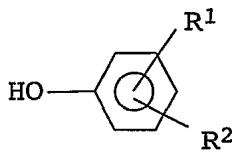
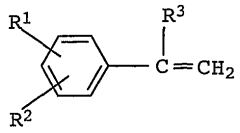
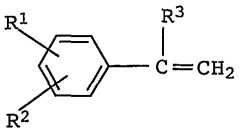
Isopentane's Impacts on Functional Group Compatibility in Polymers
JUL 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isopentane and Polymer Compatibility Overview
Isopentane, a branched alkane with the molecular formula C5H12, plays a significant role in polymer chemistry, particularly in the context of functional group compatibility. This volatile organic compound, known for its low boiling point and high vapor pressure, interacts with various polymer structures in ways that can profoundly affect their properties and applications.
The compatibility between isopentane and polymers is a complex interplay of molecular structures, chemical properties, and physical interactions. Polymers, being long-chain molecules composed of repeating subunits, exhibit a wide range of functional groups that can interact with isopentane. These interactions can lead to changes in polymer morphology, mechanical properties, and overall performance characteristics.
One of the primary considerations in isopentane-polymer compatibility is the nature of the functional groups present in the polymer structure. Polar functional groups, such as hydroxyl (-OH), carboxyl (-COOH), or amine (-NH2), tend to have limited compatibility with the non-polar isopentane molecule. This can result in phase separation or reduced miscibility, potentially affecting the polymer's physical properties and processing characteristics.
Conversely, polymers with predominantly non-polar structures or those containing aliphatic side chains may exhibit better compatibility with isopentane. This improved compatibility can lead to enhanced solubility, plasticization effects, or even the formation of stable blends or composites. The degree of compatibility can significantly influence the polymer's thermal properties, mechanical strength, and gas permeability.
The impact of isopentane on functional group compatibility in polymers extends beyond simple solubility considerations. It can affect the polymer's crystallinity, glass transition temperature, and overall molecular mobility. These changes can have profound implications for the polymer's end-use properties, such as flexibility, toughness, and barrier properties.
Understanding the relationship between isopentane and polymer functional groups is crucial for various industrial applications. In foam production, for instance, isopentane is often used as a blowing agent. Its compatibility with the polymer matrix determines the foam's cell structure, density, and insulation properties. Similarly, in the development of polymer-based membranes or films, isopentane interactions can influence gas permeation rates and selectivity.
The study of isopentane's impacts on functional group compatibility in polymers is an ongoing area of research in materials science. It involves a multidisciplinary approach, combining principles from organic chemistry, polymer physics, and materials engineering. Advanced characterization techniques, such as spectroscopy, thermal analysis, and microscopy, are employed to elucidate the intricate relationships between isopentane and various polymer systems.
The compatibility between isopentane and polymers is a complex interplay of molecular structures, chemical properties, and physical interactions. Polymers, being long-chain molecules composed of repeating subunits, exhibit a wide range of functional groups that can interact with isopentane. These interactions can lead to changes in polymer morphology, mechanical properties, and overall performance characteristics.
One of the primary considerations in isopentane-polymer compatibility is the nature of the functional groups present in the polymer structure. Polar functional groups, such as hydroxyl (-OH), carboxyl (-COOH), or amine (-NH2), tend to have limited compatibility with the non-polar isopentane molecule. This can result in phase separation or reduced miscibility, potentially affecting the polymer's physical properties and processing characteristics.
Conversely, polymers with predominantly non-polar structures or those containing aliphatic side chains may exhibit better compatibility with isopentane. This improved compatibility can lead to enhanced solubility, plasticization effects, or even the formation of stable blends or composites. The degree of compatibility can significantly influence the polymer's thermal properties, mechanical strength, and gas permeability.
The impact of isopentane on functional group compatibility in polymers extends beyond simple solubility considerations. It can affect the polymer's crystallinity, glass transition temperature, and overall molecular mobility. These changes can have profound implications for the polymer's end-use properties, such as flexibility, toughness, and barrier properties.
