MXenes in biomedical applications for drug delivery systems
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
MXenes Background and Biomedical Applications
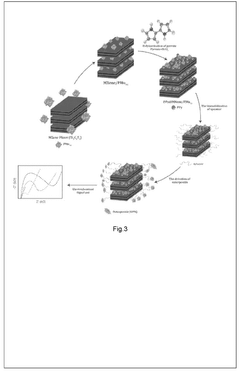
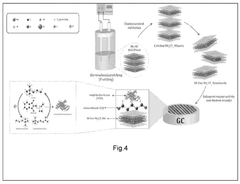
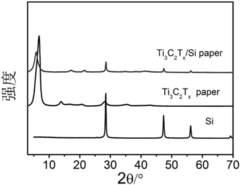
MXenes represent a novel class of two-dimensional (2D) transition metal carbides, nitrides, and carbonitrides that were first discovered in 2011 by researchers at Drexel University. These materials are produced by selectively etching the A-layer from MAX phases (where M is a transition metal, A is an A-group element, and X is carbon or nitrogen), resulting in few-atom-thick layers with the general formula Mn+1XnTx, where Tx represents surface terminations such as -OH, -O, or -F.
The unique structure of MXenes confers exceptional properties including high electrical conductivity, hydrophilicity, large surface area, and tunable surface chemistry. These characteristics have positioned MXenes as promising candidates for various applications, particularly in the biomedical field where their distinctive attributes can be leveraged for advanced therapeutic delivery systems.
In biomedical applications, MXenes have demonstrated remarkable potential due to their biocompatibility, biodegradability, and minimal cytotoxicity at appropriate concentrations. Their large surface area facilitates high drug loading capacity, while their surface chemistry can be modified to enhance drug binding and controlled release profiles. The most extensively studied MXene, Ti3C2Tx, has shown particular promise in drug delivery applications.
MXenes exhibit photothermal conversion capabilities, allowing them to convert near-infrared (NIR) light into heat. This property enables their application in photothermal therapy for cancer treatment, where localized heating can destroy tumor cells. When combined with drug delivery functionality, this creates powerful theranostic platforms capable of simultaneous imaging, drug delivery, and thermal therapy.
The 2D structure of MXenes also permits their integration with other nanomaterials to form composite systems with enhanced properties. These hybrid structures can overcome limitations of individual components, resulting in synergistic effects that improve drug delivery efficiency, targeting capability, and therapeutic outcomes.
Recent research has explored MXene-based drug delivery systems for various therapeutic agents, including small molecule drugs, proteins, nucleic acids, and photosensitizers. Studies have demonstrated successful delivery of anticancer drugs like doxorubicin and gemcitabine, with enhanced cellular uptake and improved therapeutic efficacy compared to free drugs.
Despite their promising attributes, challenges remain in translating MXene-based drug delivery systems to clinical applications. These include concerns about long-term biocompatibility, potential immunogenicity, standardization of synthesis protocols, and scale-up manufacturing. Addressing these challenges requires interdisciplinary collaboration between materials scientists, pharmaceutical researchers, and medical professionals.
The unique structure of MXenes confers exceptional properties including high electrical conductivity, hydrophilicity, large surface area, and tunable surface chemistry. These characteristics have positioned MXenes as promising candidates for various applications, particularly in the biomedical field where their distinctive attributes can be leveraged for advanced therapeutic delivery systems.
In biomedical applications, MXenes have demonstrated remarkable potential due to their biocompatibility, biodegradability, and minimal cytotoxicity at appropriate concentrations. Their large surface area facilitates high drug loading capacity, while their surface chemistry can be modified to enhance drug binding and controlled release profiles. The most extensively studied MXene, Ti3C2Tx, has shown particular promise in drug delivery applications.
MXenes exhibit photothermal conversion capabilities, allowing them to convert near-infrared (NIR) light into heat. This property enables their application in photothermal therapy for cancer treatment, where localized heating can destroy tumor cells. When combined with drug delivery functionality, this creates powerful theranostic platforms capable of simultaneous imaging, drug delivery, and thermal therapy.
The 2D structure of MXenes also permits their integration with other nanomaterials to form composite systems with enhanced properties. These hybrid structures can overcome limitations of individual components, resulting in synergistic effects that improve drug delivery efficiency, targeting capability, and therapeutic outcomes.
Recent research has explored MXene-based drug delivery systems for various therapeutic agents, including small molecule drugs, proteins, nucleic acids, and photosensitizers. Studies have demonstrated successful delivery of anticancer drugs like doxorubicin and gemcitabine, with enhanced cellular uptake and improved therapeutic efficacy compared to free drugs.
