Isopentane’s Influence on Carcinogenicity in Vapor Phases
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isopentane Carcinogenicity Background and Objectives
Isopentane, a branched-chain alkane with the molecular formula C5H12, has been a subject of increasing interest in the field of environmental and occupational health. The study of its potential carcinogenic effects, particularly in vapor phases, has gained prominence due to its widespread use in various industrial applications and its presence in consumer products.
The historical context of isopentane research dates back to the mid-20th century when concerns about volatile organic compounds (VOCs) and their impact on human health began to emerge. Initially, isopentane was primarily studied for its physical and chemical properties, with limited focus on its biological effects. However, as analytical techniques advanced and epidemiological studies became more sophisticated, the scientific community started to investigate the potential health risks associated with isopentane exposure.
The evolution of isopentane research has been closely tied to the broader field of toxicology and cancer research. As our understanding of carcinogenesis mechanisms improved, researchers began to examine the potential role of isopentane in initiating or promoting cancer development. This shift in focus was partly driven by observations of increased cancer incidence in certain occupational settings where isopentane exposure was common.
The primary objective of current research into isopentane's carcinogenic potential in vapor phases is to establish a comprehensive understanding of its biological interactions and potential health risks. This includes investigating the mechanisms by which isopentane vapor may induce cellular changes that could lead to cancer development. Researchers aim to determine the dose-response relationship, identify susceptible populations, and assess the long-term effects of chronic low-level exposure.
Another critical goal is to evaluate the synergistic effects of isopentane with other environmental pollutants or occupational exposures. This is particularly important given that isopentane is often present in complex mixtures of hydrocarbons in both industrial and environmental settings. Understanding these interactions could provide valuable insights into the overall carcinogenic potential of isopentane in real-world scenarios.
Furthermore, the research aims to inform regulatory decisions and occupational safety guidelines. By elucidating the carcinogenic potential of isopentane vapor, policymakers and industry leaders can develop evidence-based standards for exposure limits and implement appropriate safety measures to protect workers and the general public.
In conclusion, the background and objectives of isopentane carcinogenicity research reflect a growing recognition of the need for comprehensive toxicological assessments of common industrial chemicals. The findings from these studies will not only contribute to our scientific knowledge but also have significant implications for public health, occupational safety, and environmental protection.
The historical context of isopentane research dates back to the mid-20th century when concerns about volatile organic compounds (VOCs) and their impact on human health began to emerge. Initially, isopentane was primarily studied for its physical and chemical properties, with limited focus on its biological effects. However, as analytical techniques advanced and epidemiological studies became more sophisticated, the scientific community started to investigate the potential health risks associated with isopentane exposure.
The evolution of isopentane research has been closely tied to the broader field of toxicology and cancer research. As our understanding of carcinogenesis mechanisms improved, researchers began to examine the potential role of isopentane in initiating or promoting cancer development. This shift in focus was partly driven by observations of increased cancer incidence in certain occupational settings where isopentane exposure was common.
The primary objective of current research into isopentane's carcinogenic potential in vapor phases is to establish a comprehensive understanding of its biological interactions and potential health risks. This includes investigating the mechanisms by which isopentane vapor may induce cellular changes that could lead to cancer development. Researchers aim to determine the dose-response relationship, identify susceptible populations, and assess the long-term effects of chronic low-level exposure.
Another critical goal is to evaluate the synergistic effects of isopentane with other environmental pollutants or occupational exposures. This is particularly important given that isopentane is often present in complex mixtures of hydrocarbons in both industrial and environmental settings. Understanding these interactions could provide valuable insights into the overall carcinogenic potential of isopentane in real-world scenarios.
Furthermore, the research aims to inform regulatory decisions and occupational safety guidelines. By elucidating the carcinogenic potential of isopentane vapor, policymakers and industry leaders can develop evidence-based standards for exposure limits and implement appropriate safety measures to protect workers and the general public.
In conclusion, the background and objectives of isopentane carcinogenicity research reflect a growing recognition of the need for comprehensive toxicological assessments of common industrial chemicals. The findings from these studies will not only contribute to our scientific knowledge but also have significant implications for public health, occupational safety, and environmental protection.
Market Analysis for Isopentane Applications
The market for isopentane applications has been experiencing significant growth in recent years, driven by its versatile properties and increasing demand across various industries. Isopentane, a branched-chain alkane with the molecular formula C5H12, finds extensive use as a blowing agent, aerosol propellant, and in the production of polystyrene foam.
