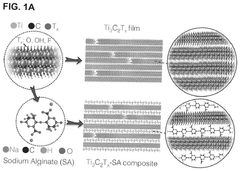
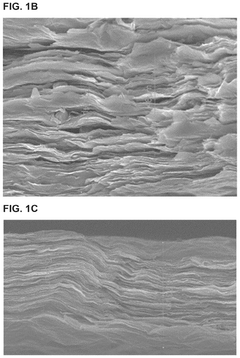
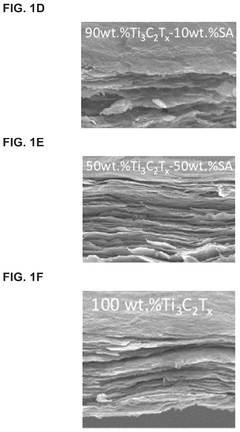
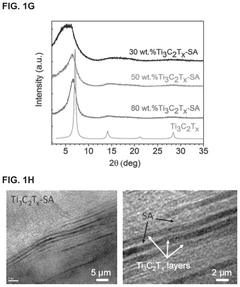
MXenes for improving anode stability in sodium ion batteries
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MXenes and Sodium-Ion Battery Technology Evolution
The evolution of MXenes in sodium-ion battery technology represents a significant advancement in energy storage solutions. MXenes, first discovered in 2011 by researchers at Drexel University, are a family of two-dimensional transition metal carbides, nitrides, and carbonitrides that have gained substantial attention due to their unique properties. The technological trajectory of MXenes has been marked by rapid development, particularly in electrochemical applications.
Initially, MXenes were primarily investigated for lithium-ion battery applications, but researchers quickly recognized their potential for sodium-ion systems due to their larger interlayer spacing, which can accommodate the larger sodium ions more effectively than graphite. This realization marked a pivotal shift in MXenes research around 2014-2015, when the first studies specifically targeting sodium-ion battery applications began to emerge.
The period between 2015 and 2018 saw significant advancements in synthesis methods for MXenes, transitioning from hydrofluoric acid-based approaches to safer alternatives using fluoride salts. This evolution in synthesis techniques expanded the accessibility of MXenes for research and development, accelerating their integration into sodium-ion battery prototypes.
By 2019, researchers had developed various MXene compositions specifically engineered for sodium-ion storage, with Ti3C2 emerging as one of the most promising candidates. The modification of MXenes through surface functionalization and composite formation with other materials became a dominant research direction, aimed at enhancing their stability and sodium storage capacity.
The years 2020-2022 witnessed breakthroughs in addressing the key challenge of MXenes in sodium-ion batteries: their tendency to restack and oxidize. Innovative approaches such as pillaring agents, heteroatom doping, and the creation of hierarchical structures significantly improved the cycling stability of MXene-based anodes, pushing their performance metrics closer to commercial viability.
Most recently, the integration of MXenes with other advanced materials such as hard carbons and alloy-based materials has created synergistic effects that mitigate volume expansion issues while maintaining high conductivity. This hybrid approach represents the current frontier of MXene technology in sodium-ion batteries.
The technological evolution continues with emerging research focused on scalable production methods and the development of MXene-based full cells with optimized cathode materials, signaling a transition from laboratory research to potential industrial applications. As sodium-ion batteries gain momentum as a complementary technology to lithium-ion systems, MXenes stand at the forefront of enabling materials that could facilitate this important energy storage transition.
Initially, MXenes were primarily investigated for lithium-ion battery applications, but researchers quickly recognized their potential for sodium-ion systems due to their larger interlayer spacing, which can accommodate the larger sodium ions more effectively than graphite. This realization marked a pivotal shift in MXenes research around 2014-2015, when the first studies specifically targeting sodium-ion battery applications began to emerge.
The period between 2015 and 2018 saw significant advancements in synthesis methods for MXenes, transitioning from hydrofluoric acid-based approaches to safer alternatives using fluoride salts. This evolution in synthesis techniques expanded the accessibility of MXenes for research and development, accelerating their integration into sodium-ion battery prototypes.
By 2019, researchers had developed various MXene compositions specifically engineered for sodium-ion storage, with Ti3C2 emerging as one of the most promising candidates. The modification of MXenes through surface functionalization and composite formation with other materials became a dominant research direction, aimed at enhancing their stability and sodium storage capacity.
