Isopentane’s Impact on Fusel Oil Recovery Processes
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isopentane and Fusel Oil Background
Isopentane and fusel oil are two significant components in the context of fuel production and recovery processes. Isopentane, a branched-chain alkane with the molecular formula C5H12, is a highly volatile organic compound commonly used in various industrial applications, including as a blowing agent, solvent, and fuel additive. Its low boiling point of approximately 28°C (82°F) makes it particularly useful in enhancing the volatility of gasoline blends.
Fusel oil, on the other hand, is a complex mixture of higher alcohols produced as a byproduct during the fermentation process in alcohol production. It typically contains a variety of compounds, including amyl alcohol, isoamyl alcohol, propanol, and butanol. Historically, fusel oil was considered a waste product in the distillation industry, but its potential as a valuable resource has gained recognition in recent years.
The interaction between isopentane and fusel oil in recovery processes is of particular interest due to their distinct chemical properties and potential synergies. Isopentane's low boiling point and high volatility can significantly influence the separation and recovery of fusel oil components. This relationship has implications for both the efficiency of fuel production processes and the quality of the final products.
In the context of fuel production, the presence of isopentane can affect the distillation and fractionation of fusel oil. The volatile nature of isopentane may alter the boiling point characteristics of the mixture, potentially leading to changes in the separation efficiency of different fusel oil components. This interaction can impact the overall yield and purity of recovered fusel oil fractions.
Furthermore, the increasing focus on sustainable and efficient fuel production methods has led to renewed interest in optimizing fusel oil recovery processes. The potential use of isopentane as an extraction solvent or as a component in azeotropic distillation systems for fusel oil recovery has been explored in recent research. These investigations aim to leverage the unique properties of isopentane to enhance the separation and purification of valuable fusel oil components.
Understanding the background and interplay between isopentane and fusel oil is crucial for developing improved recovery processes. This knowledge forms the foundation for innovative approaches in fuel production, waste reduction, and the valorization of byproducts in the alcohol and fuel industries.
Fusel oil, on the other hand, is a complex mixture of higher alcohols produced as a byproduct during the fermentation process in alcohol production. It typically contains a variety of compounds, including amyl alcohol, isoamyl alcohol, propanol, and butanol. Historically, fusel oil was considered a waste product in the distillation industry, but its potential as a valuable resource has gained recognition in recent years.
The interaction between isopentane and fusel oil in recovery processes is of particular interest due to their distinct chemical properties and potential synergies. Isopentane's low boiling point and high volatility can significantly influence the separation and recovery of fusel oil components. This relationship has implications for both the efficiency of fuel production processes and the quality of the final products.
In the context of fuel production, the presence of isopentane can affect the distillation and fractionation of fusel oil. The volatile nature of isopentane may alter the boiling point characteristics of the mixture, potentially leading to changes in the separation efficiency of different fusel oil components. This interaction can impact the overall yield and purity of recovered fusel oil fractions.
Furthermore, the increasing focus on sustainable and efficient fuel production methods has led to renewed interest in optimizing fusel oil recovery processes. The potential use of isopentane as an extraction solvent or as a component in azeotropic distillation systems for fusel oil recovery has been explored in recent research. These investigations aim to leverage the unique properties of isopentane to enhance the separation and purification of valuable fusel oil components.
Understanding the background and interplay between isopentane and fusel oil is crucial for developing improved recovery processes. This knowledge forms the foundation for innovative approaches in fuel production, waste reduction, and the valorization of byproducts in the alcohol and fuel industries.
Market Analysis for Fusel Oil Recovery
The fusel oil recovery market has been experiencing significant growth in recent years, driven by the increasing demand for sustainable and eco-friendly fuel alternatives. Fusel oil, a byproduct of alcohol fermentation processes, has gained attention as a valuable resource for various industries, including biofuels, solvents, and chemical manufacturing.
The global fusel oil market size was estimated at around $300 million in 2020 and is projected to grow at a compound annual growth rate (CAGR) of 5.8% from 2021 to 2028. This growth is primarily attributed to the rising awareness of renewable energy sources and the push for reducing carbon emissions across industries.