Understanding the relationship between isopentane and polymer functional groups is crucial for various industrial applications. In foam production, for instance, isopentane is often used as a blowing agent. Its compatibility with the polymer matrix determines the foam's cell structure, density, and insulation properties. Similarly, in the development of polymer-based membranes or films, isopentane interactions can influence gas permeation rates and selectivity.
The study of isopentane's impacts on functional group compatibility in polymers is an ongoing area of research in materials science. It involves a multidisciplinary approach, combining principles from organic chemistry, polymer physics, and materials engineering. Advanced characterization techniques, such as spectroscopy, thermal analysis, and microscopy, are employed to elucidate the intricate relationships between isopentane and various polymer systems.
Market Demand Analysis for Isopentane-Compatible Polymers
The market demand for isopentane-compatible polymers has been steadily increasing due to the growing awareness of environmental concerns and the need for more sustainable materials in various industries. Isopentane, a volatile organic compound, is widely used as a blowing agent in the production of foam insulation and packaging materials. However, its compatibility with certain polymers has become a critical factor in determining the performance and durability of end products.
In the construction industry, there is a significant demand for isopentane-compatible polymers in the production of rigid foam insulation panels. These panels are essential for improving energy efficiency in buildings, and the market for such materials is expected to grow as energy conservation regulations become more stringent worldwide. The automotive sector also presents a substantial market opportunity, as manufacturers seek lightweight materials that can withstand exposure to isopentane in fuel systems and other components.
The packaging industry is another key driver of demand for isopentane-compatible polymers. With the rise of e-commerce and the need for protective packaging solutions, there is a growing market for foam materials that can withstand the presence of isopentane without degradation. This is particularly important for the transportation of sensitive electronic components and perishable goods.
In the appliance sector, isopentane-compatible polymers are crucial for the production of refrigerators and freezers. As manufacturers phase out hydrofluorocarbons (HFCs) due to their high global warming potential, isopentane has emerged as a more environmentally friendly alternative. This transition has created a substantial market for polymers that can maintain their structural integrity and insulating properties when exposed to isopentane.
The medical device industry also contributes to the demand for isopentane-compatible polymers, particularly in the production of diagnostic equipment and storage containers. These applications require materials that can resist chemical degradation while maintaining strict hygiene standards.
Market analysis indicates that the Asia-Pacific region is expected to be the fastest-growing market for isopentane-compatible polymers, driven by rapid industrialization and urbanization. North America and Europe are also significant markets, with a focus on sustainable and energy-efficient solutions driving demand.
As environmental regulations become more stringent and industries continue to seek sustainable alternatives, the market for isopentane-compatible polymers is projected to expand further. This growth is supported by ongoing research and development efforts to improve the performance and cost-effectiveness of these materials, ensuring their wider adoption across various sectors.
In the construction industry, there is a significant demand for isopentane-compatible polymers in the production of rigid foam insulation panels. These panels are essential for improving energy efficiency in buildings, and the market for such materials is expected to grow as energy conservation regulations become more stringent worldwide. The automotive sector also presents a substantial market opportunity, as manufacturers seek lightweight materials that can withstand exposure to isopentane in fuel systems and other components.
The packaging industry is another key driver of demand for isopentane-compatible polymers. With the rise of e-commerce and the need for protective packaging solutions, there is a growing market for foam materials that can withstand the presence of isopentane without degradation. This is particularly important for the transportation of sensitive electronic components and perishable goods.
In the appliance sector, isopentane-compatible polymers are crucial for the production of refrigerators and freezers. As manufacturers phase out hydrofluorocarbons (HFCs) due to their high global warming potential, isopentane has emerged as a more environmentally friendly alternative. This transition has created a substantial market for polymers that can maintain their structural integrity and insulating properties when exposed to isopentane.
