with a Ca/P molar ratio greater than 1.67, followed by a series of steps to determine and adjust the phosphoric acid addition amount based on the CaO/HAp peak intensity ratio, ensuring the production of single-phase hydroxyapatite.
Production method and particles of hydroxyapatite
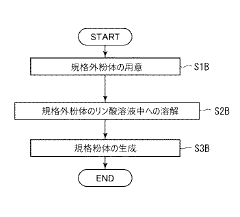
PatentActiveJP2021070619A
Innovation
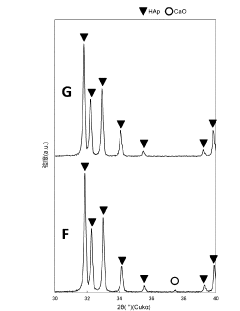
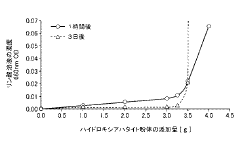
- A production method involving the dissolution of non-standard hydroxyapatite particles in a phosphoric acid solution, followed by controlled adjustment of dissolution conditions to recover and regenerate hydroxyapatite particles within the desired size range, utilizing a wet synthesis process to produce standardized particles.
Environmental Impact Assessment
The environmental impact assessment of simplified production methods for hydroxyapatite (HA) is crucial for evaluating their sustainability and potential for mass applications. Traditional HA production methods often involve energy-intensive processes and the use of hazardous chemicals, raising concerns about their environmental footprint. Simplified production methods aim to address these issues by reducing energy consumption, minimizing waste generation, and utilizing more environmentally friendly precursors.
One of the primary environmental benefits of simplified HA production methods is the potential reduction in energy consumption. Many conventional techniques require high-temperature sintering or prolonged reaction times, leading to significant energy expenditure. Simplified methods often employ lower reaction temperatures or shorter processing times, resulting in decreased energy requirements and associated greenhouse gas emissions. This energy efficiency not only reduces the carbon footprint of HA production but also contributes to overall cost-effectiveness, making mass applications more feasible.
Water usage and wastewater generation are critical environmental factors to consider in HA production. Simplified methods frequently incorporate strategies to minimize water consumption and improve water recycling within the production process. By optimizing reaction conditions and employing more efficient separation techniques, these methods can significantly reduce the volume of wastewater generated and the associated treatment requirements. This not only conserves water resources but also minimizes the potential for water pollution from chemical contaminants.
The choice of precursor materials in simplified HA production methods can have a substantial impact on environmental sustainability. Many simplified approaches utilize bio-based or waste-derived precursors, such as eggshells or seashells, as calcium sources. This not only reduces reliance on mined mineral resources but also provides a pathway for valorizing waste materials that might otherwise be discarded. The use of such alternative precursors can contribute to circular economy principles and reduce the overall environmental burden of HA production.
Waste generation and management are key considerations in the environmental assessment of HA production methods. Simplified techniques often aim to improve reaction yields and reduce the formation of unwanted by-products. This results in less waste material requiring disposal or treatment, thereby minimizing the environmental impact associated with waste management. Additionally, some simplified methods incorporate strategies for recovering and reusing unreacted precursors or by-products, further enhancing resource efficiency and reducing waste streams.
The potential for reduced chemical usage in simplified HA production methods is another significant environmental benefit. By optimizing reaction conditions and employing more efficient synthesis routes, these methods can often achieve the desired HA properties with lower quantities of chemical reagents. This not only reduces the environmental risks associated with chemical handling and storage but also decreases the potential for chemical contamination in waste streams. Furthermore, the use of less hazardous or more environmentally benign chemicals in simplified methods can contribute to improved worker safety and reduced environmental risks.
One of the primary environmental benefits of simplified HA production methods is the potential reduction in energy consumption. Many conventional techniques require high-temperature sintering or prolonged reaction times, leading to significant energy expenditure. Simplified methods often employ lower reaction temperatures or shorter processing times, resulting in decreased energy requirements and associated greenhouse gas emissions. This energy efficiency not only reduces the carbon footprint of HA production but also contributes to overall cost-effectiveness, making mass applications more feasible.
Water usage and wastewater generation are critical environmental factors to consider in HA production. Simplified methods frequently incorporate strategies to minimize water consumption and improve water recycling within the production process. By optimizing reaction conditions and employing more efficient separation techniques, these methods can significantly reduce the volume of wastewater generated and the associated treatment requirements. This not only conserves water resources but also minimizes the potential for water pollution from chemical contaminants.
The choice of precursor materials in simplified HA production methods can have a substantial impact on environmental sustainability. Many simplified approaches utilize bio-based or waste-derived precursors, such as eggshells or seashells, as calcium sources. This not only reduces reliance on mined mineral resources but also provides a pathway for valorizing waste materials that might otherwise be discarded. The use of such alternative precursors can contribute to circular economy principles and reduce the overall environmental burden of HA production.
Waste generation and management are key considerations in the environmental assessment of HA production methods. Simplified techniques often aim to improve reaction yields and reduce the formation of unwanted by-products. This results in less waste material requiring disposal or treatment, thereby minimizing the environmental impact associated with waste management. Additionally, some simplified methods incorporate strategies for recovering and reusing unreacted precursors or by-products, further enhancing resource efficiency and reducing waste streams.
