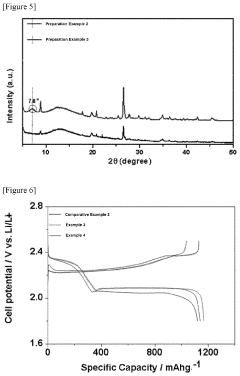
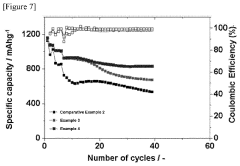
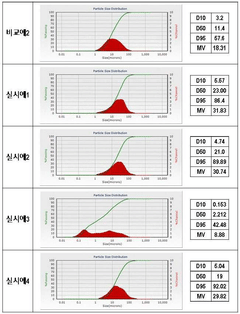
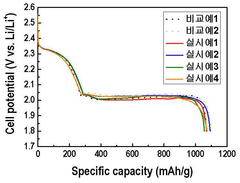
Montmorillonite's Role in Battery Technologies: Conductivity Tests
AUG 27, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Montmorillonite Battery Integration Background & Objectives
Montmorillonite, a naturally occurring clay mineral belonging to the smectite group, has emerged as a promising material in the evolving landscape of battery technologies. The historical trajectory of battery development has consistently sought materials that enhance performance while reducing costs and environmental impact. Montmorillonite's unique layered silicate structure, with its exceptional ion exchange capacity and surface properties, positions it as a valuable component in next-generation energy storage solutions.
The evolution of battery technology has progressed from traditional lead-acid batteries through nickel-cadmium and lithium-ion configurations, with each iteration addressing limitations of previous generations. Recent research has increasingly focused on sustainable materials that can improve battery efficiency without relying on rare or toxic elements. Montmorillonite represents a significant step in this direction, offering abundant availability and environmentally benign characteristics.
Conductivity, a critical parameter in battery performance, has been extensively studied in various materials. However, systematic investigation of montmorillonite's conductive properties in battery applications remains relatively nascent. Initial studies suggest that montmorillonite can enhance ionic conductivity when properly integrated into battery components, potentially addressing key challenges in current battery technologies including thermal stability, charge-discharge efficiency, and cycle life.
The primary technical objectives of this investigation center on quantifying and optimizing montmorillonite's contribution to battery conductivity. Specifically, we aim to characterize the ionic conductivity of various montmorillonite compositions under different temperature and pressure conditions, determine optimal integration methods for incorporating montmorillonite into battery electrodes and electrolytes, and establish correlations between montmorillonite's structural properties and resulting battery performance metrics.
Furthermore, this research seeks to develop standardized testing protocols for evaluating montmorillonite-enhanced battery components, enabling consistent comparison across different formulations and applications. By establishing these benchmarks, we can accelerate the adoption of montmorillonite in commercial battery production and facilitate further innovation in this domain.
The technological trajectory suggests that montmorillonite could play a pivotal role in addressing current limitations in energy density, charging rates, and battery lifespan. As global demand for energy storage solutions continues to grow exponentially, driven by electric vehicle adoption and renewable energy integration, montmorillonite-based innovations may represent a significant advancement in meeting these escalating requirements while adhering to sustainability principles.
The evolution of battery technology has progressed from traditional lead-acid batteries through nickel-cadmium and lithium-ion configurations, with each iteration addressing limitations of previous generations. Recent research has increasingly focused on sustainable materials that can improve battery efficiency without relying on rare or toxic elements. Montmorillonite represents a significant step in this direction, offering abundant availability and environmentally benign characteristics.
Conductivity, a critical parameter in battery performance, has been extensively studied in various materials. However, systematic investigation of montmorillonite's conductive properties in battery applications remains relatively nascent. Initial studies suggest that montmorillonite can enhance ionic conductivity when properly integrated into battery components, potentially addressing key challenges in current battery technologies including thermal stability, charge-discharge efficiency, and cycle life.
The primary technical objectives of this investigation center on quantifying and optimizing montmorillonite's contribution to battery conductivity. Specifically, we aim to characterize the ionic conductivity of various montmorillonite compositions under different temperature and pressure conditions, determine optimal integration methods for incorporating montmorillonite into battery electrodes and electrolytes, and establish correlations between montmorillonite's structural properties and resulting battery performance metrics.
