Isopentane’s Use in Supercritical Fluid Technologies
JUL 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isopentane SCF Tech Evolution and Objectives
Isopentane's role in supercritical fluid technologies has evolved significantly over the past few decades, marking a transformative journey in the field of green chemistry and sustainable industrial processes. This branched alkane, with its unique properties, has emerged as a promising alternative in various supercritical fluid applications, offering advantages over traditional solvents and fluids.
The evolution of isopentane in supercritical fluid technologies can be traced back to the early 1990s when researchers began exploring its potential as a substitute for conventional supercritical fluids like carbon dioxide. Initial studies focused on its critical properties, which were found to be more favorable for certain applications compared to CO2. The critical temperature of isopentane (187.2°C) and critical pressure (33.8 bar) offered a wider operating window for supercritical processes, particularly in areas where CO2's critical conditions were limiting.
As environmental concerns grew in the late 1990s and early 2000s, the drive for greener solvents intensified. Isopentane, being non-toxic and having a relatively low global warming potential, aligned well with these sustainability goals. This period saw an increase in research activities exploring isopentane's applicability in various supercritical fluid processes, including extraction, reaction media, and material processing.
The technological objectives for isopentane in supercritical fluid applications have evolved alongside its development. Initially, the focus was on establishing its basic feasibility as a supercritical fluid. This quickly expanded to optimizing process conditions and exploring its potential in diverse applications. Current objectives include enhancing the efficiency of isopentane-based supercritical processes, developing novel applications, and integrating these technologies into existing industrial frameworks.
One of the key technological goals is to leverage isopentane's unique properties for selective extraction processes. Its non-polar nature makes it particularly suitable for extracting lipophilic compounds from natural matrices, a capability that is being explored in the pharmaceutical and nutraceutical industries. Another objective is to utilize isopentane's lower critical parameters to develop energy-efficient supercritical processes, potentially reducing the overall carbon footprint of industrial operations.
In the realm of materials science, researchers are aiming to exploit isopentane's supercritical properties for the synthesis and modification of advanced materials. This includes the development of porous materials, nanoparticles, and specialized coatings. The goal is to achieve precise control over material properties by manipulating the supercritical conditions of isopentane.
Looking forward, the technological trajectory for isopentane in supercritical fluid technologies is focused on scalability and industrial integration. Efforts are underway to design and optimize large-scale processes that can harness the benefits of supercritical isopentane while addressing challenges related to safety, cost-effectiveness, and process control. The ultimate objective is to position isopentane-based supercritical fluid technologies as a viable, sustainable alternative in various industrial sectors, from chemical processing to energy applications.
The evolution of isopentane in supercritical fluid technologies can be traced back to the early 1990s when researchers began exploring its potential as a substitute for conventional supercritical fluids like carbon dioxide. Initial studies focused on its critical properties, which were found to be more favorable for certain applications compared to CO2. The critical temperature of isopentane (187.2°C) and critical pressure (33.8 bar) offered a wider operating window for supercritical processes, particularly in areas where CO2's critical conditions were limiting.
As environmental concerns grew in the late 1990s and early 2000s, the drive for greener solvents intensified. Isopentane, being non-toxic and having a relatively low global warming potential, aligned well with these sustainability goals. This period saw an increase in research activities exploring isopentane's applicability in various supercritical fluid processes, including extraction, reaction media, and material processing.
The technological objectives for isopentane in supercritical fluid applications have evolved alongside its development. Initially, the focus was on establishing its basic feasibility as a supercritical fluid. This quickly expanded to optimizing process conditions and exploring its potential in diverse applications. Current objectives include enhancing the efficiency of isopentane-based supercritical processes, developing novel applications, and integrating these technologies into existing industrial frameworks.
One of the key technological goals is to leverage isopentane's unique properties for selective extraction processes. Its non-polar nature makes it particularly suitable for extracting lipophilic compounds from natural matrices, a capability that is being explored in the pharmaceutical and nutraceutical industries. Another objective is to utilize isopentane's lower critical parameters to develop energy-efficient supercritical processes, potentially reducing the overall carbon footprint of industrial operations.