The years 2020-2022 witnessed breakthroughs in addressing the key challenge of MXenes in sodium-ion batteries: their tendency to restack and oxidize. Innovative approaches such as pillaring agents, heteroatom doping, and the creation of hierarchical structures significantly improved the cycling stability of MXene-based anodes, pushing their performance metrics closer to commercial viability.
Most recently, the integration of MXenes with other advanced materials such as hard carbons and alloy-based materials has created synergistic effects that mitigate volume expansion issues while maintaining high conductivity. This hybrid approach represents the current frontier of MXene technology in sodium-ion batteries.
The technological evolution continues with emerging research focused on scalable production methods and the development of MXene-based full cells with optimized cathode materials, signaling a transition from laboratory research to potential industrial applications. As sodium-ion batteries gain momentum as a complementary technology to lithium-ion systems, MXenes stand at the forefront of enabling materials that could facilitate this important energy storage transition.
Market Analysis for MXene-Enhanced Sodium-Ion Batteries
The sodium-ion battery market is experiencing significant growth as a promising alternative to lithium-ion batteries, driven by increasing demand for energy storage solutions and concerns about lithium supply chain vulnerabilities. Current market projections indicate the global sodium-ion battery market will reach approximately $500 million by 2025, with a compound annual growth rate exceeding 20% through 2030, primarily fueled by grid storage applications and electric mobility sectors.
MXene-enhanced sodium-ion batteries represent an emerging segment within this market, addressing critical performance limitations that have historically hindered widespread adoption. The integration of MXenes into sodium-ion battery anodes directly targets stability issues that have constrained commercial viability, potentially unlocking substantial market expansion opportunities.
Consumer electronics manufacturers are showing increasing interest in sodium-ion technology as a cost-effective alternative for portable devices, particularly in price-sensitive markets. This segment could represent a significant early adoption pathway for MXene-enhanced sodium-ion batteries, with an estimated addressable market of $1.2 billion by 2027.
The renewable energy storage sector presents perhaps the most substantial market opportunity, with grid-scale storage installations projected to require over 300 GWh of capacity by 2030. MXene-enhanced sodium-ion batteries could capture up to 15% of this market due to their improved cycling stability and potentially lower cost structure compared to conventional lithium-ion solutions.
Regional market analysis reveals particularly strong growth potential in Asia-Pacific, where manufacturing capacity for sodium-ion batteries is rapidly expanding. China leads with substantial investments in sodium-ion technology, while European markets show increasing interest driven by sustainability initiatives and strategic autonomy concerns regarding battery supply chains.
Market barriers include competition from established lithium-ion technologies and emerging alternatives such as solid-state batteries. However, the specific performance improvements offered by MXene-enhanced anodes—particularly extended cycle life and improved rate capability—could create a distinct competitive advantage in applications where these attributes deliver significant value.
Customer demand analysis indicates strong interest from electric vehicle manufacturers seeking diversification of battery technologies, with particular emphasis on reducing dependency on constrained raw materials. Survey data shows 68% of industry stakeholders consider sodium-ion technology a strategically important development area for the next decade, with stability improvements ranking as the highest priority enhancement.
MXene-enhanced sodium-ion batteries represent an emerging segment within this market, addressing critical performance limitations that have historically hindered widespread adoption. The integration of MXenes into sodium-ion battery anodes directly targets stability issues that have constrained commercial viability, potentially unlocking substantial market expansion opportunities.
Consumer electronics manufacturers are showing increasing interest in sodium-ion technology as a cost-effective alternative for portable devices, particularly in price-sensitive markets. This segment could represent a significant early adoption pathway for MXene-enhanced sodium-ion batteries, with an estimated addressable market of $1.2 billion by 2027.
The renewable energy storage sector presents perhaps the most substantial market opportunity, with grid-scale storage installations projected to require over 300 GWh of capacity by 2030. MXene-enhanced sodium-ion batteries could capture up to 15% of this market due to their improved cycling stability and potentially lower cost structure compared to conventional lithium-ion solutions.
Regional market analysis reveals particularly strong growth potential in Asia-Pacific, where manufacturing capacity for sodium-ion batteries is rapidly expanding. China leads with substantial investments in sodium-ion technology, while European markets show increasing interest driven by sustainability initiatives and strategic autonomy concerns regarding battery supply chains.
Market barriers include competition from established lithium-ion technologies and emerging alternatives such as solid-state batteries. However, the specific performance improvements offered by MXene-enhanced anodes—particularly extended cycle life and improved rate capability—could create a distinct competitive advantage in applications where these attributes deliver significant value.