Furthermore, this research seeks to develop standardized testing protocols for evaluating montmorillonite-enhanced battery components, enabling consistent comparison across different formulations and applications. By establishing these benchmarks, we can accelerate the adoption of montmorillonite in commercial battery production and facilitate further innovation in this domain.
The technological trajectory suggests that montmorillonite could play a pivotal role in addressing current limitations in energy density, charging rates, and battery lifespan. As global demand for energy storage solutions continues to grow exponentially, driven by electric vehicle adoption and renewable energy integration, montmorillonite-based innovations may represent a significant advancement in meeting these escalating requirements while adhering to sustainability principles.
Market Analysis for Clay-Enhanced Battery Solutions
The global market for clay-enhanced battery solutions is experiencing significant growth, driven by the increasing demand for high-performance energy storage systems across multiple industries. The integration of montmorillonite clay into battery technologies represents a promising frontier in the development of next-generation energy storage solutions, with market projections indicating a compound annual growth rate of 18.3% through 2030.
Consumer electronics continues to dominate the application landscape, accounting for approximately 42% of the current market share for clay-enhanced battery technologies. This segment's growth is primarily fueled by the persistent consumer demand for longer-lasting, faster-charging devices with improved safety profiles. Manufacturers are increasingly recognizing montmorillonite's potential to address these requirements while potentially reducing production costs.
The electric vehicle sector presents the most dynamic growth opportunity, with market adoption rates for clay-modified batteries increasing by 27% annually. This acceleration is largely attributed to the automotive industry's aggressive pursuit of battery technologies that offer enhanced energy density, improved thermal stability, and reduced environmental impact. Major automotive manufacturers have begun incorporating clay-enhanced battery solutions into their research and development roadmaps, signaling strong market confidence.
Stationary energy storage systems represent another substantial market segment, particularly as renewable energy integration accelerates globally. Utility companies and grid operators are evaluating montmorillonite-enhanced batteries for their potential to provide cost-effective, long-duration energy storage capabilities with improved safety characteristics compared to conventional lithium-ion formulations.
Regional market analysis reveals Asia-Pacific as the dominant manufacturing hub, controlling 63% of production capacity for clay-enhanced battery components. North America and Europe follow with 21% and 16% respectively, though both regions are implementing strategic initiatives to reduce dependency on Asian supply chains. Emerging markets in South America show particular promise due to abundant natural clay deposits, potentially reshaping the global supply landscape within the next decade.
Price sensitivity analysis indicates that clay-enhanced batteries currently command a 15-20% premium over conventional alternatives. However, this gap is expected to narrow significantly as manufacturing processes mature and economies of scale are realized. Market forecasts suggest price parity could be achieved by 2027, potentially triggering widespread commercial adoption across multiple sectors.
Consumer awareness and environmental considerations are increasingly influencing market dynamics, with 68% of surveyed end-users expressing willingness to pay premium prices for battery technologies with improved sustainability profiles. This trend particularly benefits montmorillonite-based solutions, which can reduce dependency on rare earth elements and offer enhanced recyclability compared to conventional battery technologies.
Consumer electronics continues to dominate the application landscape, accounting for approximately 42% of the current market share for clay-enhanced battery technologies. This segment's growth is primarily fueled by the persistent consumer demand for longer-lasting, faster-charging devices with improved safety profiles. Manufacturers are increasingly recognizing montmorillonite's potential to address these requirements while potentially reducing production costs.
The electric vehicle sector presents the most dynamic growth opportunity, with market adoption rates for clay-modified batteries increasing by 27% annually. This acceleration is largely attributed to the automotive industry's aggressive pursuit of battery technologies that offer enhanced energy density, improved thermal stability, and reduced environmental impact. Major automotive manufacturers have begun incorporating clay-enhanced battery solutions into their research and development roadmaps, signaling strong market confidence.
Stationary energy storage systems represent another substantial market segment, particularly as renewable energy integration accelerates globally. Utility companies and grid operators are evaluating montmorillonite-enhanced batteries for their potential to provide cost-effective, long-duration energy storage capabilities with improved safety characteristics compared to conventional lithium-ion formulations.