The biofuel sector represents the largest end-use segment for fusel oil, accounting for approximately 40% of the market share. The increasing adoption of biofuels in the transportation sector, coupled with government initiatives promoting renewable energy, has been a key driver for this segment's growth.
Geographically, Asia Pacific dominates the fusel oil market, with countries like China and India leading the demand due to their rapidly expanding industrial sectors and growing emphasis on sustainable practices. North America and Europe follow closely, driven by stringent environmental regulations and a strong focus on reducing dependence on fossil fuels.
The market for fusel oil recovery processes is highly competitive, with several key players investing in research and development to improve extraction efficiency and product quality. The introduction of advanced technologies, such as membrane separation and molecular distillation, has significantly enhanced the recovery rates and purity of fusel oil.
However, the market faces challenges related to the high initial investment costs for setting up recovery facilities and the fluctuating prices of raw materials. Additionally, the limited availability of feedstock and the need for specialized equipment pose barriers to market entry for smaller players.
The impact of isopentane on fusel oil recovery processes has garnered attention in recent years. Isopentane, a branched-chain alkane, has shown potential in enhancing the separation and purification of fusel oil components. Its low boiling point and unique chemical properties make it an attractive solvent for extracting valuable compounds from fusel oil mixtures.
Research indicates that incorporating isopentane in the recovery process can lead to improved yields and higher purity of recovered fusel oil fractions. This development has sparked interest among industry players, potentially opening new avenues for product differentiation and market expansion.
As environmental concerns continue to drive the shift towards sustainable practices, the demand for efficient fusel oil recovery processes is expected to grow. The market is likely to witness increased investments in technological advancements and process optimizations, with a focus on maximizing resource utilization and minimizing waste generation.
The global fusel oil market size was estimated at around $300 million in 2020 and is projected to grow at a compound annual growth rate (CAGR) of 5.8% from 2021 to 2028. This growth is primarily attributed to the rising awareness of renewable energy sources and the push for reducing carbon emissions across industries.
The biofuel sector represents the largest end-use segment for fusel oil, accounting for approximately 40% of the market share. The increasing adoption of biofuels in the transportation sector, coupled with government initiatives promoting renewable energy, has been a key driver for this segment's growth.
Geographically, Asia Pacific dominates the fusel oil market, with countries like China and India leading the demand due to their rapidly expanding industrial sectors and growing emphasis on sustainable practices. North America and Europe follow closely, driven by stringent environmental regulations and a strong focus on reducing dependence on fossil fuels.
The market for fusel oil recovery processes is highly competitive, with several key players investing in research and development to improve extraction efficiency and product quality. The introduction of advanced technologies, such as membrane separation and molecular distillation, has significantly enhanced the recovery rates and purity of fusel oil.
However, the market faces challenges related to the high initial investment costs for setting up recovery facilities and the fluctuating prices of raw materials. Additionally, the limited availability of feedstock and the need for specialized equipment pose barriers to market entry for smaller players.
The impact of isopentane on fusel oil recovery processes has garnered attention in recent years. Isopentane, a branched-chain alkane, has shown potential in enhancing the separation and purification of fusel oil components. Its low boiling point and unique chemical properties make it an attractive solvent for extracting valuable compounds from fusel oil mixtures.
Research indicates that incorporating isopentane in the recovery process can lead to improved yields and higher purity of recovered fusel oil fractions. This development has sparked interest among industry players, potentially opening new avenues for product differentiation and market expansion.
As environmental concerns continue to drive the shift towards sustainable practices, the demand for efficient fusel oil recovery processes is expected to grow. The market is likely to witness increased investments in technological advancements and process optimizations, with a focus on maximizing resource utilization and minimizing waste generation.
Current Challenges in Isopentane-Fusel Oil Separation
The separation of isopentane from fusel oil presents several significant challenges in current recovery processes. One of the primary difficulties lies in the similar boiling points of isopentane and certain components of fusel oil, particularly ethanol and other light alcohols. This proximity in boiling points makes traditional distillation techniques less effective, as achieving a clean separation requires precise temperature control and multiple distillation stages.