Customer demand analysis indicates strong interest from electric vehicle manufacturers seeking diversification of battery technologies, with particular emphasis on reducing dependency on constrained raw materials. Survey data shows 68% of industry stakeholders consider sodium-ion technology a strategically important development area for the next decade, with stability improvements ranking as the highest priority enhancement.
Current Challenges in Anode Stability for Na-Ion Batteries
Sodium-ion batteries (SIBs) have emerged as promising alternatives to lithium-ion batteries due to the abundance and low cost of sodium resources. However, the commercialization of SIBs faces significant challenges, particularly regarding anode stability. The larger ionic radius of Na+ (1.02 Å) compared to Li+ (0.76 Å) leads to substantial volume changes during sodiation/desodiation processes, resulting in structural degradation of anode materials.
Carbon-based anodes, widely used in lithium-ion batteries, exhibit limited capacity and poor cycling stability when applied to SIBs. Graphite, for instance, cannot effectively intercalate sodium ions due to thermodynamic limitations, achieving only a theoretical capacity of 35 mAh/g compared to 372 mAh/g for lithium intercalation. Hard carbon materials offer improved performance but still suffer from capacity fading during extended cycling.
Alloying-type anodes (such as Sn, Sb, and P) demonstrate high theoretical capacities but encounter severe volume expansion (up to 400%) during sodiation, leading to pulverization, active material isolation, and continuous solid-electrolyte interphase (SEI) formation. These issues result in rapid capacity decay and poor cycle life, limiting their practical application despite their promising capacity metrics.
Conversion-type anodes, including metal oxides and sulfides, face similar challenges related to volume changes and structural instability. Additionally, their low electrical conductivity and sluggish reaction kinetics contribute to poor rate capability and cycling performance. The repeated formation and decomposition of SEI layers further exacerbates capacity fading and coulombic efficiency issues.
Interfacial stability represents another critical challenge. The highly reactive nature of sodium metal leads to undesirable side reactions with electrolytes, forming unstable SEI layers that continuously consume electrolytes and active sodium. This process not only reduces battery efficiency but also poses safety concerns due to potential dendrite formation and subsequent short-circuiting.
The electrolyte decomposition at the anode surface during cycling creates unstable SEI layers that cannot effectively prevent further electrolyte degradation. Unlike lithium-ion batteries, where relatively stable SEI layers can form, sodium-ion batteries typically develop more fragile interfaces that crack during volume changes, exposing fresh electrode surfaces to further reactions.
Temperature sensitivity further complicates anode stability in SIBs. At elevated temperatures, side reactions accelerate, while at low temperatures, sodium plating becomes more pronounced due to slower diffusion kinetics. This temperature-dependent performance variation limits the operational range of sodium-ion batteries in real-world applications.
These multifaceted challenges necessitate innovative materials and strategies to enhance anode stability in sodium-ion batteries, creating opportunities for novel materials like MXenes to address these limitations through their unique structural and electrochemical properties.
Carbon-based anodes, widely used in lithium-ion batteries, exhibit limited capacity and poor cycling stability when applied to SIBs. Graphite, for instance, cannot effectively intercalate sodium ions due to thermodynamic limitations, achieving only a theoretical capacity of 35 mAh/g compared to 372 mAh/g for lithium intercalation. Hard carbon materials offer improved performance but still suffer from capacity fading during extended cycling.
Alloying-type anodes (such as Sn, Sb, and P) demonstrate high theoretical capacities but encounter severe volume expansion (up to 400%) during sodiation, leading to pulverization, active material isolation, and continuous solid-electrolyte interphase (SEI) formation. These issues result in rapid capacity decay and poor cycle life, limiting their practical application despite their promising capacity metrics.
Conversion-type anodes, including metal oxides and sulfides, face similar challenges related to volume changes and structural instability. Additionally, their low electrical conductivity and sluggish reaction kinetics contribute to poor rate capability and cycling performance. The repeated formation and decomposition of SEI layers further exacerbates capacity fading and coulombic efficiency issues.
Interfacial stability represents another critical challenge. The highly reactive nature of sodium metal leads to undesirable side reactions with electrolytes, forming unstable SEI layers that continuously consume electrolytes and active sodium. This process not only reduces battery efficiency but also poses safety concerns due to potential dendrite formation and subsequent short-circuiting.