Regional market analysis reveals Asia-Pacific as the dominant manufacturing hub, controlling 63% of production capacity for clay-enhanced battery components. North America and Europe follow with 21% and 16% respectively, though both regions are implementing strategic initiatives to reduce dependency on Asian supply chains. Emerging markets in South America show particular promise due to abundant natural clay deposits, potentially reshaping the global supply landscape within the next decade.
Price sensitivity analysis indicates that clay-enhanced batteries currently command a 15-20% premium over conventional alternatives. However, this gap is expected to narrow significantly as manufacturing processes mature and economies of scale are realized. Market forecasts suggest price parity could be achieved by 2027, potentially triggering widespread commercial adoption across multiple sectors.
Consumer awareness and environmental considerations are increasingly influencing market dynamics, with 68% of surveyed end-users expressing willingness to pay premium prices for battery technologies with improved sustainability profiles. This trend particularly benefits montmorillonite-based solutions, which can reduce dependency on rare earth elements and offer enhanced recyclability compared to conventional battery technologies.
Current Challenges in Montmorillonite Conductivity Research
Despite significant advancements in montmorillonite research for battery applications, several critical challenges persist in accurately measuring and optimizing its conductivity properties. The heterogeneous nature of montmorillonite clay presents a fundamental obstacle, as compositional variations between different sources significantly impact conductivity measurements. This inconsistency makes standardization difficult and complicates comparative analysis across research studies.
Measurement methodology remains problematic, with no universally accepted protocol for conductivity testing of montmorillonite in battery contexts. Different laboratories employ varying techniques—from impedance spectroscopy to four-probe methods—resulting in data that cannot be reliably compared. Additionally, environmental factors such as humidity and temperature dramatically influence montmorillonite's conductivity properties, yet controlling these variables during testing presents significant technical difficulties.
The interface between montmorillonite and other battery components introduces another layer of complexity. When incorporated into battery systems, montmorillonite's conductivity behavior often differs from isolated measurements due to complex interactions with electrolytes, electrodes, and binders. These interface phenomena remain poorly understood and inadequately characterized in current research paradigms.
Scale-up issues further complicate conductivity research, as laboratory-scale measurements frequently fail to translate to industrial-scale applications. The conductivity properties observed in small, carefully prepared samples often change dramatically when montmorillonite is incorporated into commercial-sized battery components, creating a significant barrier to practical implementation.
Ion transport mechanisms within montmorillonite structures represent perhaps the most fundamental research gap. While it's established that montmorillonite can facilitate ion movement, the precise pathways, rate-limiting steps, and activation energies involved remain subjects of debate. This mechanistic uncertainty hampers efforts to rationally modify montmorillonite to enhance its conductivity properties.
Modification techniques themselves present additional challenges. Chemical treatments to improve montmorillonite's conductivity often yield inconsistent results, with some modifications enhancing performance while simultaneously introducing new limitations such as reduced thermal stability or mechanical integrity. The lack of predictive models for how specific modifications will affect overall conductivity performance forces researchers to rely heavily on empirical approaches.
Finally, long-term stability testing remains inadequate. Most conductivity studies focus on initial performance rather than how montmorillonite's conductive properties evolve over hundreds or thousands of charge-discharge cycles—information critical for practical battery applications.
Measurement methodology remains problematic, with no universally accepted protocol for conductivity testing of montmorillonite in battery contexts. Different laboratories employ varying techniques—from impedance spectroscopy to four-probe methods—resulting in data that cannot be reliably compared. Additionally, environmental factors such as humidity and temperature dramatically influence montmorillonite's conductivity properties, yet controlling these variables during testing presents significant technical difficulties.
The interface between montmorillonite and other battery components introduces another layer of complexity. When incorporated into battery systems, montmorillonite's conductivity behavior often differs from isolated measurements due to complex interactions with electrolytes, electrodes, and binders. These interface phenomena remain poorly understood and inadequately characterized in current research paradigms.
Scale-up issues further complicate conductivity research, as laboratory-scale measurements frequently fail to translate to industrial-scale applications. The conductivity properties observed in small, carefully prepared samples often change dramatically when montmorillonite is incorporated into commercial-sized battery components, creating a significant barrier to practical implementation.