Another challenge is the formation of azeotropes between isopentane and some fusel oil components. These azeotropic mixtures have a constant boiling point, making it impossible to separate the constituents by simple distillation. This phenomenon necessitates the use of more complex separation techniques, such as extractive distillation or pressure swing adsorption, which can significantly increase process complexity and operational costs.
The presence of water in the fusel oil-isopentane mixture further complicates the separation process. Water forms azeotropes with both isopentane and ethanol, creating a ternary mixture that is particularly difficult to separate. The water content can vary depending on the source of the fusel oil, adding an element of unpredictability to the separation process and requiring adaptive separation strategies.
Energy efficiency is another critical challenge in isopentane-fusel oil separation. The need for multiple distillation stages or advanced separation techniques often results in high energy consumption. This not only increases operational costs but also raises environmental concerns, particularly in an era where sustainability is becoming increasingly important in industrial processes.
Scale-up issues present additional hurdles when moving from laboratory-scale separations to industrial-scale processes. The behavior of isopentane-fusel oil mixtures can change significantly at larger volumes, affecting separation efficiency and product purity. Designing and optimizing large-scale separation equipment that can maintain the required level of separation while handling industrial volumes is a complex engineering challenge.
Lastly, the recovery of high-purity isopentane from fusel oil is crucial for many applications, particularly in the petrochemical industry. Achieving the necessary purity levels without excessive energy input or complex process designs remains a significant challenge. The presence of trace impurities can have substantial impacts on downstream processes, making the development of efficient and cost-effective purification methods a priority in this field.
Another challenge is the formation of azeotropes between isopentane and some fusel oil components. These azeotropic mixtures have a constant boiling point, making it impossible to separate the constituents by simple distillation. This phenomenon necessitates the use of more complex separation techniques, such as extractive distillation or pressure swing adsorption, which can significantly increase process complexity and operational costs.
The presence of water in the fusel oil-isopentane mixture further complicates the separation process. Water forms azeotropes with both isopentane and ethanol, creating a ternary mixture that is particularly difficult to separate. The water content can vary depending on the source of the fusel oil, adding an element of unpredictability to the separation process and requiring adaptive separation strategies.
Energy efficiency is another critical challenge in isopentane-fusel oil separation. The need for multiple distillation stages or advanced separation techniques often results in high energy consumption. This not only increases operational costs but also raises environmental concerns, particularly in an era where sustainability is becoming increasingly important in industrial processes.
Scale-up issues present additional hurdles when moving from laboratory-scale separations to industrial-scale processes. The behavior of isopentane-fusel oil mixtures can change significantly at larger volumes, affecting separation efficiency and product purity. Designing and optimizing large-scale separation equipment that can maintain the required level of separation while handling industrial volumes is a complex engineering challenge.
Lastly, the recovery of high-purity isopentane from fusel oil is crucial for many applications, particularly in the petrochemical industry. Achieving the necessary purity levels without excessive energy input or complex process designs remains a significant challenge. The presence of trace impurities can have substantial impacts on downstream processes, making the development of efficient and cost-effective purification methods a priority in this field.