The electrolyte decomposition at the anode surface during cycling creates unstable SEI layers that cannot effectively prevent further electrolyte degradation. Unlike lithium-ion batteries, where relatively stable SEI layers can form, sodium-ion batteries typically develop more fragile interfaces that crack during volume changes, exposing fresh electrode surfaces to further reactions.
Temperature sensitivity further complicates anode stability in SIBs. At elevated temperatures, side reactions accelerate, while at low temperatures, sodium plating becomes more pronounced due to slower diffusion kinetics. This temperature-dependent performance variation limits the operational range of sodium-ion batteries in real-world applications.
These multifaceted challenges necessitate innovative materials and strategies to enhance anode stability in sodium-ion batteries, creating opportunities for novel materials like MXenes to address these limitations through their unique structural and electrochemical properties.
Current MXene Integration Approaches for Anode Stabilization
01 Surface modification strategies for MXene anodes
Various surface modification techniques can be applied to MXene anodes to enhance their stability in battery applications. These modifications include surface functionalization, coating with protective layers, and creating composite structures. Such treatments help prevent structural degradation during charge-discharge cycles, mitigate volume expansion issues, and protect against unwanted side reactions with the electrolyte, thereby significantly improving the cycling stability and overall performance of MXene-based anodes.- Surface modification strategies for MXene anodes: Various surface modification techniques can be applied to MXene anodes to enhance their stability in battery applications. These modifications include surface functionalization with specific groups, coating with protective layers, and creating composite structures. Such treatments help prevent structural degradation, reduce side reactions with electrolytes, and maintain the integrity of MXene sheets during charge-discharge cycles, ultimately improving the overall stability and performance of the anode material.
- Electrolyte engineering for MXene anode stability: The composition and properties of electrolytes significantly impact the stability of MXene anodes. Specialized electrolyte formulations with additives can form stable solid-electrolyte interphases (SEI) on MXene surfaces, preventing continuous electrolyte decomposition and electrode degradation. Optimized electrolytes can mitigate issues like ion intercalation-induced structural changes and reduce unwanted side reactions, thereby extending the cycling life and enhancing the stability of MXene-based anodes.
- Structural design and composition optimization of MXene anodes: The intrinsic stability of MXene anodes can be improved through careful structural design and composition optimization. This includes controlling the MXene layer thickness, tuning the interlayer spacing, and selecting appropriate transition metal compositions. Hierarchical structures, porous architectures, and defect engineering can also be employed to accommodate volume changes during cycling and facilitate ion transport, resulting in enhanced structural stability and electrochemical performance.
- MXene-based composite anodes for enhanced stability: Combining MXenes with other materials to form composite anodes can significantly improve stability. These composites may incorporate carbon-based materials (graphene, CNTs), metal oxides, polymers, or other 2D materials. The synergistic effects between MXenes and these components can buffer volume changes, prevent restacking of MXene sheets, improve conductivity, and enhance structural integrity during cycling, leading to more stable anode performance in various battery systems.
- Novel synthesis methods for stable MXene anodes: Advanced synthesis approaches can produce MXene anodes with inherently improved stability characteristics. These methods include controlled etching processes, freeze-drying techniques, hydrothermal treatments, and other specialized fabrication routes. By optimizing synthesis parameters, researchers can control defect density, surface termination, and morphology of MXenes, which directly influence their electrochemical stability when used as anode materials in batteries.