The medical device industry also contributes to the demand for isopentane-compatible polymers, particularly in the production of diagnostic equipment and storage containers. These applications require materials that can resist chemical degradation while maintaining strict hygiene standards.
Market analysis indicates that the Asia-Pacific region is expected to be the fastest-growing market for isopentane-compatible polymers, driven by rapid industrialization and urbanization. North America and Europe are also significant markets, with a focus on sustainable and energy-efficient solutions driving demand.
As environmental regulations become more stringent and industries continue to seek sustainable alternatives, the market for isopentane-compatible polymers is projected to expand further. This growth is supported by ongoing research and development efforts to improve the performance and cost-effectiveness of these materials, ensuring their wider adoption across various sectors.
Current Challenges in Isopentane-Polymer Interactions
The interaction between isopentane and polymers presents several significant challenges in the field of polymer science and engineering. One of the primary issues is the potential for isopentane to disrupt the structural integrity of polymers, particularly those with sensitive functional groups. This disruption can lead to changes in the polymer's physical properties, including its mechanical strength, thermal stability, and overall performance.
A major challenge lies in understanding and predicting the exact nature of these interactions at a molecular level. The branched structure of isopentane can lead to complex interactions with various polymer functional groups, making it difficult to develop comprehensive models for predicting behavior across different polymer systems. This complexity is further compounded by the fact that the effects can vary significantly depending on the concentration of isopentane and the specific polymer composition.
Another critical challenge is the potential for isopentane to act as a plasticizer in certain polymer systems. While this can be beneficial in some applications, it can also lead to undesired changes in material properties, such as decreased glass transition temperature or increased polymer chain mobility. These changes can significantly impact the polymer's intended functionality, particularly in applications requiring specific mechanical or thermal properties.
The volatility of isopentane poses additional challenges in polymer processing and product stability. During manufacturing processes that involve heat or pressure, isopentane can rapidly vaporize, leading to defects such as voids or bubbles in the final product. This not only affects the aesthetic quality but can also compromise the structural integrity and performance of the polymer-based product.
Environmental and safety concerns also present significant challenges in the use of isopentane with polymers. As a volatile organic compound (VOC), isopentane can contribute to air pollution and poses potential health risks in industrial settings. Developing safe handling procedures and effective containment methods is crucial, especially in large-scale manufacturing environments.
Furthermore, the long-term effects of isopentane on polymer degradation and aging are not fully understood. This gap in knowledge presents challenges in predicting the lifespan and performance of polymer products exposed to isopentane over extended periods. Research is needed to elucidate the mechanisms of polymer degradation in the presence of isopentane and to develop strategies for mitigating these effects.
Lastly, the compatibility of isopentane with different polymer additives and processing aids presents another layer of complexity. The presence of isopentane can potentially interfere with the effectiveness of stabilizers, flame retardants, or other functional additives commonly used in polymer formulations. Developing new additive systems or modifying existing ones to maintain their functionality in the presence of isopentane is an ongoing challenge for polymer scientists and engineers.
A major challenge lies in understanding and predicting the exact nature of these interactions at a molecular level. The branched structure of isopentane can lead to complex interactions with various polymer functional groups, making it difficult to develop comprehensive models for predicting behavior across different polymer systems. This complexity is further compounded by the fact that the effects can vary significantly depending on the concentration of isopentane and the specific polymer composition.
Another critical challenge is the potential for isopentane to act as a plasticizer in certain polymer systems. While this can be beneficial in some applications, it can also lead to undesired changes in material properties, such as decreased glass transition temperature or increased polymer chain mobility. These changes can significantly impact the polymer's intended functionality, particularly in applications requiring specific mechanical or thermal properties.
The volatility of isopentane poses additional challenges in polymer processing and product stability. During manufacturing processes that involve heat or pressure, isopentane can rapidly vaporize, leading to defects such as voids or bubbles in the final product. This not only affects the aesthetic quality but can also compromise the structural integrity and performance of the polymer-based product.