In the automotive sector, isopentane is utilized in the manufacturing of lightweight components, contributing to improved fuel efficiency and reduced emissions. The growing emphasis on sustainable transportation solutions has led to an increased adoption of isopentane-based materials in vehicle production.
The construction industry represents another major market for isopentane applications. Its excellent insulation properties make it a preferred choice for producing foam insulation materials used in buildings and infrastructure projects. As energy efficiency regulations become more stringent globally, the demand for high-performance insulation materials containing isopentane is expected to rise.
The electronics industry also relies on isopentane for various applications, including the production of circuit boards and semiconductor cleaning. The rapid growth of the electronics sector, particularly in emerging economies, is likely to drive the demand for isopentane in this market segment.
Environmental concerns and regulations have played a crucial role in shaping the isopentane market. As a more environmentally friendly alternative to certain chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs), isopentane has gained traction in refrigeration and air conditioning applications. This shift towards greener solutions has opened up new opportunities for isopentane manufacturers and suppliers.
The global isopentane market is projected to witness steady growth in the coming years. Factors such as urbanization, industrialization, and the increasing focus on energy-efficient solutions are expected to drive demand across various end-use industries. However, the market faces challenges related to price volatility of raw materials and potential health and safety concerns associated with isopentane exposure.
Regional market dynamics play a significant role in the isopentane industry. Asia-Pacific is emerging as a key growth region, driven by rapid industrialization and infrastructure development in countries like China and India. North America and Europe continue to be important markets, with a strong focus on sustainable and energy-efficient solutions driving demand for isopentane-based products.
In the automotive sector, isopentane is utilized in the manufacturing of lightweight components, contributing to improved fuel efficiency and reduced emissions. The growing emphasis on sustainable transportation solutions has led to an increased adoption of isopentane-based materials in vehicle production.
The construction industry represents another major market for isopentane applications. Its excellent insulation properties make it a preferred choice for producing foam insulation materials used in buildings and infrastructure projects. As energy efficiency regulations become more stringent globally, the demand for high-performance insulation materials containing isopentane is expected to rise.
The electronics industry also relies on isopentane for various applications, including the production of circuit boards and semiconductor cleaning. The rapid growth of the electronics sector, particularly in emerging economies, is likely to drive the demand for isopentane in this market segment.
Environmental concerns and regulations have played a crucial role in shaping the isopentane market. As a more environmentally friendly alternative to certain chlorofluorocarbons (CFCs) and hydrochlorofluorocarbons (HCFCs), isopentane has gained traction in refrigeration and air conditioning applications. This shift towards greener solutions has opened up new opportunities for isopentane manufacturers and suppliers.
The global isopentane market is projected to witness steady growth in the coming years. Factors such as urbanization, industrialization, and the increasing focus on energy-efficient solutions are expected to drive demand across various end-use industries. However, the market faces challenges related to price volatility of raw materials and potential health and safety concerns associated with isopentane exposure.
Regional market dynamics play a significant role in the isopentane industry. Asia-Pacific is emerging as a key growth region, driven by rapid industrialization and infrastructure development in countries like China and India. North America and Europe continue to be important markets, with a strong focus on sustainable and energy-efficient solutions driving demand for isopentane-based products.
Current Understanding of Isopentane Vapor Carcinogenicity
The current understanding of isopentane vapor carcinogenicity is limited, with ongoing research efforts to elucidate its potential health impacts. Isopentane, a volatile organic compound (VOC) commonly used in various industrial applications, has been the subject of increasing scrutiny due to its widespread use and potential for human exposure.
Recent studies have focused on the inhalation toxicity of isopentane vapors, as this is the primary route of exposure in occupational and environmental settings. While acute toxicity data suggest relatively low immediate health risks, the long-term effects, particularly regarding carcinogenicity, remain a subject of debate among researchers and regulatory bodies.
Experimental studies on laboratory animals have provided mixed results. Some studies have reported no significant increase in tumor incidence following prolonged exposure to isopentane vapors, while others have observed subtle changes in cellular structures that could potentially lead to neoplastic transformations. These conflicting findings highlight the complexity of assessing the carcinogenic potential of isopentane in its vapor phase.
Mechanistic studies have explored the potential pathways through which isopentane vapors might exert carcinogenic effects. One hypothesis suggests that isopentane may act as a tumor promoter rather than a direct carcinogen, potentially enhancing the effects of other known carcinogens. This theory is based on observations of increased cellular proliferation and alterations in gene expression patterns following exposure to isopentane vapors.