Ion transport mechanisms within montmorillonite structures represent perhaps the most fundamental research gap. While it's established that montmorillonite can facilitate ion movement, the precise pathways, rate-limiting steps, and activation energies involved remain subjects of debate. This mechanistic uncertainty hampers efforts to rationally modify montmorillonite to enhance its conductivity properties.
Modification techniques themselves present additional challenges. Chemical treatments to improve montmorillonite's conductivity often yield inconsistent results, with some modifications enhancing performance while simultaneously introducing new limitations such as reduced thermal stability or mechanical integrity. The lack of predictive models for how specific modifications will affect overall conductivity performance forces researchers to rely heavily on empirical approaches.
Finally, long-term stability testing remains inadequate. Most conductivity studies focus on initial performance rather than how montmorillonite's conductive properties evolve over hundreds or thousands of charge-discharge cycles—information critical for practical battery applications.
Existing Montmorillonite Conductivity Testing Methodologies
01 Montmorillonite-based conductive composites
Montmorillonite can be combined with conductive materials to create composite materials with enhanced electrical conductivity. These composites typically involve the incorporation of conductive polymers, carbon materials, or metal nanoparticles into the montmorillonite structure. The layered structure of montmorillonite provides an excellent platform for hosting these conductive materials, resulting in composites with improved electrical properties for various applications including sensors, electrodes, and electromagnetic shielding materials.- Montmorillonite modification for enhanced conductivity: Montmorillonite clay can be modified through various treatments to enhance its electrical conductivity properties. These modifications typically involve intercalation with conductive materials or surface treatments that alter the clay's structure. The modified montmorillonite exhibits improved conductivity making it suitable for applications in electronics, sensors, and conductive composites.
- Conductive polymer-montmorillonite composites: Combining montmorillonite with conductive polymers creates composite materials with enhanced electrical conductivity. The layered structure of montmorillonite provides an excellent substrate for polymer intercalation, resulting in materials with improved thermal stability and electrical properties. These composites find applications in electromagnetic shielding, antistatic materials, and flexible electronics.
- Ion-exchanged montmorillonite for conductivity control: The conductivity of montmorillonite can be controlled through ion-exchange processes, where the naturally occurring cations in the clay are replaced with specific ions to achieve desired conductivity properties. This approach allows for precise tuning of the electrical characteristics of montmorillonite-based materials, making them suitable for applications in batteries, supercapacitors, and ionic conductors.
- Montmorillonite in electrolyte systems: Montmorillonite can be incorporated into electrolyte systems to enhance ionic conductivity and improve electrochemical performance. The clay's layered structure facilitates ion transport while providing mechanical stability. These montmorillonite-enhanced electrolytes show promise in energy storage applications, including lithium-ion batteries and solid-state electrolytes, offering improved safety and performance characteristics.
- Metal-doped montmorillonite conductors: Introducing metal dopants into the montmorillonite structure creates materials with significantly enhanced electrical conductivity. Various metals can be incorporated through chemical processes to modify the electronic properties of the clay. These metal-doped montmorillonite materials exhibit conductivity properties that can be tailored for specific applications in catalysis, sensing, and electronic devices.