Environmental and safety concerns also present significant challenges in the use of isopentane with polymers. As a volatile organic compound (VOC), isopentane can contribute to air pollution and poses potential health risks in industrial settings. Developing safe handling procedures and effective containment methods is crucial, especially in large-scale manufacturing environments.
Furthermore, the long-term effects of isopentane on polymer degradation and aging are not fully understood. This gap in knowledge presents challenges in predicting the lifespan and performance of polymer products exposed to isopentane over extended periods. Research is needed to elucidate the mechanisms of polymer degradation in the presence of isopentane and to develop strategies for mitigating these effects.
Lastly, the compatibility of isopentane with different polymer additives and processing aids presents another layer of complexity. The presence of isopentane can potentially interfere with the effectiveness of stabilizers, flame retardants, or other functional additives commonly used in polymer formulations. Developing new additive systems or modifying existing ones to maintain their functionality in the presence of isopentane is an ongoing challenge for polymer scientists and engineers.
Existing Solutions for Isopentane-Polymer Compatibility
01 Compatibility with hydrocarbon processes
Isopentane shows compatibility with various hydrocarbon processes, including alkylation, isomerization, and dehydrogenation. Its functional groups allow for efficient integration in refining and petrochemical applications, particularly in processes involving C5 hydrocarbons.- Compatibility with hydrocarbon compounds: Isopentane shows good compatibility with various hydrocarbon compounds, including alkanes, alkenes, and aromatic hydrocarbons. This compatibility allows for its use in fuel blends, solvents, and as a blowing agent in polymer foams. The functional groups present in isopentane, primarily the alkyl groups, interact favorably with similar hydrocarbon structures.
- Compatibility with halogenated compounds: Isopentane exhibits compatibility with certain halogenated compounds, particularly fluorinated and chlorinated hydrocarbons. This compatibility is useful in refrigeration systems, aerosol propellants, and as a replacement for ozone-depleting substances. The non-polar nature of isopentane allows for mixing with these halogenated compounds without significant chemical interaction.
- Compatibility in polymer systems: Isopentane is compatible with various polymer systems, making it suitable for use as a blowing agent in the production of foams and as a processing aid in polymer manufacturing. Its low boiling point and non-polar nature allow for easy incorporation and subsequent removal from polymer matrices without affecting the polymer's chemical structure.
- Compatibility with oxygenated compounds: Isopentane shows limited compatibility with oxygenated compounds such as alcohols, ethers, and ketones. While it can form mixtures with some of these compounds, the compatibility is generally lower compared to other hydrocarbons. This property is important in formulating certain solvents and in separation processes involving oxygenated compounds.
- Compatibility in catalytic systems: Isopentane's functional groups are compatible with various catalytic systems, particularly in isomerization and dehydrogenation reactions. The molecule's structure allows for interaction with metal catalysts and supports, enabling its transformation into other valuable compounds. This compatibility is crucial in petroleum refining and the production of high-octane fuel components.
02 Use in polymer formulations
The functional groups of isopentane make it compatible with various polymer formulations. It can be used as a blowing agent in foam production, as a solvent in adhesive formulations, and as a component in thermoplastic compositions, enhancing processability and final product properties.Expand Specific Solutions03 Compatibility with refrigeration systems
Isopentane's functional groups are compatible with refrigeration systems, making it suitable as a refrigerant or component in refrigerant blends. Its thermodynamic properties and chemical stability contribute to efficient heat transfer in cooling applications.Expand Specific Solutions04 Application in fuel compositions
The functional groups of isopentane make it compatible with various fuel compositions. It can be used as an additive to improve octane ratings in gasoline, as a component in alternative fuels, and in fuel blends for internal combustion engines.Expand Specific Solutions05 Compatibility in separation processes
Isopentane's functional groups allow for its use in various separation processes. It can be employed in extractive distillation, as an entrainer in azeotropic distillation, and in membrane separation technologies for hydrocarbon mixtures.Expand Specific Solutions
Key Players in Isopentane and Polymer Industries
The market for isopentane's impacts on functional group compatibility in polymers is in a growth phase, driven by increasing demand for advanced polymer materials across industries. The global market size is expanding, with projections indicating significant growth potential in the coming years. Technologically, the field is advancing rapidly, with companies like ExxonMobil Chemical Patents, BASF, and Covestro Deutschland AG leading innovation. These firms, along with others such as Sika Technology and PPG Industries Ohio, are investing heavily in R&D to enhance polymer functionality and performance. The competitive landscape is characterized by a mix of established chemical giants and specialized polymer companies, all vying to develop novel solutions that leverage isopentane's unique properties to improve polymer compatibility and performance.