In the realm of materials science, researchers are aiming to exploit isopentane's supercritical properties for the synthesis and modification of advanced materials. This includes the development of porous materials, nanoparticles, and specialized coatings. The goal is to achieve precise control over material properties by manipulating the supercritical conditions of isopentane.
Looking forward, the technological trajectory for isopentane in supercritical fluid technologies is focused on scalability and industrial integration. Efforts are underway to design and optimize large-scale processes that can harness the benefits of supercritical isopentane while addressing challenges related to safety, cost-effectiveness, and process control. The ultimate objective is to position isopentane-based supercritical fluid technologies as a viable, sustainable alternative in various industrial sectors, from chemical processing to energy applications.
Market Demand for Isopentane-based SCF Applications
The market demand for isopentane-based supercritical fluid (SCF) applications has been steadily growing across various industries. This growth is primarily driven by the unique properties of isopentane when used in supercritical fluid technologies, offering advantages over traditional solvents and processes.
In the pharmaceutical industry, isopentane-based SCF applications have gained significant traction for drug delivery systems and particle engineering. The ability to control particle size and morphology using supercritical fluid technology has led to improved bioavailability and efficacy of pharmaceutical products. This has resulted in a surge in demand for isopentane-based SCF processes in drug formulation and development.
The food and beverage sector has also shown increasing interest in isopentane-based SCF applications. The technology's potential for extracting natural compounds, such as flavors, fragrances, and nutraceuticals, without leaving harmful residues has made it an attractive option for manufacturers seeking clean-label products. This trend aligns with the growing consumer demand for natural and organic food ingredients.
In the cosmetics and personal care industry, isopentane-based SCF technologies have found applications in the production of high-quality extracts and the development of novel formulations. The ability to process heat-sensitive materials and create micro and nanoparticles has opened up new possibilities for product innovation, driving market demand in this sector.
The environmental benefits of isopentane-based SCF applications have also contributed to their increasing adoption. As industries face stricter regulations on emissions and waste reduction, the use of supercritical fluids as a green alternative to traditional organic solvents has become more appealing. This has led to a growing market for isopentane-based SCF technologies in waste treatment and recycling applications.
The electronics industry has shown interest in isopentane-based SCF applications for cleaning and drying delicate components. The technology's ability to penetrate small spaces and remove contaminants without damaging sensitive parts has made it valuable in the production of high-precision electronic devices.
As research and development efforts continue to expand the potential applications of isopentane-based SCF technologies, new market opportunities are emerging. These include advanced materials processing, nanotechnology, and biotechnology, where the unique properties of supercritical fluids can enable novel processes and products.
In the pharmaceutical industry, isopentane-based SCF applications have gained significant traction for drug delivery systems and particle engineering. The ability to control particle size and morphology using supercritical fluid technology has led to improved bioavailability and efficacy of pharmaceutical products. This has resulted in a surge in demand for isopentane-based SCF processes in drug formulation and development.
The food and beverage sector has also shown increasing interest in isopentane-based SCF applications. The technology's potential for extracting natural compounds, such as flavors, fragrances, and nutraceuticals, without leaving harmful residues has made it an attractive option for manufacturers seeking clean-label products. This trend aligns with the growing consumer demand for natural and organic food ingredients.
In the cosmetics and personal care industry, isopentane-based SCF technologies have found applications in the production of high-quality extracts and the development of novel formulations. The ability to process heat-sensitive materials and create micro and nanoparticles has opened up new possibilities for product innovation, driving market demand in this sector.
The environmental benefits of isopentane-based SCF applications have also contributed to their increasing adoption. As industries face stricter regulations on emissions and waste reduction, the use of supercritical fluids as a green alternative to traditional organic solvents has become more appealing. This has led to a growing market for isopentane-based SCF technologies in waste treatment and recycling applications.
The electronics industry has shown interest in isopentane-based SCF applications for cleaning and drying delicate components. The technology's ability to penetrate small spaces and remove contaminants without damaging sensitive parts has made it valuable in the production of high-precision electronic devices.
As research and development efforts continue to expand the potential applications of isopentane-based SCF technologies, new market opportunities are emerging. These include advanced materials processing, nanotechnology, and biotechnology, where the unique properties of supercritical fluids can enable novel processes and products.