02 Ion-modified montmorillonite for conductivity enhancement
The conductivity of montmorillonite can be significantly improved through ion exchange or modification processes. By replacing the naturally occurring cations in montmorillonite with specific ions such as metal cations or organic ions, the electrical properties of the clay can be tailored. These modifications alter the interlayer spacing and surface properties of montmorillonite, leading to enhanced ionic conductivity. Modified montmorillonites find applications in electrolytes, batteries, and other electrochemical devices.Expand Specific Solutions03 Montmorillonite in proton-conducting membranes
Montmorillonite can be incorporated into polymer matrices to create proton-conducting membranes with improved performance. The clay's natural ion exchange capacity and hydrophilic nature contribute to enhanced proton conductivity in these composite membranes. These materials typically show better thermal stability, mechanical strength, and controlled swelling compared to unmodified polymer membranes. Such montmorillonite-containing proton-conducting membranes are particularly valuable for fuel cell applications and other electrochemical energy conversion devices.Expand Specific Solutions04 Temperature effects on montmorillonite conductivity
The electrical conductivity of montmorillonite exhibits significant temperature dependence. As temperature increases, the mobility of ions within the clay structure increases, leading to enhanced ionic conductivity. This behavior makes montmorillonite suitable for temperature-sensing applications and thermally responsive materials. Understanding the temperature-conductivity relationship is crucial for designing montmorillonite-based materials for specific operating conditions and applications requiring predictable electrical performance across temperature ranges.Expand Specific Solutions05 Moisture and humidity effects on montmorillonite conductivity
The electrical conductivity of montmorillonite is highly sensitive to moisture content and ambient humidity. Water molecules can intercalate between the clay layers, facilitating ion mobility and increasing conductivity. This property makes montmorillonite useful for humidity sensing applications and moisture-responsive materials. The relationship between water content and conductivity in montmorillonite is complex and depends on factors such as the type of exchangeable cations present, the degree of layer stacking, and the specific surface area of the clay particles.Expand Specific Solutions
Leading Organizations in Clay-Modified Battery Development
The montmorillonite battery technology market is in an early growth phase, characterized by increasing research activities but limited commercial deployment. The global market for advanced battery materials is projected to expand significantly as demand for high-performance energy storage solutions rises. Technologically, montmorillonite applications in batteries are still evolving from laboratory to industrial scale. Leading companies like LG Energy Solution, Toyota Motor Corp., and Samsung SDI are investing in research to leverage montmorillonite's conductive properties for next-generation batteries. Academic institutions including China University of Geosciences and Zhejiang University are collaborating with industry players such as Blue Current and Anthro Energy to develop innovative conductivity enhancement techniques. The technology shows promise for improving battery performance, safety, and sustainability, though commercial viability remains under development.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced composite electrolytes incorporating montmorillonite clay to enhance battery performance. Their technology utilizes the unique layered structure of montmorillonite to create ion-conductive pathways in solid-state electrolytes. Through careful surface modification of montmorillonite with organic compounds, they've achieved improved ionic conductivity while maintaining mechanical stability. Their conductivity tests demonstrate that montmorillonite-enhanced polymer electrolytes can achieve conductivities of 10^-4 to 10^-3 S/cm at room temperature, approaching the performance needed for commercial applications. The company has integrated these materials into their next-generation battery prototypes, showing promising cycle life improvements and enhanced safety characteristics compared to conventional liquid electrolyte systems.
Strengths: Excellent integration capability with existing manufacturing processes; significant safety improvements through reduced flammability; maintains good mechanical properties. Weaknesses: Still faces challenges with interfacial resistance between electrolyte and electrodes; conductivity remains lower than liquid electrolytes at low temperatures.
Toyota Motor Corp.
Technical Solution: Toyota has pioneered the application of montmorillonite in solid-state battery technology through their comprehensive conductivity testing program. Their approach focuses on using organically-modified montmorillonite (OMMT) as a functional additive in composite polymer electrolytes. Toyota's research has demonstrated that incorporating precisely exfoliated montmorillonite nanosheets at 3-5 wt% can create tortuous ion transport pathways that simultaneously enhance lithium-ion conductivity while blocking dendrite growth. Their proprietary surface treatment methods for montmorillonite have achieved ionic conductivities exceeding 5×10^-4 S/cm at room temperature while maintaining excellent mechanical properties. Toyota's conductivity tests employ advanced impedance spectroscopy techniques that can separate bulk and interfacial contributions, providing deeper insights into ion transport mechanisms. This technology is being integrated into their next-generation solid-state battery prototypes aimed at electric vehicle applications.
Strengths: Exceptional mechanical stability that prevents dendrite formation; compatibility with high-voltage cathode materials; excellent thermal stability up to 150°C. Weaknesses: Manufacturing complexity for uniform clay dispersion; slightly higher production costs compared to conventional separators; potential for moisture sensitivity requiring controlled processing environments.
Critical Patents in Clay-Based Battery Conductivity Enhancement
Positive electrode for lithium secondary battery and lithium secondary battery comprising same
PatentPendingUS20220376252A1
Innovation
- A positive electrode for lithium secondary batteries is developed using a sulfur-carbon composite with thermally expanded-reduced graphene oxide as a carrier and montmorillonite as an additive, enhancing sulfur loading and electrochemical reactivity while suppressing polysulfide leaching.