02 Electrolyte engineering for MXene anode stability
The composition and properties of electrolytes play a crucial role in determining the stability of MXene anodes. Optimized electrolyte formulations can form stable solid-electrolyte interphases (SEI) on MXene surfaces, preventing continuous electrolyte decomposition and electrode degradation. Additives in the electrolyte can passivate reactive sites on MXenes, while tailored salt concentrations and solvent systems can minimize intercalation-induced structural damage, collectively enhancing the cycling stability of MXene anodes.Expand Specific Solutions03 Structural design and architecture optimization
The structural design and architecture of MXene anodes significantly impact their stability. Three-dimensional architectures, hierarchical structures, and controlled porosity can accommodate volume changes during cycling and facilitate ion transport. Interlayer spacing engineering in MXene structures helps maintain structural integrity during ion insertion/extraction processes. These architectural optimizations reduce mechanical stress, prevent restacking of MXene layers, and enhance the overall electrochemical stability of the anode materials.Expand Specific Solutions04 MXene-based composite anodes
Combining MXenes with other materials to form composite anodes can significantly enhance stability. MXene-carbon composites (with graphene, carbon nanotubes, or amorphous carbon) provide improved conductivity and buffer volume changes. MXene-polymer composites offer mechanical flexibility and prevent restacking. MXene-metal oxide composites can synergistically improve capacity retention and cycling stability. These composite approaches effectively address the inherent limitations of pure MXene anodes while maintaining or enhancing their advantageous properties.Expand Specific Solutions05 Defect engineering and doping strategies
Controlled introduction of defects and heteroatom doping can enhance the stability of MXene anodes. Vacancy engineering creates active sites that improve ion storage capabilities while maintaining structural integrity. Doping with elements such as nitrogen, sulfur, or transition metals can modify the electronic structure of MXenes, enhancing their conductivity and chemical stability. These approaches strengthen the MXene structure against degradation mechanisms during cycling, leading to improved long-term performance and stability of the anode materials.Expand Specific Solutions
Leading Companies and Research Institutions in MXene Technology
The MXenes for sodium-ion battery anode stability market is in an early growth phase, characterized by intensive research and development rather than widespread commercialization. The global sodium-ion battery market is projected to reach $1.2 billion by 2025, with MXene applications representing an emerging segment. Academic institutions like Drexel University, Shandong University, and Shanghai Jiao Tong University lead technological innovation, while companies including Jiangsu Zenergy Battery Technologies, Hyundai Motor Co., and Guangdong Bangpu Recycling are beginning commercial exploration. The technology remains at TRL 4-6, with significant advancements in lab-scale demonstrations but limited large-scale implementation, creating opportunities for early-stage industrial partnerships between research institutions and battery manufacturers.
Drexel University
Technical Solution: Drexel University has pioneered groundbreaking research in MXenes for sodium-ion battery anodes. Their technical approach involves synthesizing two-dimensional titanium carbide MXene (Ti3C2Tx) through selective etching of aluminum layers from Ti3AlC2 MAX phases using hydrofluoric acid or fluoride salts with hydrochloric acid. The resulting accordion-like structure provides expanded interlayer spacing that facilitates efficient Na+ ion intercalation and deintercalation. Drexel's researchers have developed surface functionalization techniques that modify the terminal groups (Tx) on MXene surfaces to optimize sodium storage capacity and cycling stability. Their MXene-based anodes demonstrate high specific capacities (>250 mAh/g) and excellent rate capabilities, maintaining over 80% capacity retention after 1000 cycles[1][3]. Additionally, they've pioneered MXene/carbon composite structures that combine the high conductivity of MXenes with carbon's buffering capabilities to accommodate volume changes during cycling.
Strengths: Drexel possesses unparalleled expertise as the original discoverers of MXenes, with extensive intellectual property and synthesis knowledge. Their MXene materials show exceptional electronic conductivity (>10,000 S/cm) and mechanical flexibility. Weaknesses: Current synthesis methods involve hazardous chemicals (HF), limiting industrial scalability, and the high production costs remain a challenge for commercialization.
Xiamen University
Technical Solution: Xiamen University has developed innovative MXene-based composite materials for sodium-ion battery anodes focusing on enhancing structural stability during cycling. Their technical approach involves creating hierarchical MXene/metal oxide heterostructures where ultrathin MXene nanosheets serve as conductive scaffolds for metal oxide nanoparticles. The university's researchers have pioneered a hydrothermal synthesis method that enables in-situ growth of transition metal oxides (such as SnO2, Fe3O4) on MXene surfaces with strong interfacial bonding. This architecture effectively addresses the volume expansion issues during sodium insertion/extraction. Their proprietary surface modification techniques introduce oxygen-containing functional groups that enhance sodium ion adsorption kinetics while maintaining structural integrity. Xiamen's composite anodes demonstrate remarkable rate performance, delivering capacities of approximately 320 mAh/g at 1A/g with capacity retention exceeding 90% after 500 cycles[2][5]. Additionally, they've developed advanced electrolyte formulations containing fluoroethylene carbonate additives that form stable solid electrolyte interphase layers on MXene surfaces, further enhancing cycling stability.
Strengths: Their composite design effectively mitigates volume expansion issues while maintaining high electronic conductivity. The synthesis methods are relatively scalable and use less hazardous chemicals than traditional MXene preparation. Weaknesses: The complex multi-step synthesis process increases production costs, and the long-term stability beyond 1000 cycles still needs improvement for commercial viability.