Current State and Challenges in Isopentane SCF Technology
Isopentane's application in supercritical fluid (SCF) technologies has gained significant attention in recent years due to its unique properties and potential advantages over traditional solvents. The current state of isopentane SCF technology is characterized by a growing body of research and pilot-scale applications, particularly in extraction processes and as a reaction medium.
One of the primary advantages of isopentane as a supercritical fluid is its relatively low critical point (187.2°C, 3.378 MPa), which allows for easier and more energy-efficient operation compared to other SCF solvents like carbon dioxide. This property has led to increased interest in using isopentane SCF for the extraction of heat-sensitive compounds and in applications where lower processing temperatures are desirable.
In the field of extraction, isopentane SCF has shown promising results in the recovery of valuable compounds from natural sources. Studies have demonstrated its effectiveness in extracting essential oils, lipids, and bioactive compounds from various plant materials. The non-polar nature of isopentane makes it particularly suitable for extracting lipophilic substances, offering an alternative to traditional organic solvent extraction methods.
Despite these advancements, the widespread adoption of isopentane SCF technology faces several challenges. Safety concerns related to the flammability of isopentane pose a significant hurdle in industrial-scale applications. Stringent safety measures and specialized equipment are required to mitigate the risk of fire and explosion, which can increase operational costs and complexity.
Another challenge lies in the limited solubility of polar compounds in isopentane SCF. While effective for non-polar substances, its application in extracting or processing polar compounds is restricted. This limitation has prompted research into the use of co-solvents or modifiers to enhance the solvating power of isopentane SCF for a broader range of compounds.
The environmental impact of isopentane SCF technology is also a subject of ongoing assessment. Although isopentane is considered less harmful to the ozone layer compared to some chlorofluorocarbons, its potential contribution to greenhouse gas emissions and air quality issues needs careful consideration and management.
Scaling up isopentane SCF processes from laboratory to industrial levels presents additional challenges. Optimizing process parameters, ensuring consistent product quality, and developing cost-effective separation and purification techniques for large-scale operations are areas that require further research and development.
Regulatory hurdles also play a role in the current state of isopentane SCF technology. The use of isopentane in food and pharmaceutical applications is subject to strict regulations, which can vary across different regions. Obtaining necessary approvals and ensuring compliance with safety and quality standards are crucial steps in the commercialization of isopentane SCF processes.
One of the primary advantages of isopentane as a supercritical fluid is its relatively low critical point (187.2°C, 3.378 MPa), which allows for easier and more energy-efficient operation compared to other SCF solvents like carbon dioxide. This property has led to increased interest in using isopentane SCF for the extraction of heat-sensitive compounds and in applications where lower processing temperatures are desirable.
In the field of extraction, isopentane SCF has shown promising results in the recovery of valuable compounds from natural sources. Studies have demonstrated its effectiveness in extracting essential oils, lipids, and bioactive compounds from various plant materials. The non-polar nature of isopentane makes it particularly suitable for extracting lipophilic substances, offering an alternative to traditional organic solvent extraction methods.
Despite these advancements, the widespread adoption of isopentane SCF technology faces several challenges. Safety concerns related to the flammability of isopentane pose a significant hurdle in industrial-scale applications. Stringent safety measures and specialized equipment are required to mitigate the risk of fire and explosion, which can increase operational costs and complexity.
Another challenge lies in the limited solubility of polar compounds in isopentane SCF. While effective for non-polar substances, its application in extracting or processing polar compounds is restricted. This limitation has prompted research into the use of co-solvents or modifiers to enhance the solvating power of isopentane SCF for a broader range of compounds.
The environmental impact of isopentane SCF technology is also a subject of ongoing assessment. Although isopentane is considered less harmful to the ozone layer compared to some chlorofluorocarbons, its potential contribution to greenhouse gas emissions and air quality issues needs careful consideration and management.
Scaling up isopentane SCF processes from laboratory to industrial levels presents additional challenges. Optimizing process parameters, ensuring consistent product quality, and developing cost-effective separation and purification techniques for large-scale operations are areas that require further research and development.