Epidemiological data on human populations exposed to isopentane vapors are limited, making it challenging to draw definitive conclusions about its carcinogenic potential in real-world scenarios. Occupational health studies have not consistently demonstrated a clear link between isopentane exposure and increased cancer risk, but these studies often face limitations in exposure assessment and confounding factors.
Regulatory agencies have adopted a cautious approach in classifying isopentane's carcinogenicity. The International Agency for Research on Cancer (IARC) has not yet evaluated isopentane specifically, while other agencies have classified it as "not classifiable as to its carcinogenicity to humans" due to insufficient evidence.
Current research efforts are focused on developing more sensitive biomarkers of exposure and effect, as well as improving analytical methods for detecting and quantifying isopentane in environmental and biological samples. These advancements are expected to provide more accurate assessments of exposure levels and potential health risks associated with isopentane vapors.
In conclusion, while the current understanding of isopentane vapor carcinogenicity remains inconclusive, ongoing research continues to explore its potential health impacts. The scientific community acknowledges the need for more comprehensive studies to fully elucidate the long-term effects of isopentane vapor exposure and its potential role in carcinogenesis.
Recent studies have focused on the inhalation toxicity of isopentane vapors, as this is the primary route of exposure in occupational and environmental settings. While acute toxicity data suggest relatively low immediate health risks, the long-term effects, particularly regarding carcinogenicity, remain a subject of debate among researchers and regulatory bodies.
Experimental studies on laboratory animals have provided mixed results. Some studies have reported no significant increase in tumor incidence following prolonged exposure to isopentane vapors, while others have observed subtle changes in cellular structures that could potentially lead to neoplastic transformations. These conflicting findings highlight the complexity of assessing the carcinogenic potential of isopentane in its vapor phase.
Mechanistic studies have explored the potential pathways through which isopentane vapors might exert carcinogenic effects. One hypothesis suggests that isopentane may act as a tumor promoter rather than a direct carcinogen, potentially enhancing the effects of other known carcinogens. This theory is based on observations of increased cellular proliferation and alterations in gene expression patterns following exposure to isopentane vapors.
Epidemiological data on human populations exposed to isopentane vapors are limited, making it challenging to draw definitive conclusions about its carcinogenic potential in real-world scenarios. Occupational health studies have not consistently demonstrated a clear link between isopentane exposure and increased cancer risk, but these studies often face limitations in exposure assessment and confounding factors.
Regulatory agencies have adopted a cautious approach in classifying isopentane's carcinogenicity. The International Agency for Research on Cancer (IARC) has not yet evaluated isopentane specifically, while other agencies have classified it as "not classifiable as to its carcinogenicity to humans" due to insufficient evidence.
Current research efforts are focused on developing more sensitive biomarkers of exposure and effect, as well as improving analytical methods for detecting and quantifying isopentane in environmental and biological samples. These advancements are expected to provide more accurate assessments of exposure levels and potential health risks associated with isopentane vapors.
In conclusion, while the current understanding of isopentane vapor carcinogenicity remains inconclusive, ongoing research continues to explore its potential health impacts. The scientific community acknowledges the need for more comprehensive studies to fully elucidate the long-term effects of isopentane vapor exposure and its potential role in carcinogenesis.