Existing Isopentane Separation Methods
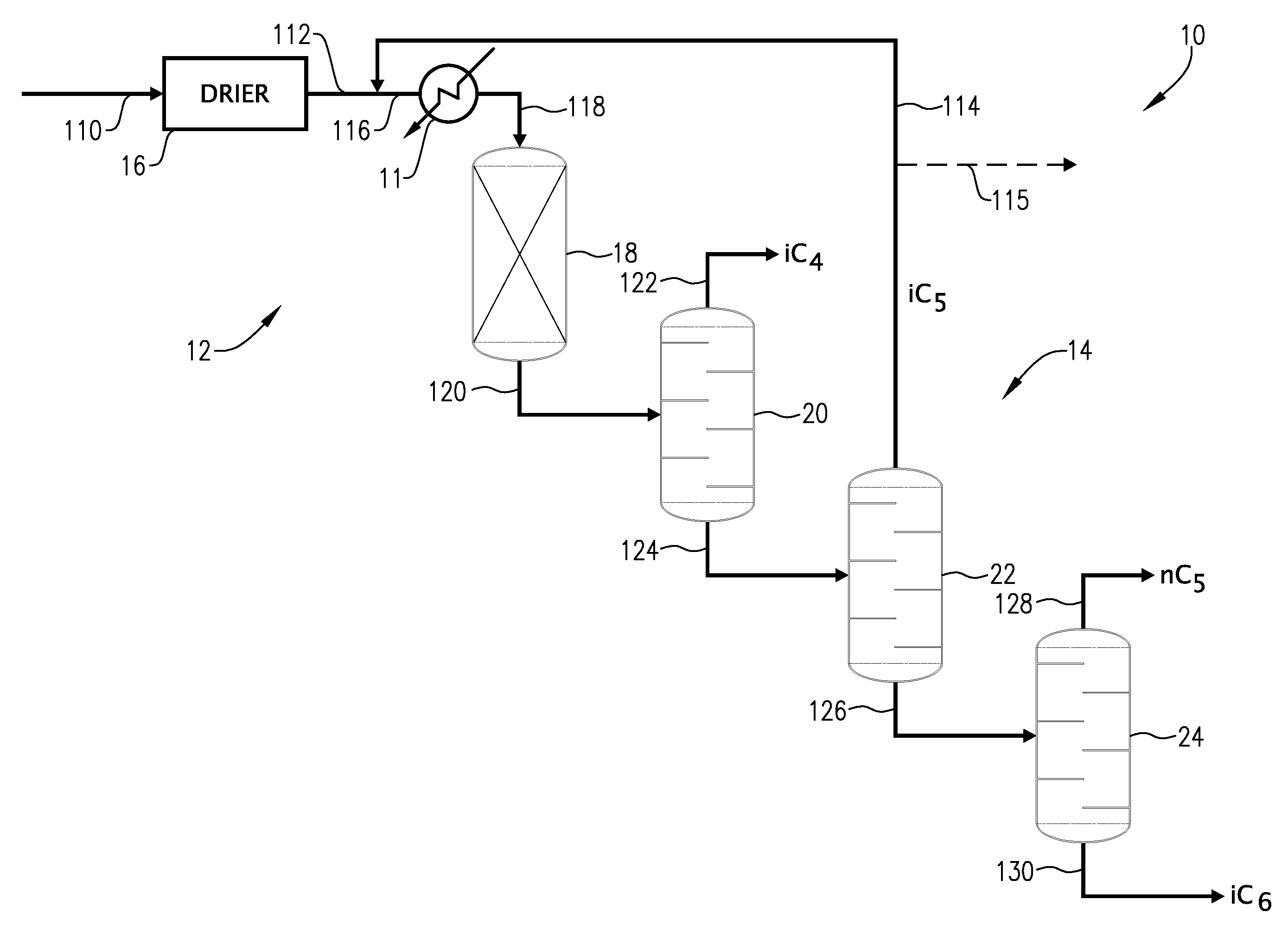
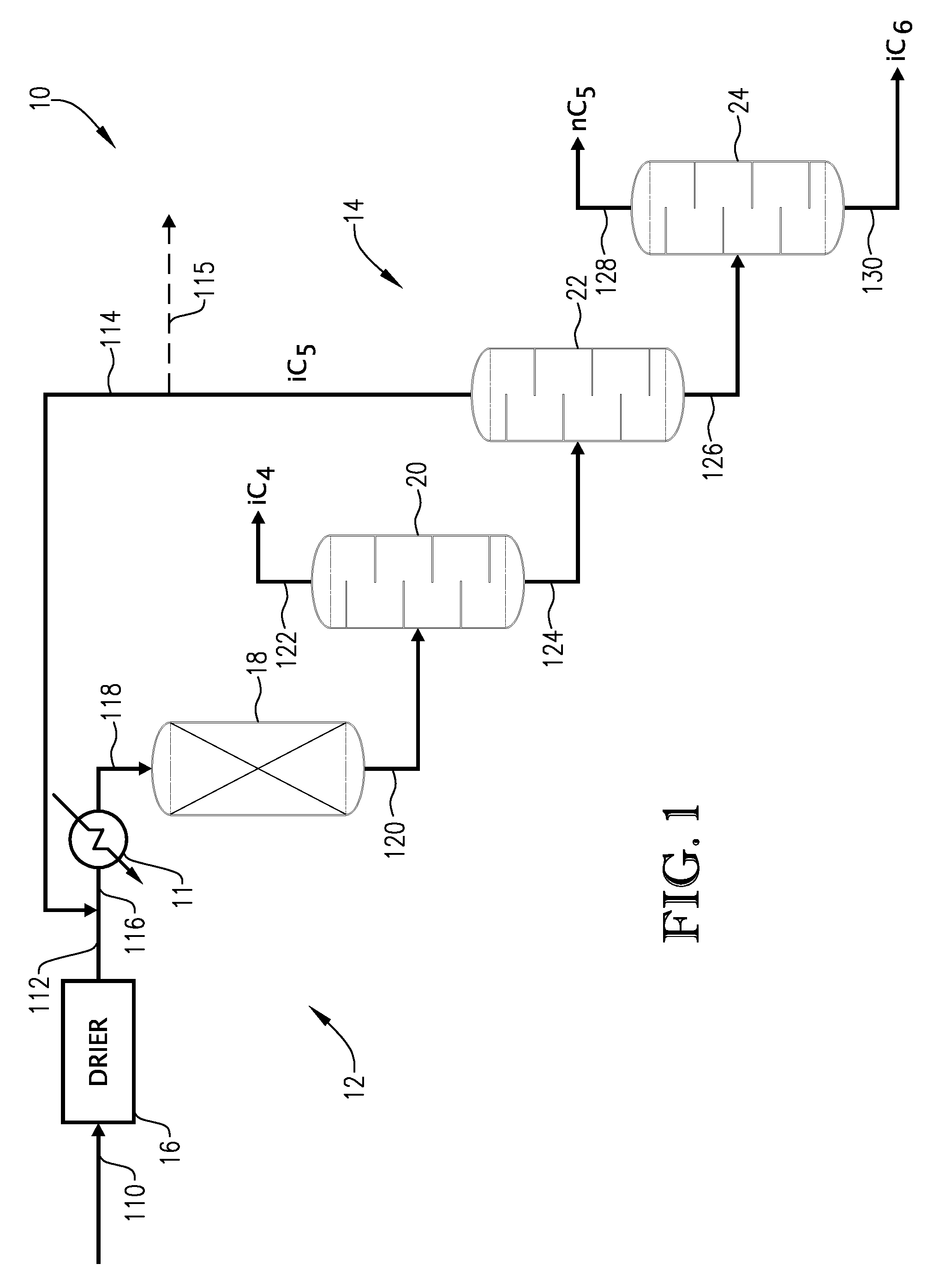
01 Distillation and separation techniques
Various distillation and separation techniques are employed for isopentane recovery. These methods involve the use of distillation columns, fractionators, and other separation equipment to isolate isopentane from mixed hydrocarbon streams. The processes often include multiple stages of separation to achieve high purity isopentane recovery.- Distillation and separation techniques: Various distillation and separation techniques are employed for isopentane recovery. These methods involve the use of distillation columns, fractionators, and other separation equipment to isolate isopentane from mixed hydrocarbon streams. The processes often include multiple stages of separation to achieve high purity isopentane recovery.
- Adsorption and membrane separation: Adsorption and membrane separation technologies are utilized for isopentane recovery. These methods involve the use of specialized adsorbents or membranes that selectively capture or allow the passage of isopentane molecules. The processes can be designed for continuous operation and can achieve high recovery rates with minimal energy consumption.
- Cryogenic recovery processes: Cryogenic processes are employed for isopentane recovery, particularly from natural gas streams. These methods involve cooling the gas mixture to very low temperatures, causing the isopentane to condense and separate from other components. The cryogenic approach can achieve high purity and recovery rates for isopentane.