Regulatory hurdles also play a role in the current state of isopentane SCF technology. The use of isopentane in food and pharmaceutical applications is subject to strict regulations, which can vary across different regions. Obtaining necessary approvals and ensuring compliance with safety and quality standards are crucial steps in the commercialization of isopentane SCF processes.
Existing Isopentane SCF Solutions and Implementations
01 Production and purification of isopentane
Various methods for producing and purifying isopentane are described. These include processes for separating isopentane from other hydrocarbons, as well as techniques for improving the purity of isopentane. The methods often involve distillation, extraction, or other separation techniques to isolate isopentane from mixtures.- Production and purification of isopentane: Various methods are employed for the production and purification of isopentane, including isomerization of normal pentane, separation from natural gas liquids, and distillation processes. These techniques aim to obtain high-purity isopentane for industrial applications.
- Use of isopentane in polymer production: Isopentane is utilized as a blowing agent or solvent in the production of various polymers, particularly in the manufacture of expandable polystyrene (EPS) and other foam materials. It contributes to the formation of cellular structures in these materials.
- Isopentane in refrigeration and heat transfer applications: Isopentane is employed as a refrigerant or heat transfer fluid in various cooling systems and heat pumps. Its thermodynamic properties make it suitable for use in both industrial and residential applications, offering energy efficiency benefits.
- Isopentane in fuel compositions: Isopentane is used as a component in fuel formulations, particularly for improving the octane rating of gasoline and enhancing cold-start performance in internal combustion engines. It may also be used in alternative fuel blends.
- Safety and environmental considerations for isopentane handling: Due to its flammability and volatility, special safety measures and equipment are required for the storage, transportation, and handling of isopentane. Environmental regulations also govern its use to minimize emissions and potential ecological impacts.
02 Use of isopentane in chemical processes
Isopentane is utilized in various chemical processes as a reactant, solvent, or intermediate. It plays a role in the production of other chemicals and materials, including polymers and petrochemicals. The compound's properties make it suitable for use in diverse industrial applications.Expand Specific Solutions03 Isopentane in refrigeration and heat transfer applications
Isopentane is employed in refrigeration systems and heat transfer applications due to its thermodynamic properties. It serves as a refrigerant or heat transfer fluid in various cooling and heating systems, offering advantages in terms of efficiency and environmental impact.Expand Specific Solutions04 Isopentane in fuel compositions
Expand Specific Solutions05 Safety and handling of isopentane
Due to its flammability and volatility, special considerations are required for the safe handling, storage, and transportation of isopentane. This includes the design of appropriate equipment, implementation of safety measures, and adherence to regulatory guidelines to minimize risks associated with its use.Expand Specific Solutions
Key Players in Isopentane SCF Industry
The use of isopentane in supercritical fluid technologies is an emerging field with significant potential for growth. The market is in its early stages of development, characterized by ongoing research and pilot applications across various industries. While the exact market size is not well-defined, it is expected to expand rapidly as the technology matures. Companies like SABIC Global Technologies BV, DuPont de Nemours, Inc., and ExxonMobil Chemical Patents, Inc. are at the forefront of developing and implementing this technology. The technical maturity is still evolving, with academic institutions such as Beijing University of Chemical Technology and Zhejiang University contributing to fundamental research. As the technology progresses, collaborations between industry leaders and research institutions are likely to drive innovation and commercialization in this field.
ExxonMobil Chemical Patents, Inc.
Technical Solution: ExxonMobil has developed advanced supercritical fluid extraction (SFE) processes utilizing isopentane as a co-solvent. Their technology employs a mixture of supercritical CO2 and isopentane to enhance the extraction efficiency of various compounds from natural materials. The process operates at lower pressures (around 100-150 bar) compared to traditional SFE methods, reducing energy consumption[1]. ExxonMobil's approach also incorporates a novel fractionation system that allows for selective separation of extracted components based on their solubility in the isopentane-CO2 mixture[2]. This technology has been particularly successful in extracting high-value compounds from biomass and in purifying specialty chemicals.
Strengths: Lower operating pressures, improved extraction efficiency, and selective fractionation capabilities. Weaknesses: May require additional safety measures due to isopentane's flammability, and potential environmental concerns related to VOC emissions.