Existing Methods for Assessing Vapor Phase Carcinogenicity
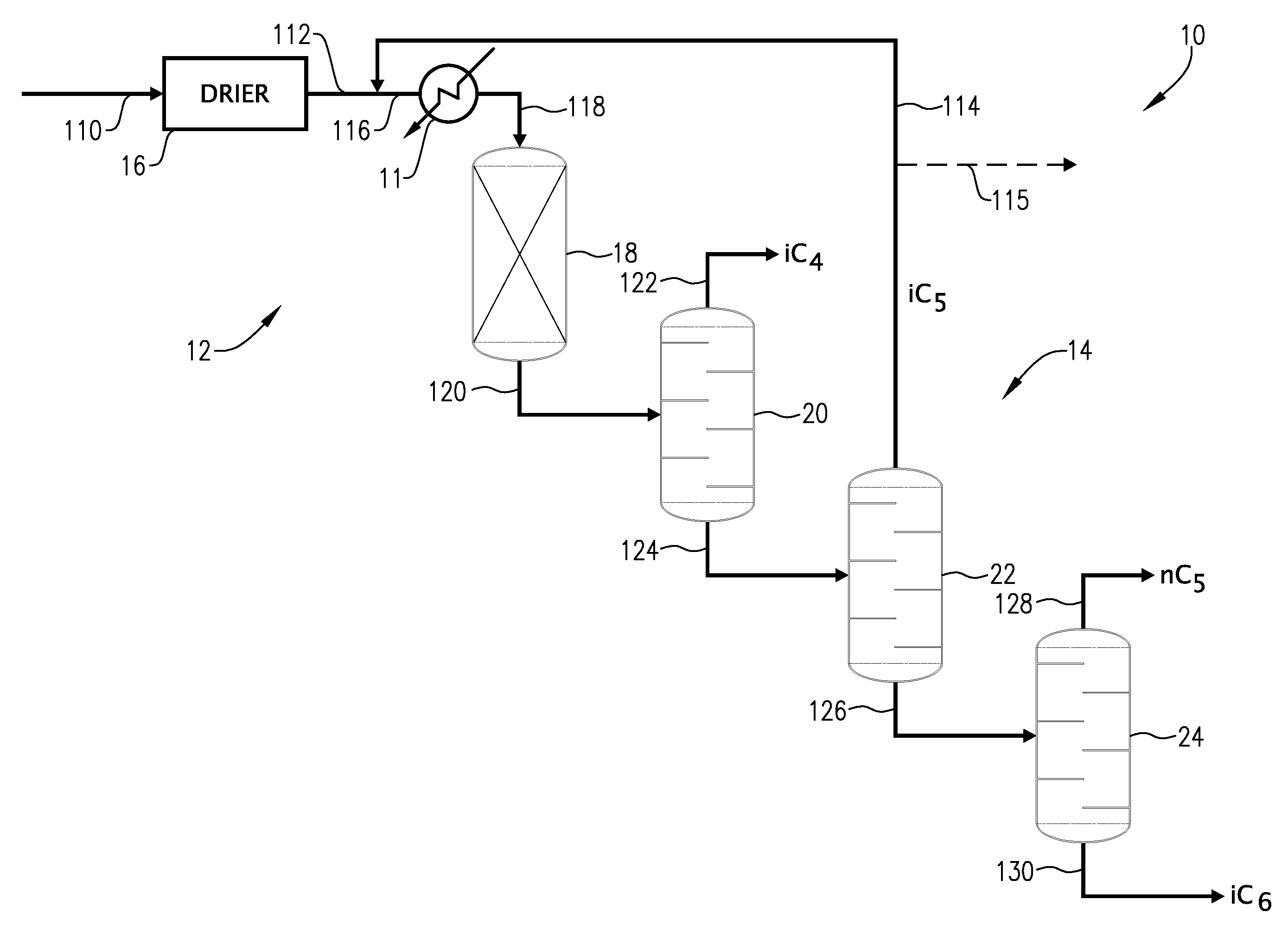
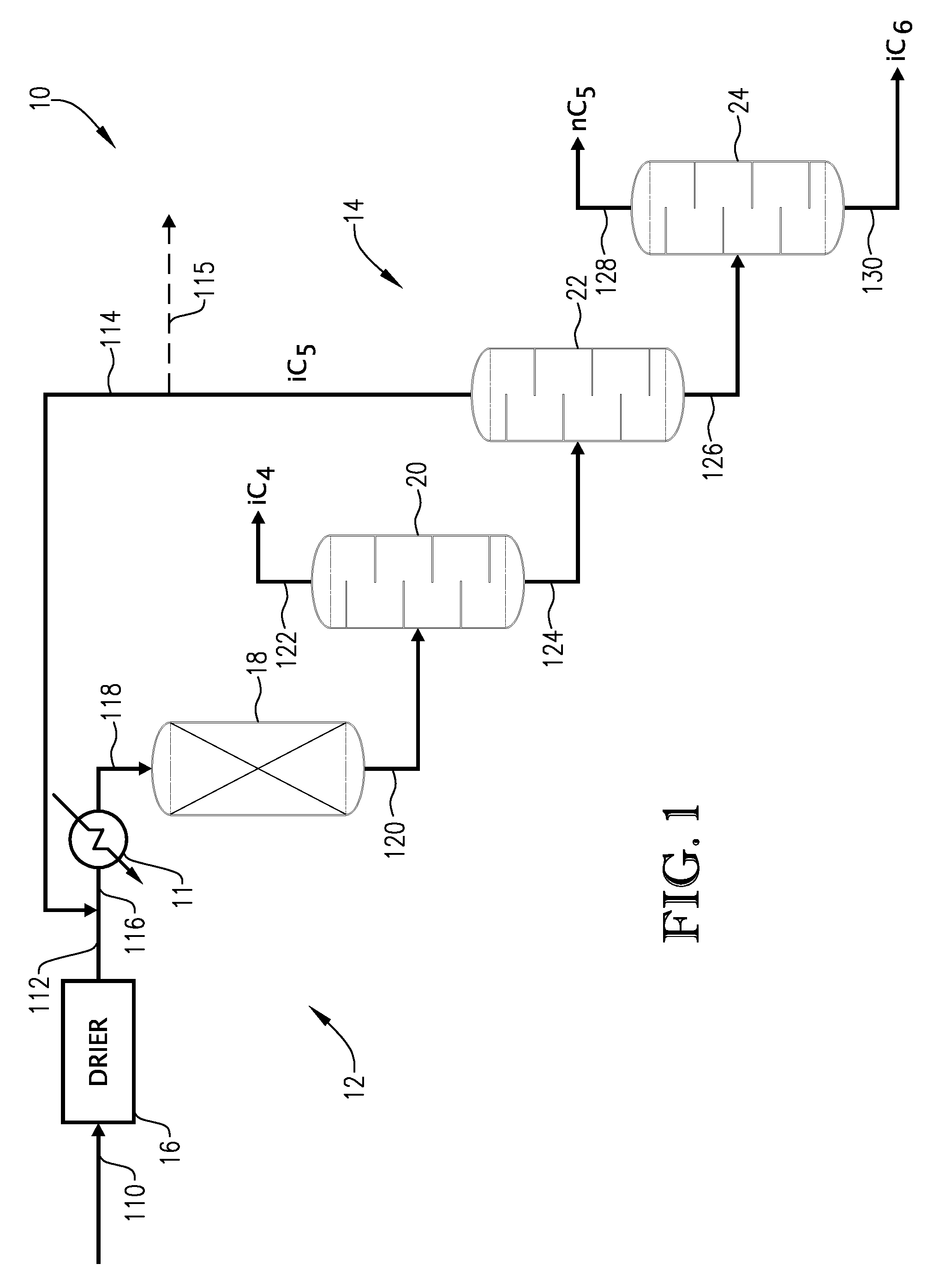
01 Isopentane in chemical processes
Isopentane is used in various chemical processes, including as a reactant or solvent in organic synthesis, polymerization reactions, and petrochemical applications. While its use is widespread, the potential carcinogenicity of isopentane in these processes is not explicitly addressed, suggesting that it may not be a significant concern in controlled industrial settings.- Isopentane in chemical processes: Isopentane is used in various chemical processes, including as a reactant or solvent in organic synthesis, polymerization, and catalytic reactions. While its use in these processes does not directly address carcinogenicity, it highlights the widespread industrial applications of isopentane, which may contribute to potential exposure risks.
- Safety considerations in isopentane handling: Due to its flammability and volatility, special safety measures are required when handling isopentane in industrial settings. This includes the use of specialized equipment, storage facilities, and handling procedures to minimize the risk of fire, explosion, and worker exposure. These safety considerations indirectly relate to potential health risks, including carcinogenicity.
- Isopentane in fuel compositions: Isopentane is used as a component in various fuel compositions, including gasoline and specialized fuels. While the focus is on fuel properties rather than carcinogenicity, the widespread use of isopentane in fuels may contribute to environmental and occupational exposure, which could be relevant to long-term health effects studies.
- Purification and analysis of isopentane: Methods for purifying and analyzing isopentane are important for ensuring its quality and purity in various applications. These processes may involve techniques such as distillation, chromatography, and spectroscopic analysis. While not directly addressing carcinogenicity, high-purity isopentane may be used in toxicological studies or other research related to its potential health effects.
- Environmental impact of isopentane: The use and release of isopentane in industrial processes and products can have environmental implications. This includes its potential as a volatile organic compound (VOC) and its role in atmospheric chemistry. While not directly related to carcinogenicity, understanding the environmental fate and transport of isopentane is important for assessing potential routes of human exposure and ecological impacts.