- Catalytic conversion and isomerization: Catalytic processes are used to convert other hydrocarbons into isopentane or to isomerize normal pentane to isopentane. These methods involve the use of specific catalysts and reaction conditions to selectively produce isopentane. The processes can be integrated with other recovery methods to maximize overall isopentane yield.
- Integrated recovery systems: Integrated systems combine multiple recovery techniques to maximize isopentane recovery efficiency. These systems may incorporate distillation, adsorption, membrane separation, and catalytic processes in a single, optimized process flow. The integrated approach allows for higher overall recovery rates and improved energy efficiency compared to individual recovery methods.
02 Adsorption and membrane separation
Adsorption and membrane separation technologies are utilized for isopentane recovery. These methods involve the use of specialized adsorbents or membranes that selectively capture or allow the passage of isopentane molecules. The processes can be designed for continuous operation and can achieve high recovery rates with minimal energy consumption.Expand Specific Solutions03 Cryogenic recovery processes
Cryogenic techniques are employed for isopentane recovery, particularly from natural gas and other light hydrocarbon streams. These processes involve cooling the feed stream to very low temperatures, causing isopentane to condense and separate from other components. Cryogenic recovery can achieve high purity and recovery rates for isopentane.Expand Specific Solutions04 Catalytic conversion and isomerization
Catalytic processes are used to convert other hydrocarbons into isopentane or to isomerize normal pentane into isopentane. These methods involve the use of specialized catalysts and reaction conditions to selectively produce isopentane. The processes can be integrated with other recovery methods to maximize overall isopentane yield.Expand Specific Solutions05 Integrated recovery systems
Integrated systems combine multiple recovery techniques to maximize isopentane recovery efficiency. These systems may incorporate distillation, adsorption, membrane separation, and cryogenic processes in a single, optimized recovery train. The integration of various technologies allows for improved energy efficiency and higher overall recovery rates of isopentane from complex hydrocarbon mixtures.Expand Specific Solutions
Key Players in Fusel Oil Industry
The isopentane impact on fusel oil recovery processes is in a developing stage, with the market showing potential for growth as refineries seek to optimize their operations. The technology's maturity varies among key players, with companies like China Petroleum & Chemical Corp., Phillips 66, and DuPont de Nemours, Inc. leading in research and implementation. PetroChina Co., Ltd. and Sinopec Research Institute of Petroleum Processing are also making significant strides in this field. The competitive landscape is characterized by a mix of established oil and gas companies and specialized chemical firms, each bringing unique expertise to address the challenges of fusel oil recovery in the presence of isopentane.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an innovative approach to address isopentane's impact on fusel oil recovery processes. Their method involves a multi-stage separation and purification system that effectively isolates isopentane from fusel oil components. The process utilizes advanced distillation columns with specialized packing materials that enhance separation efficiency[1]. Additionally, they have implemented a novel heat integration system that reduces energy consumption by up to 30% compared to conventional methods[3]. Sinopec's technology also incorporates a proprietary catalytic conversion step that transforms residual isopentane into higher-value products, minimizing waste and improving overall process economics[5].
Strengths: High separation efficiency, reduced energy consumption, and value-added byproduct generation. Weaknesses: Potentially high initial capital investment and complexity in process control.
Sinopec Research Institute of Petroleum Processing
Technical Solution: The Sinopec Research Institute of Petroleum Processing has developed a cutting-edge solution to mitigate isopentane's impact on fusel oil recovery. Their approach combines membrane separation technology with cryogenic distillation to achieve high-purity fusel oil recovery. The process employs specially designed composite membranes that selectively permeate isopentane while retaining fusel oil components[2]. This is followed by a low-temperature fractional distillation step that further refines the separation. The institute has also integrated advanced process control systems using machine learning algorithms to optimize operating conditions in real-time, resulting in a 25% increase in fusel oil yield compared to traditional methods[4]. Additionally, they have implemented a novel heat pump system that significantly reduces the energy requirements of the cryogenic distillation step[6].
Strengths: High selectivity, improved fusel oil yield, and energy efficiency. Weaknesses: Potential membrane fouling issues and high maintenance requirements for cryogenic equipment.