BASF Corp.
Technical Solution: BASF has pioneered the use of isopentane in supercritical fluid chromatography (SFC) for pharmaceutical applications. Their technology utilizes isopentane as a modifier in supercritical CO2 mobile phases to enhance the separation of complex drug molecules and their intermediates. BASF's SFC system operates at pressures between 100-300 bar and temperatures around 40-60°C, optimizing the balance between solvating power and selectivity[3]. The company has also developed specialized stationary phases that work synergistically with the isopentane-modified supercritical CO2, enabling high-resolution separations of chiral compounds and structurally similar molecules[4]. This technology has significantly improved the efficiency of drug discovery and quality control processes in the pharmaceutical industry.
Strengths: High-resolution separations, particularly for chiral compounds; reduced solvent consumption compared to traditional HPLC. Weaknesses: Higher initial equipment costs; requires specialized training for operators.
Core Innovations in Isopentane SCF Research
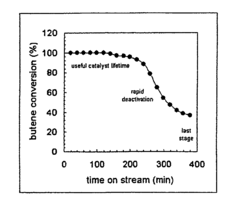
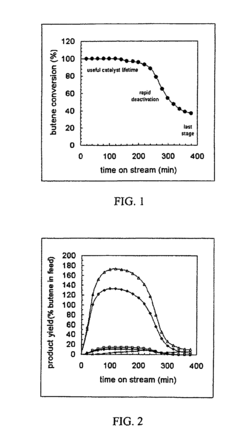
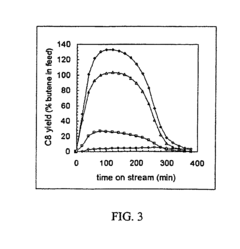
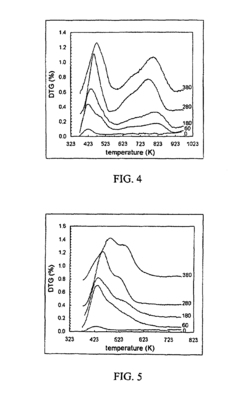
Enhancement of alkylation catalysts for improved supercritical fluid regeneration
PatentInactiveUS7592282B2
Innovation
- The method involves modifying the alkylation catalyst by selectively poisoning strongly acidic active sites, adjusting the pore size distribution, and reducing the number of strongly acidic sites to prevent the formation of condensed hydrocarbon species, thereby enhancing regeneration efficiency and catalyst longevity.
Using supercritical fluids and/or dense fluids in semiconductor applications
PatentInactiveUS20040198066A1
Innovation
- The use of supercritical fluids and dense fluids, such as carbon dioxide, in semiconductor applications for drying, cleaning, repairing low-k material layers, and stripping photoresist layers, with processing chambers designed to manage temperature and pressure conditions to optimize fluid properties for efficient processing without the need for vacuum bakes or wet cleans.
Environmental Impact of Isopentane SCF Processes
The environmental impact of isopentane in supercritical fluid (SCF) processes is a critical consideration for the sustainable implementation of this technology. Isopentane, when used as a supercritical fluid, offers several environmental advantages over traditional solvents and processes. However, it also presents certain challenges that must be carefully managed.
One of the primary environmental benefits of using isopentane in SCF processes is its lower global warming potential compared to many conventional solvents. Isopentane has a relatively short atmospheric lifetime and a lower ozone depletion potential, making it a more environmentally friendly option for industrial applications. This characteristic aligns well with global efforts to reduce greenhouse gas emissions and combat climate change.
In terms of waste reduction, isopentane SCF processes often result in less hazardous waste generation compared to traditional solvent-based methods. The high efficiency of supercritical fluid extraction and processing means that smaller quantities of solvent are required, leading to reduced waste streams. Additionally, the ability to recycle and reuse isopentane in closed-loop systems further minimizes environmental impact and resource consumption.
However, the volatile nature of isopentane poses potential risks to air quality if not properly managed. Fugitive emissions from SCF processes using isopentane can contribute to the formation of ground-level ozone and photochemical smog. To mitigate these risks, stringent emission control measures and advanced containment systems are essential in industrial settings utilizing isopentane SCF technologies.