The potential for reduced chemical usage in simplified HA production methods is another significant environmental benefit. By optimizing reaction conditions and employing more efficient synthesis routes, these methods can often achieve the desired HA properties with lower quantities of chemical reagents. This not only reduces the environmental risks associated with chemical handling and storage but also decreases the potential for chemical contamination in waste streams. Furthermore, the use of less hazardous or more environmentally benign chemicals in simplified methods can contribute to improved worker safety and reduced environmental risks.
Cost-Benefit Analysis of Production Methods
The cost-benefit analysis of hydroxyapatite (HA) production methods is crucial for determining the feasibility of mass applications. Traditional methods, such as wet chemical precipitation and sol-gel synthesis, often involve complex processes and high costs, limiting large-scale production. However, recent advancements in simplified production techniques have shown promise in reducing expenses while maintaining product quality.
One of the most cost-effective approaches is the mechanochemical method, which utilizes high-energy ball milling to synthesize HA from calcium and phosphate precursors. This technique eliminates the need for expensive equipment and complex chemical processes, significantly reducing production costs. The initial investment in milling equipment is offset by lower operational expenses and increased production efficiency. Additionally, the mechanochemical method allows for the use of cheaper raw materials, further enhancing its cost-effectiveness.
Another simplified production method gaining traction is the microwave-assisted synthesis of HA. This technique offers substantial benefits in terms of energy consumption and processing time. Compared to conventional heating methods, microwave synthesis can reduce reaction times from hours to minutes, leading to significant energy savings. The rapid heating also results in more uniform particle size distribution, potentially improving the quality of the final product. While the initial investment in microwave equipment may be higher, the long-term energy savings and increased production rates provide a favorable cost-benefit ratio.
The hydrothermal method has also shown promise for cost-effective HA production. This technique utilizes high-pressure and high-temperature conditions to synthesize HA from aqueous solutions. The main advantage of this method is its ability to produce highly crystalline HA with controlled morphology. Although the initial setup costs for hydrothermal reactors can be substantial, the method offers benefits such as lower reaction temperatures compared to solid-state reactions and the ability to produce large quantities of HA in a single batch. These factors contribute to reduced energy consumption and improved production efficiency, offsetting the initial investment over time.
When considering the cost-benefit analysis of these simplified production methods, it is essential to factor in not only the direct production costs but also the potential savings in downstream processing. For instance, methods that produce HA with more consistent particle size and morphology may reduce the need for additional purification or processing steps, leading to overall cost savings in the production chain. Furthermore, the ability to produce HA with tailored properties using these simplified methods can open up new market opportunities, potentially increasing the return on investment.
In conclusion, the cost-benefit analysis of simplified HA production methods reveals significant potential for reducing production costs while maintaining or even improving product quality. The choice of method will depend on factors such as desired production volume, available resources, and specific application requirements. As research in this field continues, further improvements in cost-effectiveness are expected, paving the way for broader adoption of HA in mass applications.
One of the most cost-effective approaches is the mechanochemical method, which utilizes high-energy ball milling to synthesize HA from calcium and phosphate precursors. This technique eliminates the need for expensive equipment and complex chemical processes, significantly reducing production costs. The initial investment in milling equipment is offset by lower operational expenses and increased production efficiency. Additionally, the mechanochemical method allows for the use of cheaper raw materials, further enhancing its cost-effectiveness.
Another simplified production method gaining traction is the microwave-assisted synthesis of HA. This technique offers substantial benefits in terms of energy consumption and processing time. Compared to conventional heating methods, microwave synthesis can reduce reaction times from hours to minutes, leading to significant energy savings. The rapid heating also results in more uniform particle size distribution, potentially improving the quality of the final product. While the initial investment in microwave equipment may be higher, the long-term energy savings and increased production rates provide a favorable cost-benefit ratio.
The hydrothermal method has also shown promise for cost-effective HA production. This technique utilizes high-pressure and high-temperature conditions to synthesize HA from aqueous solutions. The main advantage of this method is its ability to produce highly crystalline HA with controlled morphology. Although the initial setup costs for hydrothermal reactors can be substantial, the method offers benefits such as lower reaction temperatures compared to solid-state reactions and the ability to produce large quantities of HA in a single batch. These factors contribute to reduced energy consumption and improved production efficiency, offsetting the initial investment over time.
When considering the cost-benefit analysis of these simplified production methods, it is essential to factor in not only the direct production costs but also the potential savings in downstream processing. For instance, methods that produce HA with more consistent particle size and morphology may reduce the need for additional purification or processing steps, leading to overall cost savings in the production chain. Furthermore, the ability to produce HA with tailored properties using these simplified methods can open up new market opportunities, potentially increasing the return on investment.
In conclusion, the cost-benefit analysis of simplified HA production methods reveals significant potential for reducing production costs while maintaining or even improving product quality. The choice of method will depend on factors such as desired production volume, available resources, and specific application requirements. As research in this field continues, further improvements in cost-effectiveness are expected, paving the way for broader adoption of HA in mass applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!