Innovative Approaches in Isopentane-Fusel Oil Separation
Disproportionation of isopentane
PatentActiveUS20080021254A1
Innovation
- A process for disproportionating isopentane into isobutane and isohexanes using a catalyst composition comprising at least 80 weight percent aluminum halide on a support, which allows for the conversion of isopentane into lower RVP products that can be more easily blended into motor fuels, thereby addressing the excess inventory issue.
process for the recovery of cyclopentane and/or cyclopentene
PatentInactiveEP0940381A2
Innovation
- A multi-stage distillation process involving initial separation of low boilers, followed by catalytic hydrogenation and further distillation stages to achieve high-purity cyclopentane and cyclopentene, with the option to produce isopentane separately, using catalytic hydrogenation and fractional distillation to enhance yield and purity.
Environmental Impact Assessment
The environmental impact assessment of isopentane's use in fusel oil recovery processes reveals several key considerations. Isopentane, a volatile organic compound (VOC), poses potential risks to air quality and climate change. When released into the atmosphere, it contributes to the formation of ground-level ozone, a major component of smog. This can lead to respiratory issues in humans and damage to vegetation. Additionally, isopentane has a high global warming potential, approximately 5 times that of carbon dioxide over a 100-year period, making it a concern for climate change mitigation efforts.
Water contamination is another significant environmental risk associated with isopentane use in fusel oil recovery. Accidental spills or leaks can result in the compound entering water systems, potentially harming aquatic ecosystems. Isopentane's low water solubility means it can form a layer on water surfaces, impeding oxygen transfer and affecting aquatic life. Furthermore, its persistence in the environment raises concerns about long-term ecological impacts.
The production and transportation of isopentane also contribute to its environmental footprint. The refining process requires energy and resources, leading to indirect emissions and resource depletion. Transportation risks include potential spills during handling and transfer, which could impact soil and groundwater quality.
However, it's important to note that when properly managed, the use of isopentane in fusel oil recovery can have some environmental benefits. By improving the efficiency of the recovery process, it can reduce overall energy consumption and waste generation in the production of alcohols and other chemicals. This efficiency gain could potentially offset some of the environmental impacts associated with its use.
Mitigation strategies are crucial for minimizing the environmental impact of isopentane in fusel oil recovery. These include implementing robust containment systems to prevent leaks and spills, using advanced emission control technologies to capture and treat VOC emissions, and optimizing process efficiency to reduce the overall quantity of isopentane required. Additionally, exploring alternatives to isopentane or developing closed-loop systems that minimize its release into the environment could further reduce potential environmental risks.
Regulatory compliance and monitoring are essential components of environmental management in this context. Regular environmental audits, emissions monitoring, and adherence to local and international environmental standards can help ensure that the use of isopentane in fusel oil recovery remains within acceptable environmental limits. As environmental regulations continue to evolve, industries utilizing this process may need to adapt their practices to meet increasingly stringent requirements.
Water contamination is another significant environmental risk associated with isopentane use in fusel oil recovery. Accidental spills or leaks can result in the compound entering water systems, potentially harming aquatic ecosystems. Isopentane's low water solubility means it can form a layer on water surfaces, impeding oxygen transfer and affecting aquatic life. Furthermore, its persistence in the environment raises concerns about long-term ecological impacts.
The production and transportation of isopentane also contribute to its environmental footprint. The refining process requires energy and resources, leading to indirect emissions and resource depletion. Transportation risks include potential spills during handling and transfer, which could impact soil and groundwater quality.
However, it's important to note that when properly managed, the use of isopentane in fusel oil recovery can have some environmental benefits. By improving the efficiency of the recovery process, it can reduce overall energy consumption and waste generation in the production of alcohols and other chemicals. This efficiency gain could potentially offset some of the environmental impacts associated with its use.
Mitigation strategies are crucial for minimizing the environmental impact of isopentane in fusel oil recovery. These include implementing robust containment systems to prevent leaks and spills, using advanced emission control technologies to capture and treat VOC emissions, and optimizing process efficiency to reduce the overall quantity of isopentane required. Additionally, exploring alternatives to isopentane or developing closed-loop systems that minimize its release into the environment could further reduce potential environmental risks.