Covestro Deutschland AG
Technical Solution: Covestro has developed a novel approach to address isopentane's impacts on functional group compatibility in polymers. Their technology focuses on creating specialized polyurethane formulations that maintain structural integrity when exposed to isopentane. They have engineered polymer chains with enhanced intermolecular forces that resist isopentane-induced swelling [2]. Covestro's research has also led to the development of hybrid materials that combine the benefits of different polymer types to create isopentane-resistant composites [4]. These materials find applications in insulation panels, refrigeration systems, and automotive components where isopentane exposure is common.
Strengths: Tailored solutions for specific applications, improved durability in isopentane environments. Weaknesses: May have limitations in extreme temperature conditions, potentially higher material costs.
BASF SE
Technical Solution: BASF SE has developed innovative polymer solutions that address isopentane compatibility issues. Their approach involves modifying polymer structures to enhance resistance to isopentane-induced swelling and degradation. They have implemented cross-linking techniques to create more stable polymer networks [1]. Additionally, BASF has introduced specialized additives that act as isopentane barriers within the polymer matrix, significantly reducing permeation and maintaining structural integrity [3]. Their research has also focused on developing co-polymers with specific functional groups that exhibit improved compatibility with isopentane, allowing for better performance in applications such as insulation foams and automotive components [5].
Strengths: Comprehensive polymer modification techniques, wide range of applications. Weaknesses: Potential increased production costs, may require reformulation of existing products.
Core Innovations in Functional Group Engineering
Polymer composition containing at least one middle molecular weight reactive polyisobutene
PatentInactiveEP1423474A2
Innovation
- A polymer composition comprising medium molecular weight reactive polyisobutene with terminal double bonds and another polymer, where the components interact through physical phenomena or form covalent bonds, enhancing compatibility and stability.
Polymer composition containing at least one middle molecular weight reactive polyisobutene
PatentWO2003020822A2
Innovation
- A polymer composition comprising a medium molecular weight reactive polyisobutene with terminal double bonds and a complementary polymer, where the components interact either physically or form covalent bonds, enhancing compatibility and stability through specific functionalization reactions such as alkylation, epoxidation, ene reactions, hydroformylation, and others.
Environmental Impact of Isopentane-Polymer Systems
The environmental impact of isopentane-polymer systems is a critical consideration in the development and application of these materials. Isopentane, a volatile organic compound, is often used as a blowing agent in polymer foam production. Its interaction with various functional groups in polymers can lead to significant environmental consequences.
One of the primary environmental concerns is the potential for isopentane to contribute to air pollution. When released into the atmosphere, isopentane can participate in photochemical reactions, leading to the formation of ground-level ozone and smog. These air quality issues can have detrimental effects on human health and ecosystems, particularly in urban areas where polymer manufacturing facilities are often located.
Furthermore, the production and use of isopentane-polymer systems can result in greenhouse gas emissions. Isopentane itself is a potent greenhouse gas with a global warming potential significantly higher than carbon dioxide. The manufacturing processes involved in creating these polymer systems often require substantial energy inputs, contributing to indirect carbon emissions.