Water pollution is another environmental concern associated with isopentane SCF processes. While the technology generally requires less water compared to conventional methods, any accidental releases or improper disposal of isopentane-containing waste can lead to water contamination. This necessitates robust wastewater treatment systems and strict handling protocols to protect aquatic ecosystems.
Energy efficiency is a notable environmental advantage of isopentane SCF processes. The lower critical temperature and pressure of isopentane compared to other supercritical fluids like carbon dioxide mean that less energy is required to reach and maintain supercritical conditions. This translates to reduced energy consumption and, consequently, lower indirect greenhouse gas emissions associated with power generation.
From a life cycle perspective, the environmental impact of isopentane production must also be considered. While isopentane can be derived from both petroleum and renewable sources, the method of production significantly influences its overall environmental footprint. Efforts to develop and scale up bio-based isopentane production could further enhance the sustainability profile of isopentane SCF technologies.
In conclusion, the environmental impact of isopentane SCF processes is multifaceted, offering significant benefits in terms of reduced global warming potential, waste generation, and energy consumption. However, careful management of air emissions, water pollution risks, and production methods is crucial to maximize the environmental advantages of this technology. As research and implementation of isopentane SCF processes continue to advance, ongoing assessment and optimization of their environmental performance will be essential for ensuring sustainable industrial practices.
One of the primary environmental benefits of using isopentane in SCF processes is its lower global warming potential compared to many conventional solvents. Isopentane has a relatively short atmospheric lifetime and a lower ozone depletion potential, making it a more environmentally friendly option for industrial applications. This characteristic aligns well with global efforts to reduce greenhouse gas emissions and combat climate change.
In terms of waste reduction, isopentane SCF processes often result in less hazardous waste generation compared to traditional solvent-based methods. The high efficiency of supercritical fluid extraction and processing means that smaller quantities of solvent are required, leading to reduced waste streams. Additionally, the ability to recycle and reuse isopentane in closed-loop systems further minimizes environmental impact and resource consumption.
However, the volatile nature of isopentane poses potential risks to air quality if not properly managed. Fugitive emissions from SCF processes using isopentane can contribute to the formation of ground-level ozone and photochemical smog. To mitigate these risks, stringent emission control measures and advanced containment systems are essential in industrial settings utilizing isopentane SCF technologies.
Water pollution is another environmental concern associated with isopentane SCF processes. While the technology generally requires less water compared to conventional methods, any accidental releases or improper disposal of isopentane-containing waste can lead to water contamination. This necessitates robust wastewater treatment systems and strict handling protocols to protect aquatic ecosystems.
Energy efficiency is a notable environmental advantage of isopentane SCF processes. The lower critical temperature and pressure of isopentane compared to other supercritical fluids like carbon dioxide mean that less energy is required to reach and maintain supercritical conditions. This translates to reduced energy consumption and, consequently, lower indirect greenhouse gas emissions associated with power generation.
From a life cycle perspective, the environmental impact of isopentane production must also be considered. While isopentane can be derived from both petroleum and renewable sources, the method of production significantly influences its overall environmental footprint. Efforts to develop and scale up bio-based isopentane production could further enhance the sustainability profile of isopentane SCF technologies.
In conclusion, the environmental impact of isopentane SCF processes is multifaceted, offering significant benefits in terms of reduced global warming potential, waste generation, and energy consumption. However, careful management of air emissions, water pollution risks, and production methods is crucial to maximize the environmental advantages of this technology. As research and implementation of isopentane SCF processes continue to advance, ongoing assessment and optimization of their environmental performance will be essential for ensuring sustainable industrial practices.
Safety Regulations for Isopentane SCF Applications
The safety regulations for isopentane in supercritical fluid (SCF) applications are crucial due to the compound's flammability and potential environmental impact. These regulations typically cover storage, handling, and operational procedures to minimize risks associated with its use in SCF technologies.
Storage regulations for isopentane in SCF applications often mandate the use of explosion-proof containers and storage areas. These containers must be kept in well-ventilated spaces, away from sources of ignition and direct sunlight. Temperature control is essential, as isopentane has a low boiling point and can easily vaporize. Facilities are required to implement proper grounding and bonding procedures to prevent static electricity buildup, which could lead to ignition.