Regulatory compliance and monitoring are essential components of environmental management in this context. Regular environmental audits, emissions monitoring, and adherence to local and international environmental standards can help ensure that the use of isopentane in fusel oil recovery remains within acceptable environmental limits. As environmental regulations continue to evolve, industries utilizing this process may need to adapt their practices to meet increasingly stringent requirements.
Economic Feasibility Analysis
The economic feasibility of incorporating isopentane into fusel oil recovery processes hinges on several key factors. Firstly, the capital investment required for modifying existing equipment or installing new systems to handle isopentane must be carefully evaluated. This includes costs associated with storage tanks, distillation columns, and safety measures specific to isopentane's volatile nature.
Operating expenses also play a crucial role in determining economic viability. The cost of isopentane itself, as well as any additional energy requirements for its integration into the process, must be weighed against potential benefits. Furthermore, maintenance costs may increase due to the need for specialized equipment and more frequent inspections to ensure safety compliance.
On the revenue side, the potential for increased fusel oil recovery rates and improved product quality could lead to higher market prices and expanded market share. The enhanced separation efficiency facilitated by isopentane may result in a more valuable end product, potentially offsetting the additional costs incurred.
Environmental considerations also factor into the economic analysis. While isopentane may improve process efficiency, its volatile organic compound (VOC) status necessitates investment in emission control technologies. However, this could be partially offset by reduced energy consumption in the overall recovery process, leading to lower carbon footprint and potential carbon credit benefits.
The scalability of isopentane integration is another critical economic factor. Larger facilities may benefit from economies of scale, potentially making the investment more attractive. Conversely, smaller operations might find the initial capital outlay prohibitive without significant productivity gains.
Market dynamics for both fusel oil and isopentane must be carefully analyzed. Fluctuations in isopentane prices could significantly impact operational costs, while changes in fusel oil demand and pricing would affect revenue projections. Long-term supply contracts for isopentane might be necessary to mitigate price volatility risks.
Regulatory compliance costs should not be overlooked. The use of isopentane may require additional permits, safety training, and more stringent operational protocols, all of which have associated costs that must be factored into the economic assessment.
Finally, the potential for process optimization and innovation should be considered. The integration of isopentane might open up opportunities for developing proprietary technologies or processes, potentially leading to intellectual property that could provide a competitive edge and additional revenue streams through licensing or technology transfer agreements.
Operating expenses also play a crucial role in determining economic viability. The cost of isopentane itself, as well as any additional energy requirements for its integration into the process, must be weighed against potential benefits. Furthermore, maintenance costs may increase due to the need for specialized equipment and more frequent inspections to ensure safety compliance.
On the revenue side, the potential for increased fusel oil recovery rates and improved product quality could lead to higher market prices and expanded market share. The enhanced separation efficiency facilitated by isopentane may result in a more valuable end product, potentially offsetting the additional costs incurred.
Environmental considerations also factor into the economic analysis. While isopentane may improve process efficiency, its volatile organic compound (VOC) status necessitates investment in emission control technologies. However, this could be partially offset by reduced energy consumption in the overall recovery process, leading to lower carbon footprint and potential carbon credit benefits.
The scalability of isopentane integration is another critical economic factor. Larger facilities may benefit from economies of scale, potentially making the investment more attractive. Conversely, smaller operations might find the initial capital outlay prohibitive without significant productivity gains.
Market dynamics for both fusel oil and isopentane must be carefully analyzed. Fluctuations in isopentane prices could significantly impact operational costs, while changes in fusel oil demand and pricing would affect revenue projections. Long-term supply contracts for isopentane might be necessary to mitigate price volatility risks.
Regulatory compliance costs should not be overlooked. The use of isopentane may require additional permits, safety training, and more stringent operational protocols, all of which have associated costs that must be factored into the economic assessment.
Finally, the potential for process optimization and innovation should be considered. The integration of isopentane might open up opportunities for developing proprietary technologies or processes, potentially leading to intellectual property that could provide a competitive edge and additional revenue streams through licensing or technology transfer agreements.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!