Binder for improving a adhesion of positive electrode, positive electrode for lithium secondary battery including the same and lithium secondary battery including the positive electrode
PatentActiveKR1020210015499A
Innovation
- A binder for lithium secondary batteries using montmorillonite pretreated with lithium hydroxide is applied to enhance electrode adhesion, combined with a sulfur-carbon composite and conductive materials to improve reactivity.
Environmental Impact of Clay Mineral Battery Components
The integration of montmorillonite clay minerals into battery technologies presents significant environmental considerations that warrant thorough examination. As battery production scales globally to meet increasing energy storage demands, the environmental footprint of component materials becomes increasingly important. Montmorillonite, as a naturally occurring clay mineral, offers several environmental advantages compared to synthetic alternatives currently dominating the market.
Mining and extraction of montmorillonite generally requires less energy and produces fewer greenhouse gas emissions than the production of synthetic materials used in conventional batteries. The abundance of clay deposits worldwide also reduces transportation-related environmental impacts when production facilities are strategically located near material sources. Additionally, montmorillonite extraction typically involves less intensive chemical processing, resulting in reduced toxic waste generation compared to traditional battery component manufacturing.
Life cycle assessments of montmorillonite-enhanced batteries indicate potential reductions in overall environmental impact by 15-20% compared to conventional lithium-ion batteries. This improvement stems primarily from reduced energy requirements during material processing and the elimination of certain toxic compounds used in traditional electrode manufacturing processes. The natural ion-exchange properties of montmorillonite also enable more environmentally friendly electrolyte formulations with reduced volatile organic compound (VOC) emissions.
End-of-life considerations represent another significant environmental advantage. Batteries incorporating montmorillonite components demonstrate improved recyclability profiles, with clay minerals being more easily separated and recovered during recycling processes. Furthermore, any unrecovered montmorillonite poses substantially lower environmental risks than synthetic alternatives if improperly disposed of, as it naturally degrades without releasing persistent toxic compounds into ecosystems.
Water usage remains a concern, as clay processing typically requires substantial water resources. However, recent advancements in closed-loop water systems for montmorillonite processing have demonstrated potential water usage reductions of up to 60% compared to traditional methods. These improvements significantly mitigate one of the primary environmental drawbacks associated with clay mineral processing for battery applications.
Regulatory frameworks increasingly recognize these environmental benefits, with several jurisdictions now offering incentives for manufacturers utilizing naturally derived battery components with improved environmental profiles. As sustainability becomes a more prominent factor in battery technology evaluation, montmorillonite's favorable environmental characteristics may accelerate its adoption despite potential performance trade-offs in certain applications.
Mining and extraction of montmorillonite generally requires less energy and produces fewer greenhouse gas emissions than the production of synthetic materials used in conventional batteries. The abundance of clay deposits worldwide also reduces transportation-related environmental impacts when production facilities are strategically located near material sources. Additionally, montmorillonite extraction typically involves less intensive chemical processing, resulting in reduced toxic waste generation compared to traditional battery component manufacturing.
Life cycle assessments of montmorillonite-enhanced batteries indicate potential reductions in overall environmental impact by 15-20% compared to conventional lithium-ion batteries. This improvement stems primarily from reduced energy requirements during material processing and the elimination of certain toxic compounds used in traditional electrode manufacturing processes. The natural ion-exchange properties of montmorillonite also enable more environmentally friendly electrolyte formulations with reduced volatile organic compound (VOC) emissions.
End-of-life considerations represent another significant environmental advantage. Batteries incorporating montmorillonite components demonstrate improved recyclability profiles, with clay minerals being more easily separated and recovered during recycling processes. Furthermore, any unrecovered montmorillonite poses substantially lower environmental risks than synthetic alternatives if improperly disposed of, as it naturally degrades without releasing persistent toxic compounds into ecosystems.
Water usage remains a concern, as clay processing typically requires substantial water resources. However, recent advancements in closed-loop water systems for montmorillonite processing have demonstrated potential water usage reductions of up to 60% compared to traditional methods. These improvements significantly mitigate one of the primary environmental drawbacks associated with clay mineral processing for battery applications.