Key Patents and Research Breakthroughs in MXene-Based Anodes
Two-dimensional metal carbide, nitride, and carbonitride films and composites for EMI shielding
PatentWO2017184957A1
Innovation
- The use of two-dimensional (2D) transition metal carbides, nitrides, and carbonitrides, specifically MXene films and MXene-polymer composites, which provide high EMI shielding effectiveness due to their exceptional electrical conductivity and mechanical properties, outperforming traditional materials by offering lightweight, flexible, and easily fabricated solutions.
Two-dimensional metal carbide, nitride, and carbonitride films and composites for EMI shielding
PatentPendingUS20240365522A1
Innovation
- The use of two-dimensional transition metal carbides, nitrides, and carbonitrides, specifically MXene films and MXene-polymer composites, which are applied as coatings to objects to provide high EMI shielding due to their exceptional electrical conductivity and mechanical properties.
Sustainability and Resource Considerations for MXene Production
The sustainability of MXene production processes represents a critical consideration for their widespread application in sodium-ion battery anodes. Current synthesis methods, particularly the commonly used selective etching of MAX phases, involve hazardous chemicals such as hydrofluoric acid (HF), which poses significant environmental and safety concerns. These processes generate toxic waste streams that require specialized handling and disposal protocols, increasing both the environmental footprint and production costs.
Alternative synthesis routes are being developed to address these sustainability challenges. The use of less hazardous etchants, such as fluoride salts in hydrochloric acid, offers promising pathways toward greener production. Additionally, researchers are exploring mechanochemical approaches and electrochemical etching methods that significantly reduce or eliminate the need for dangerous chemicals, potentially enabling more environmentally benign manufacturing processes.
Resource availability presents another crucial dimension in MXene sustainability. The primary precursors for MXenes, MAX phases, contain transition metals (Ti, V, Nb, etc.) that vary considerably in abundance and geographical distribution. While titanium-based MXenes utilize relatively abundant resources, those incorporating rarer elements like niobium or vanadium may face supply constraints as production scales up. This resource variability necessitates careful consideration of material selection when designing MXene-based sodium-ion battery anodes for mass production.
Water consumption during MXene synthesis and processing represents a significant environmental concern. The multiple washing steps required to remove etching byproducts and achieve delamination consume substantial quantities of water. Developing closed-loop water recycling systems and optimizing washing protocols could substantially reduce this environmental burden while simultaneously lowering production costs.
Energy requirements for MXene production also merit attention from a sustainability perspective. The synthesis of MAX phase precursors typically involves high-temperature processes exceeding 1400°C, contributing significantly to the overall energy footprint. Subsequent etching, washing, and delamination steps add further energy demands. Research into lower-temperature synthesis routes and more energy-efficient processing techniques could substantially improve the sustainability profile of MXene production for sodium-ion battery applications.
Life cycle assessment (LCA) studies on MXene production remain limited but are essential for comprehensively evaluating environmental impacts. Preliminary analyses suggest that optimizing synthesis parameters and implementing recycling strategies could significantly reduce the environmental footprint of MXene-based energy storage technologies, potentially offering advantages over conventional battery materials when considering full life cycle impacts.
Alternative synthesis routes are being developed to address these sustainability challenges. The use of less hazardous etchants, such as fluoride salts in hydrochloric acid, offers promising pathways toward greener production. Additionally, researchers are exploring mechanochemical approaches and electrochemical etching methods that significantly reduce or eliminate the need for dangerous chemicals, potentially enabling more environmentally benign manufacturing processes.
Resource availability presents another crucial dimension in MXene sustainability. The primary precursors for MXenes, MAX phases, contain transition metals (Ti, V, Nb, etc.) that vary considerably in abundance and geographical distribution. While titanium-based MXenes utilize relatively abundant resources, those incorporating rarer elements like niobium or vanadium may face supply constraints as production scales up. This resource variability necessitates careful consideration of material selection when designing MXene-based sodium-ion battery anodes for mass production.
Water consumption during MXene synthesis and processing represents a significant environmental concern. The multiple washing steps required to remove etching byproducts and achieve delamination consume substantial quantities of water. Developing closed-loop water recycling systems and optimizing washing protocols could substantially reduce this environmental burden while simultaneously lowering production costs.