Handling procedures for isopentane in SCF technologies are strictly regulated. Personal protective equipment (PPE) is mandatory, including chemical-resistant gloves, safety goggles, and flame-resistant clothing. Operators must be trained in proper handling techniques and emergency response procedures. Transfer operations must be conducted in well-ventilated areas or under local exhaust ventilation to prevent the accumulation of vapors.
Operational safety regulations focus on maintaining the integrity of SCF systems using isopentane. Regular inspections and maintenance of equipment are required to prevent leaks and ensure proper functioning of safety systems. Pressure relief devices must be installed and regularly tested to prevent over-pressurization. Monitoring systems for detecting leaks and measuring isopentane concentrations in the work environment are often mandated.
Environmental regulations address the potential impact of isopentane release. Facilities must have containment systems in place to prevent spills from entering the environment. Proper disposal methods for waste isopentane and contaminated materials are specified, often requiring specialized treatment or recycling processes.
Emergency response plans are a critical component of safety regulations. These plans must outline procedures for handling spills, fires, and other potential incidents involving isopentane in SCF applications. Facilities are required to have appropriate fire suppression systems and emergency shutdown procedures in place.
Occupational exposure limits for isopentane are typically set by regulatory agencies to protect worker health. These limits dictate the maximum allowable concentration of isopentane vapors in the workplace atmosphere over specified time periods. Regular air monitoring may be required to ensure compliance with these limits.
Transportation of isopentane for SCF applications is subject to hazardous materials regulations. This includes specific packaging requirements, labeling standards, and restrictions on quantities that can be transported. Drivers and handlers must receive specialized training in the safe transport of flammable materials.
Compliance with these safety regulations is typically enforced through regular inspections and audits by regulatory agencies. Facilities using isopentane in SCF technologies must maintain detailed records of their safety procedures, training programs, and incident reports to demonstrate adherence to these regulations.
Storage regulations for isopentane in SCF applications often mandate the use of explosion-proof containers and storage areas. These containers must be kept in well-ventilated spaces, away from sources of ignition and direct sunlight. Temperature control is essential, as isopentane has a low boiling point and can easily vaporize. Facilities are required to implement proper grounding and bonding procedures to prevent static electricity buildup, which could lead to ignition.
Handling procedures for isopentane in SCF technologies are strictly regulated. Personal protective equipment (PPE) is mandatory, including chemical-resistant gloves, safety goggles, and flame-resistant clothing. Operators must be trained in proper handling techniques and emergency response procedures. Transfer operations must be conducted in well-ventilated areas or under local exhaust ventilation to prevent the accumulation of vapors.
Operational safety regulations focus on maintaining the integrity of SCF systems using isopentane. Regular inspections and maintenance of equipment are required to prevent leaks and ensure proper functioning of safety systems. Pressure relief devices must be installed and regularly tested to prevent over-pressurization. Monitoring systems for detecting leaks and measuring isopentane concentrations in the work environment are often mandated.
Environmental regulations address the potential impact of isopentane release. Facilities must have containment systems in place to prevent spills from entering the environment. Proper disposal methods for waste isopentane and contaminated materials are specified, often requiring specialized treatment or recycling processes.
Emergency response plans are a critical component of safety regulations. These plans must outline procedures for handling spills, fires, and other potential incidents involving isopentane in SCF applications. Facilities are required to have appropriate fire suppression systems and emergency shutdown procedures in place.
Occupational exposure limits for isopentane are typically set by regulatory agencies to protect worker health. These limits dictate the maximum allowable concentration of isopentane vapors in the workplace atmosphere over specified time periods. Regular air monitoring may be required to ensure compliance with these limits.
Transportation of isopentane for SCF applications is subject to hazardous materials regulations. This includes specific packaging requirements, labeling standards, and restrictions on quantities that can be transported. Drivers and handlers must receive specialized training in the safe transport of flammable materials.
Compliance with these safety regulations is typically enforced through regular inspections and audits by regulatory agencies. Facilities using isopentane in SCF technologies must maintain detailed records of their safety procedures, training programs, and incident reports to demonstrate adherence to these regulations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!