Water pollution is another environmental concern associated with isopentane-polymer systems. During the production process, there is a risk of isopentane leaching into water sources. This can lead to contamination of aquatic ecosystems, potentially harming marine life and affecting water quality for human consumption.
The disposal and end-of-life management of isopentane-containing polymers present additional environmental challenges. As these materials degrade, they may release isopentane and other potentially harmful compounds into the environment. Proper recycling and waste management strategies are crucial to mitigate these impacts.
However, it is important to note that the use of isopentane in polymer systems can also have some positive environmental implications. For instance, isopentane-blown foams often have excellent insulation properties, which can lead to improved energy efficiency in buildings and appliances. This energy-saving potential could offset some of the negative environmental impacts associated with their production and use.
The compatibility of isopentane with various functional groups in polymers also influences its environmental impact. Certain functional groups may enhance the retention of isopentane within the polymer matrix, reducing emissions during the product's lifecycle. Conversely, incompatible functional groups could lead to increased isopentane release, exacerbating environmental concerns.
To address these environmental issues, ongoing research focuses on developing more sustainable alternatives to isopentane-polymer systems. This includes exploring bio-based blowing agents, improving manufacturing processes to minimize emissions, and enhancing the recyclability of these materials. Additionally, regulatory frameworks are evolving to better manage the environmental risks associated with isopentane and similar compounds in polymer applications.
One of the primary environmental concerns is the potential for isopentane to contribute to air pollution. When released into the atmosphere, isopentane can participate in photochemical reactions, leading to the formation of ground-level ozone and smog. These air quality issues can have detrimental effects on human health and ecosystems, particularly in urban areas where polymer manufacturing facilities are often located.
Furthermore, the production and use of isopentane-polymer systems can result in greenhouse gas emissions. Isopentane itself is a potent greenhouse gas with a global warming potential significantly higher than carbon dioxide. The manufacturing processes involved in creating these polymer systems often require substantial energy inputs, contributing to indirect carbon emissions.
Water pollution is another environmental concern associated with isopentane-polymer systems. During the production process, there is a risk of isopentane leaching into water sources. This can lead to contamination of aquatic ecosystems, potentially harming marine life and affecting water quality for human consumption.
The disposal and end-of-life management of isopentane-containing polymers present additional environmental challenges. As these materials degrade, they may release isopentane and other potentially harmful compounds into the environment. Proper recycling and waste management strategies are crucial to mitigate these impacts.
However, it is important to note that the use of isopentane in polymer systems can also have some positive environmental implications. For instance, isopentane-blown foams often have excellent insulation properties, which can lead to improved energy efficiency in buildings and appliances. This energy-saving potential could offset some of the negative environmental impacts associated with their production and use.
The compatibility of isopentane with various functional groups in polymers also influences its environmental impact. Certain functional groups may enhance the retention of isopentane within the polymer matrix, reducing emissions during the product's lifecycle. Conversely, incompatible functional groups could lead to increased isopentane release, exacerbating environmental concerns.
To address these environmental issues, ongoing research focuses on developing more sustainable alternatives to isopentane-polymer systems. This includes exploring bio-based blowing agents, improving manufacturing processes to minimize emissions, and enhancing the recyclability of these materials. Additionally, regulatory frameworks are evolving to better manage the environmental risks associated with isopentane and similar compounds in polymer applications.
Safety Regulations for Isopentane in Polymer Applications
The use of isopentane in polymer applications necessitates strict adherence to safety regulations due to its highly flammable nature and potential environmental impacts. Regulatory bodies such as the Occupational Safety and Health Administration (OSHA) and the Environmental Protection Agency (EPA) in the United States have established comprehensive guidelines for the handling, storage, and disposal of isopentane in industrial settings.
OSHA mandates specific workplace safety measures for isopentane use, including proper ventilation systems, personal protective equipment (PPE), and emergency response protocols. Employers must ensure that workers are adequately trained in the safe handling of isopentane and are aware of its associated risks. Regular safety inspections and maintenance of equipment are also required to prevent leaks and minimize explosion hazards.