02 Environmental and safety considerations
The use of isopentane in industrial applications requires careful handling and safety measures due to its flammability and potential environmental impact. While carcinogenicity is not specifically mentioned, the focus on safety protocols and environmental protection suggests that any potential health risks, including carcinogenicity, are managed through proper handling and exposure control.Expand Specific Solutions03 Isopentane in fuel compositions
Isopentane is used as a component in various fuel compositions, including gasoline blends and alternative fuels. The focus on fuel efficiency and performance suggests that the potential carcinogenicity of isopentane in these applications is not a primary concern, possibly due to its rapid combustion and limited exposure during normal use.Expand Specific Solutions04 Isopentane in manufacturing processes
Isopentane is utilized in manufacturing processes for various products, including polymers, foams, and industrial materials. The emphasis on process optimization and product quality suggests that the potential carcinogenicity of isopentane is not a significant factor in these applications, possibly due to its incorporation into final products or its complete removal during processing.Expand Specific Solutions05 Isopentane in separation and purification
Isopentane is used in separation and purification processes, particularly in the petrochemical industry. The focus on efficiency and purity in these applications suggests that the potential carcinogenicity of isopentane is not a primary concern, possibly due to its volatile nature and the controlled environments in which these processes occur.Expand Specific Solutions
Key Players in Isopentane Production and Research
The competitive landscape for "Isopentane's Influence on Carcinogenicity in Vapor Phases" is in its early developmental stage, with a relatively small market size due to its specialized nature. The technology is still emerging, with varying levels of maturity among key players. Companies like ExxonMobil Chemical Patents, Inc. and Phillips 66 are likely at the forefront, leveraging their extensive experience in petrochemicals. Academic institutions such as New York University and the University of Pennsylvania are contributing to foundational research. Regulatory bodies and research organizations like the Naval Research Laboratory may play crucial roles in shaping safety standards and applications. As the field evolves, collaboration between industry and academia will be essential for advancing understanding and potential commercial applications.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed advanced analytical techniques to study the influence of isopentane on carcinogenicity in vapor phases. Their approach involves using high-resolution gas chromatography coupled with mass spectrometry (GC-MS) to accurately identify and quantify isopentane and its potential carcinogenic byproducts in complex vapor mixtures[1]. They have also implemented a novel in vitro cell exposure system that allows for controlled exposure of human lung epithelial cells to isopentane vapors, enabling the assessment of DNA damage and cellular transformation[3]. Additionally, ExxonMobil has conducted long-term inhalation studies on animal models to evaluate the chronic effects of isopentane exposure, utilizing state-of-the-art biomarkers and advanced imaging techniques to detect early signs of carcinogenesis[5].
Strengths: Comprehensive analytical capabilities, advanced in vitro and in vivo testing methods, and extensive experience in petrochemical research. Weaknesses: Potential bias due to vested interests in petrochemical industry, limited focus on alternative energy sources.
The Regents of the University of California
Technical Solution: The University of California has established a multidisciplinary research program to investigate isopentane's influence on carcinogenicity in vapor phases. Their approach combines expertise from chemistry, toxicology, and environmental health sciences. The team has developed a novel atmospheric simulation chamber that can recreate complex urban air mixtures containing isopentane and other volatile organic compounds, allowing for the study of realistic exposure scenarios[13]. They have also implemented advanced epigenomic profiling techniques to identify potential carcinogenic mechanisms induced by isopentane vapor exposure at the molecular level[15]. Additionally, the university has conducted longitudinal epidemiological studies in communities with high ambient isopentane levels, utilizing innovative biomonitoring techniques and spatial modeling to assess long-term health impacts[17].
Strengths: Multidisciplinary approach, advanced atmospheric simulation capabilities, and integration of molecular and population-level studies. Weaknesses: Potential limitations in scaling up research findings to industrial applications, dependence on grant funding for long-term studies.
Critical Studies on Isopentane's Carcinogenic Potential
Disproportionation of isopentane
PatentActiveUS20080021254A1
Innovation
- A process for disproportionating isopentane into isobutane and isohexanes using a catalyst composition comprising at least 80 weight percent aluminum halide on a support, which allows for the conversion of isopentane into lower RVP products that can be more easily blended into motor fuels, thereby addressing the excess inventory issue.
Reduction of carbon dioxide emission during isoprene production by fermentation
PatentInactiveEP2337844A1
Innovation
- Development of cells with heterologous nucleic acid encoding an isoprene synthase polypeptide, cultured in media with uncommon carbon sources, which reduces carbon dioxide emission and increases isoprene production efficiency, achieving yields greater than 400 nmole/gwcm/hr with a carbon dioxide to isoprene ratio less than 500.