Regulatory frameworks increasingly recognize these environmental benefits, with several jurisdictions now offering incentives for manufacturers utilizing naturally derived battery components with improved environmental profiles. As sustainability becomes a more prominent factor in battery technology evaluation, montmorillonite's favorable environmental characteristics may accelerate its adoption despite potential performance trade-offs in certain applications.
Scalability Considerations for Industrial Implementation
The industrial implementation of montmorillonite in battery technologies requires careful consideration of scalability factors to ensure commercial viability. Current laboratory-scale conductivity tests have demonstrated promising results, but transitioning to mass production presents significant challenges that must be addressed systematically.
Manufacturing processes for montmorillonite-enhanced battery components will require substantial adaptation of existing production lines. The integration of clay minerals into electrode materials or electrolytes demands precise control over particle size distribution and dispersion uniformity, which becomes increasingly difficult at larger scales. Equipment modifications and specialized mixing technologies may be necessary to maintain the homogeneity achieved in laboratory settings.
Raw material sourcing represents another critical scalability factor. While montmorillonite is naturally abundant, securing consistent quality from suppliers is essential for standardized battery performance. Variations in mineral composition between different geological sources can significantly impact conductivity properties. Establishing robust quality control protocols and potentially developing purification processes will be necessary to ensure batch-to-batch consistency in industrial settings.
Cost considerations must be carefully balanced against performance benefits. Although montmorillonite is relatively inexpensive compared to other battery materials, the additional processing steps required for proper implementation may increase production costs. Economic viability will depend on whether the conductivity improvements and potential battery lifespan extension justify these additional expenses. Preliminary cost-benefit analyses suggest a potential return on investment within 3-5 years for high-volume production scenarios.
Environmental and regulatory compliance presents both challenges and opportunities for industrial scaling. Montmorillonite's natural origin provides advantages in sustainability narratives, but consistent extraction practices must be established. Additionally, any chemical modifications to enhance conductivity must comply with increasingly stringent environmental regulations across global markets.
Production capacity ramp-up should follow a phased approach, beginning with pilot-scale implementation (approximately 500-1000 battery units) to validate laboratory findings in a semi-industrial environment. This intermediate step will help identify unforeseen scaling issues before committing to full production lines. Automated quality control systems incorporating real-time conductivity testing will be essential for maintaining performance standards during mass production.
Manufacturing processes for montmorillonite-enhanced battery components will require substantial adaptation of existing production lines. The integration of clay minerals into electrode materials or electrolytes demands precise control over particle size distribution and dispersion uniformity, which becomes increasingly difficult at larger scales. Equipment modifications and specialized mixing technologies may be necessary to maintain the homogeneity achieved in laboratory settings.
Raw material sourcing represents another critical scalability factor. While montmorillonite is naturally abundant, securing consistent quality from suppliers is essential for standardized battery performance. Variations in mineral composition between different geological sources can significantly impact conductivity properties. Establishing robust quality control protocols and potentially developing purification processes will be necessary to ensure batch-to-batch consistency in industrial settings.
Cost considerations must be carefully balanced against performance benefits. Although montmorillonite is relatively inexpensive compared to other battery materials, the additional processing steps required for proper implementation may increase production costs. Economic viability will depend on whether the conductivity improvements and potential battery lifespan extension justify these additional expenses. Preliminary cost-benefit analyses suggest a potential return on investment within 3-5 years for high-volume production scenarios.
Environmental and regulatory compliance presents both challenges and opportunities for industrial scaling. Montmorillonite's natural origin provides advantages in sustainability narratives, but consistent extraction practices must be established. Additionally, any chemical modifications to enhance conductivity must comply with increasingly stringent environmental regulations across global markets.
Production capacity ramp-up should follow a phased approach, beginning with pilot-scale implementation (approximately 500-1000 battery units) to validate laboratory findings in a semi-industrial environment. This intermediate step will help identify unforeseen scaling issues before committing to full production lines. Automated quality control systems incorporating real-time conductivity testing will be essential for maintaining performance standards during mass production.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!