The EPA regulates isopentane under the Clean Air Act as a volatile organic compound (VOC). Polymer manufacturers must comply with emission standards and implement best practices to reduce isopentane releases into the atmosphere. This may involve the use of closed-loop systems, vapor recovery units, and proper sealing of storage containers.
Fire safety is a critical aspect of isopentane regulations in polymer applications. The National Fire Protection Association (NFPA) provides guidelines for the storage and handling of flammable liquids, including isopentane. These guidelines specify requirements for fire suppression systems, electrical equipment classifications, and the design of storage facilities to minimize fire risks.
Transportation of isopentane is governed by the Department of Transportation (DOT) regulations, which classify it as a hazardous material. Specific packaging, labeling, and documentation requirements must be met when transporting isopentane for use in polymer production. Carriers must be certified to handle hazardous materials and follow prescribed routes to minimize risks during transit.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation applies to isopentane use in polymer applications. Manufacturers and importers must register isopentane with the European Chemicals Agency (ECHA) and provide safety data sheets detailing its properties, hazards, and safe handling procedures.
Waste management regulations also play a crucial role in the polymer industry's use of isopentane. The Resource Conservation and Recovery Act (RCRA) in the United States classifies certain isopentane-containing wastes as hazardous, requiring special disposal methods and documentation. Polymer manufacturers must implement waste reduction strategies and ensure proper disposal through licensed waste management facilities.
Compliance with these safety regulations is essential for polymer manufacturers using isopentane. Regular audits, employee training programs, and up-to-date safety management systems are necessary to maintain regulatory compliance and ensure workplace safety. As environmental concerns grow, future regulations may impose stricter controls on isopentane use, potentially driving the development of alternative blowing agents or processing methods in polymer applications.
OSHA mandates specific workplace safety measures for isopentane use, including proper ventilation systems, personal protective equipment (PPE), and emergency response protocols. Employers must ensure that workers are adequately trained in the safe handling of isopentane and are aware of its associated risks. Regular safety inspections and maintenance of equipment are also required to prevent leaks and minimize explosion hazards.
The EPA regulates isopentane under the Clean Air Act as a volatile organic compound (VOC). Polymer manufacturers must comply with emission standards and implement best practices to reduce isopentane releases into the atmosphere. This may involve the use of closed-loop systems, vapor recovery units, and proper sealing of storage containers.
Fire safety is a critical aspect of isopentane regulations in polymer applications. The National Fire Protection Association (NFPA) provides guidelines for the storage and handling of flammable liquids, including isopentane. These guidelines specify requirements for fire suppression systems, electrical equipment classifications, and the design of storage facilities to minimize fire risks.
Transportation of isopentane is governed by the Department of Transportation (DOT) regulations, which classify it as a hazardous material. Specific packaging, labeling, and documentation requirements must be met when transporting isopentane for use in polymer production. Carriers must be certified to handle hazardous materials and follow prescribed routes to minimize risks during transit.
In the European Union, the Registration, Evaluation, Authorization and Restriction of Chemicals (REACH) regulation applies to isopentane use in polymer applications. Manufacturers and importers must register isopentane with the European Chemicals Agency (ECHA) and provide safety data sheets detailing its properties, hazards, and safe handling procedures.
Waste management regulations also play a crucial role in the polymer industry's use of isopentane. The Resource Conservation and Recovery Act (RCRA) in the United States classifies certain isopentane-containing wastes as hazardous, requiring special disposal methods and documentation. Polymer manufacturers must implement waste reduction strategies and ensure proper disposal through licensed waste management facilities.
Compliance with these safety regulations is essential for polymer manufacturers using isopentane. Regular audits, employee training programs, and up-to-date safety management systems are necessary to maintain regulatory compliance and ensure workplace safety. As environmental concerns grow, future regulations may impose stricter controls on isopentane use, potentially driving the development of alternative blowing agents or processing methods in polymer applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!