Regulatory Framework for Volatile Organic Compounds
The regulatory framework for volatile organic compounds (VOCs) plays a crucial role in addressing the potential health and environmental risks associated with isopentane and other similar substances. In the United States, the Environmental Protection Agency (EPA) is the primary regulatory body responsible for establishing and enforcing standards related to VOCs. The Clean Air Act serves as the foundation for these regulations, empowering the EPA to set National Ambient Air Quality Standards (NAAQS) and implement various control measures.
Under the Clean Air Act, isopentane is classified as a VOC due to its high vapor pressure and potential to contribute to ground-level ozone formation. The EPA has established specific emission limits and control requirements for industrial processes that involve the use or production of isopentane and other VOCs. These regulations often mandate the implementation of best available control technologies (BACT) to minimize emissions and protect public health.
At the state level, many jurisdictions have adopted their own VOC regulations, which may be more stringent than federal standards. For example, California's Air Resources Board (CARB) has implemented stringent VOC regulations that affect a wide range of products and industries. These state-level regulations often focus on specific sectors or applications where isopentane and other VOCs are commonly used, such as in the production of foam insulation or as refrigerants.
Internationally, the regulatory landscape for VOCs varies significantly. The European Union has established the VOC Solvents Emissions Directive, which sets limits on VOC emissions from certain industrial activities. This directive includes specific provisions for the use of organic solvents in various processes, potentially affecting the use of isopentane in certain applications. Other countries, such as Japan and Canada, have also implemented their own VOC regulations, often focusing on specific industries or emission sources.
In recent years, there has been a growing emphasis on addressing the potential carcinogenicity of VOCs in vapor phases. This has led to increased scrutiny of substances like isopentane and efforts to better understand their long-term health effects. As a result, regulatory bodies are continuously reviewing and updating their frameworks to incorporate new scientific findings and risk assessments.
The regulatory approach to VOCs, including isopentane, often involves a combination of emission limits, monitoring requirements, and reporting obligations. Many regulations require facilities to implement leak detection and repair (LDAR) programs to identify and address fugitive emissions. Additionally, some jurisdictions have implemented permitting systems that require facilities to obtain specific authorizations before engaging in activities that may release significant quantities of VOCs.
As concerns about air quality and public health continue to grow, it is likely that the regulatory framework for VOCs will evolve. This may include more stringent emission limits, expanded monitoring requirements, and increased focus on specific compounds of concern, such as isopentane. Industry stakeholders and researchers will need to stay informed about these regulatory developments and their potential implications for various applications and processes involving VOCs.
Under the Clean Air Act, isopentane is classified as a VOC due to its high vapor pressure and potential to contribute to ground-level ozone formation. The EPA has established specific emission limits and control requirements for industrial processes that involve the use or production of isopentane and other VOCs. These regulations often mandate the implementation of best available control technologies (BACT) to minimize emissions and protect public health.
At the state level, many jurisdictions have adopted their own VOC regulations, which may be more stringent than federal standards. For example, California's Air Resources Board (CARB) has implemented stringent VOC regulations that affect a wide range of products and industries. These state-level regulations often focus on specific sectors or applications where isopentane and other VOCs are commonly used, such as in the production of foam insulation or as refrigerants.
Internationally, the regulatory landscape for VOCs varies significantly. The European Union has established the VOC Solvents Emissions Directive, which sets limits on VOC emissions from certain industrial activities. This directive includes specific provisions for the use of organic solvents in various processes, potentially affecting the use of isopentane in certain applications. Other countries, such as Japan and Canada, have also implemented their own VOC regulations, often focusing on specific industries or emission sources.
In recent years, there has been a growing emphasis on addressing the potential carcinogenicity of VOCs in vapor phases. This has led to increased scrutiny of substances like isopentane and efforts to better understand their long-term health effects. As a result, regulatory bodies are continuously reviewing and updating their frameworks to incorporate new scientific findings and risk assessments.
The regulatory approach to VOCs, including isopentane, often involves a combination of emission limits, monitoring requirements, and reporting obligations. Many regulations require facilities to implement leak detection and repair (LDAR) programs to identify and address fugitive emissions. Additionally, some jurisdictions have implemented permitting systems that require facilities to obtain specific authorizations before engaging in activities that may release significant quantities of VOCs.