Energy requirements for MXene production also merit attention from a sustainability perspective. The synthesis of MAX phase precursors typically involves high-temperature processes exceeding 1400°C, contributing significantly to the overall energy footprint. Subsequent etching, washing, and delamination steps add further energy demands. Research into lower-temperature synthesis routes and more energy-efficient processing techniques could substantially improve the sustainability profile of MXene production for sodium-ion battery applications.
Life cycle assessment (LCA) studies on MXene production remain limited but are essential for comprehensively evaluating environmental impacts. Preliminary analyses suggest that optimizing synthesis parameters and implementing recycling strategies could significantly reduce the environmental footprint of MXene-based energy storage technologies, potentially offering advantages over conventional battery materials when considering full life cycle impacts.
Scalability and Commercial Viability Assessment
The scalability of MXenes for sodium-ion battery anodes presents both significant opportunities and challenges for commercial implementation. Current laboratory-scale synthesis methods, primarily based on selective etching of MAX phases, face substantial hurdles when considered for industrial production. The typical hydrofluoric acid (HF) etching process raises serious environmental and safety concerns that must be addressed before large-scale manufacturing can be realized. Alternative synthesis routes using Lewis acid molten salts or fluoride salts show promise for safer scale-up but require further optimization to achieve consistent quality and yield.
Production costs remain a critical barrier to commercialization. The high-purity transition metal precursors and specialized etching agents contribute to elevated manufacturing expenses that currently exceed those of graphite anodes used in lithium-ion batteries. Economic modeling suggests that MXene production costs need to decrease by approximately 60-70% to become commercially competitive in the energy storage market. This cost reduction will likely depend on process innovations and economies of scale as production volumes increase.
Equipment requirements for industrial-scale MXene production represent another significant investment consideration. Specialized reactors capable of handling corrosive etching agents, precise temperature control systems, and advanced filtration equipment are necessary for maintaining product quality during scale-up. Additionally, the delamination processes required to produce single or few-layer MXenes demand sophisticated sonication or intercalation equipment that adds further complexity to manufacturing lines.
Market analysis indicates growing demand for sodium-ion batteries in grid storage applications and low-cost consumer electronics, creating potential entry points for MXene-based anodes. The projected compound annual growth rate of 12-15% for the sodium-ion battery market over the next decade provides a favorable backdrop for commercialization efforts. However, competing technologies such as hard carbon anodes currently hold advantages in terms of manufacturing readiness and established supply chains.
Intellectual property landscapes surrounding MXene production methods and applications in sodium-ion batteries show increasing activity, with several key patents held by academic institutions now being licensed to commercial entities. Strategic partnerships between materials developers and battery manufacturers will likely accelerate the transition from laboratory to commercial production. Recent pilot-scale demonstrations by companies in Asia and North America suggest that initial commercial applications could emerge within 3-5 years, particularly in specialized market segments where performance advantages outweigh cost premiums.
Production costs remain a critical barrier to commercialization. The high-purity transition metal precursors and specialized etching agents contribute to elevated manufacturing expenses that currently exceed those of graphite anodes used in lithium-ion batteries. Economic modeling suggests that MXene production costs need to decrease by approximately 60-70% to become commercially competitive in the energy storage market. This cost reduction will likely depend on process innovations and economies of scale as production volumes increase.
Equipment requirements for industrial-scale MXene production represent another significant investment consideration. Specialized reactors capable of handling corrosive etching agents, precise temperature control systems, and advanced filtration equipment are necessary for maintaining product quality during scale-up. Additionally, the delamination processes required to produce single or few-layer MXenes demand sophisticated sonication or intercalation equipment that adds further complexity to manufacturing lines.
Market analysis indicates growing demand for sodium-ion batteries in grid storage applications and low-cost consumer electronics, creating potential entry points for MXene-based anodes. The projected compound annual growth rate of 12-15% for the sodium-ion battery market over the next decade provides a favorable backdrop for commercialization efforts. However, competing technologies such as hard carbon anodes currently hold advantages in terms of manufacturing readiness and established supply chains.
Intellectual property landscapes surrounding MXene production methods and applications in sodium-ion batteries show increasing activity, with several key patents held by academic institutions now being licensed to commercial entities. Strategic partnerships between materials developers and battery manufacturers will likely accelerate the transition from laboratory to commercial production. Recent pilot-scale demonstrations by companies in Asia and North America suggest that initial commercial applications could emerge within 3-5 years, particularly in specialized market segments where performance advantages outweigh cost premiums.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!