As concerns about air quality and public health continue to grow, it is likely that the regulatory framework for VOCs will evolve. This may include more stringent emission limits, expanded monitoring requirements, and increased focus on specific compounds of concern, such as isopentane. Industry stakeholders and researchers will need to stay informed about these regulatory developments and their potential implications for various applications and processes involving VOCs.
Environmental Impact of Isopentane Emissions
The environmental impact of isopentane emissions is a critical concern in the context of its potential carcinogenicity in vapor phases. Isopentane, a volatile organic compound (VOC), is widely used in various industrial processes and consumer products. When released into the atmosphere, it contributes to the formation of ground-level ozone and photochemical smog, which can have significant adverse effects on air quality and human health.
Isopentane emissions primarily occur during its production, storage, transportation, and use in industrial applications. The petroleum and chemical industries are major sources of these emissions, particularly in refineries and petrochemical plants. Additionally, consumer products such as aerosols, solvents, and foam blowing agents can release isopentane into the environment during use and disposal.
In the atmosphere, isopentane undergoes photochemical reactions, contributing to the formation of secondary organic aerosols (SOA). These aerosols can impact climate by altering cloud formation processes and affecting the Earth's radiative balance. Furthermore, the breakdown of isopentane in the atmosphere can lead to the production of other potentially harmful compounds, including formaldehyde and acetone.
The persistence of isopentane in the environment is relatively short, with an atmospheric lifetime of a few days. However, its continuous release from various sources maintains its presence in the air. In urban areas with high industrial activity, isopentane concentrations can be significantly elevated, potentially leading to localized air quality issues and increased exposure risks for nearby populations.
Aquatic ecosystems can also be affected by isopentane emissions. When released into water bodies, isopentane can form a surface film, potentially interfering with oxygen transfer and impacting aquatic life. Although it has low water solubility, isopentane can still pose risks to aquatic organisms through direct contact or ingestion.
Efforts to mitigate the environmental impact of isopentane emissions include improved industrial processes, enhanced emission control technologies, and stricter regulations on VOC emissions. Many countries have implemented measures to reduce isopentane and other VOC emissions from industrial sources and consumer products. These efforts aim to improve air quality, reduce smog formation, and minimize potential health risks associated with isopentane exposure.
Research into alternative compounds with lower environmental impacts is ongoing, particularly in industries where isopentane is heavily used. This includes exploring more environmentally friendly blowing agents for foam production and developing less volatile solvents for industrial applications. Such innovations are crucial for reducing the overall environmental footprint of industrial processes while maintaining product performance and efficiency.
Isopentane emissions primarily occur during its production, storage, transportation, and use in industrial applications. The petroleum and chemical industries are major sources of these emissions, particularly in refineries and petrochemical plants. Additionally, consumer products such as aerosols, solvents, and foam blowing agents can release isopentane into the environment during use and disposal.
In the atmosphere, isopentane undergoes photochemical reactions, contributing to the formation of secondary organic aerosols (SOA). These aerosols can impact climate by altering cloud formation processes and affecting the Earth's radiative balance. Furthermore, the breakdown of isopentane in the atmosphere can lead to the production of other potentially harmful compounds, including formaldehyde and acetone.
The persistence of isopentane in the environment is relatively short, with an atmospheric lifetime of a few days. However, its continuous release from various sources maintains its presence in the air. In urban areas with high industrial activity, isopentane concentrations can be significantly elevated, potentially leading to localized air quality issues and increased exposure risks for nearby populations.
Aquatic ecosystems can also be affected by isopentane emissions. When released into water bodies, isopentane can form a surface film, potentially interfering with oxygen transfer and impacting aquatic life. Although it has low water solubility, isopentane can still pose risks to aquatic organisms through direct contact or ingestion.
Efforts to mitigate the environmental impact of isopentane emissions include improved industrial processes, enhanced emission control technologies, and stricter regulations on VOC emissions. Many countries have implemented measures to reduce isopentane and other VOC emissions from industrial sources and consumer products. These efforts aim to improve air quality, reduce smog formation, and minimize potential health risks associated with isopentane exposure.
Research into alternative compounds with lower environmental impacts is ongoing, particularly in industries where isopentane is heavily used. This includes exploring more environmentally friendly blowing agents for foam production and developing less volatile solvents for industrial applications. Such innovations are crucial for reducing the overall environmental footprint of industrial processes while maintaining product performance and efficiency.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!