Despite their promising attributes, challenges remain in translating MXene-based drug delivery systems to clinical applications. These include concerns about long-term biocompatibility, potential immunogenicity, standardization of synthesis protocols, and scale-up manufacturing. Addressing these challenges requires interdisciplinary collaboration between materials scientists, pharmaceutical researchers, and medical professionals.
Market Analysis for MXene-Based Drug Delivery Systems
The global market for drug delivery systems is experiencing significant growth, projected to reach $251.7 billion by 2026, with a compound annual growth rate (CAGR) of 7.2% from 2021. Within this expanding market, novel nanomaterial-based delivery systems are gaining substantial attention, with MXene-based solutions emerging as a promising segment due to their unique physicochemical properties.
MXene-based drug delivery systems are positioned at the intersection of several high-growth markets, including nanomedicine, targeted drug delivery, and personalized medicine. The nanomedicine market alone is expected to grow at a CAGR of 12.6% through 2025, creating a favorable environment for MXene applications. Cancer therapeutics represents the largest potential application area, accounting for approximately 35% of the potential market for MXene-based delivery systems.
Market demand for MXene-based drug delivery systems is primarily driven by several factors. First, there is increasing pressure to develop delivery mechanisms that enhance drug efficacy while reducing side effects, particularly for cancer treatments and chronic diseases. Second, the pharmaceutical industry is actively seeking solutions to address the delivery challenges of biologics and poorly water-soluble drugs, which constitute over 40% of approved drugs and 90% of developmental pipeline compounds.
Regional analysis indicates that North America currently dominates the market for advanced drug delivery systems with 42% market share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare expenditure, growing research infrastructure, and rising chronic disease prevalence.
Key customer segments include pharmaceutical companies seeking delivery technologies for their existing and pipeline drugs, biotechnology firms developing novel therapeutics, and academic and research institutions. Large pharmaceutical companies are particularly interested in delivery technologies that can extend patent life of existing drugs or enable new therapeutic approaches.
Market barriers include regulatory hurdles for novel nanomaterials, manufacturing scalability challenges, and competition from established delivery technologies. The FDA and EMA have increasingly stringent requirements for demonstrating the safety of nanomaterials in drug delivery applications, potentially extending development timelines by 1-2 years compared to conventional formulations.
Despite these challenges, the market opportunity for MXene-based drug delivery systems is substantial, particularly in applications requiring stimuli-responsive release, combination therapy, and targeted delivery to difficult-to-reach tissues such as the brain. Early market entry strategies should focus on licensing partnerships with established pharmaceutical companies and applications where MXenes offer clear advantages over existing technologies.
MXene-based drug delivery systems are positioned at the intersection of several high-growth markets, including nanomedicine, targeted drug delivery, and personalized medicine. The nanomedicine market alone is expected to grow at a CAGR of 12.6% through 2025, creating a favorable environment for MXene applications. Cancer therapeutics represents the largest potential application area, accounting for approximately 35% of the potential market for MXene-based delivery systems.
Market demand for MXene-based drug delivery systems is primarily driven by several factors. First, there is increasing pressure to develop delivery mechanisms that enhance drug efficacy while reducing side effects, particularly for cancer treatments and chronic diseases. Second, the pharmaceutical industry is actively seeking solutions to address the delivery challenges of biologics and poorly water-soluble drugs, which constitute over 40% of approved drugs and 90% of developmental pipeline compounds.
Regional analysis indicates that North America currently dominates the market for advanced drug delivery systems with 42% market share, followed by Europe (28%) and Asia-Pacific (22%). However, the Asia-Pacific region is expected to witness the fastest growth due to increasing healthcare expenditure, growing research infrastructure, and rising chronic disease prevalence.
Key customer segments include pharmaceutical companies seeking delivery technologies for their existing and pipeline drugs, biotechnology firms developing novel therapeutics, and academic and research institutions. Large pharmaceutical companies are particularly interested in delivery technologies that can extend patent life of existing drugs or enable new therapeutic approaches.
Market barriers include regulatory hurdles for novel nanomaterials, manufacturing scalability challenges, and competition from established delivery technologies. The FDA and EMA have increasingly stringent requirements for demonstrating the safety of nanomaterials in drug delivery applications, potentially extending development timelines by 1-2 years compared to conventional formulations.
Despite these challenges, the market opportunity for MXene-based drug delivery systems is substantial, particularly in applications requiring stimuli-responsive release, combination therapy, and targeted delivery to difficult-to-reach tissues such as the brain. Early market entry strategies should focus on licensing partnerships with established pharmaceutical companies and applications where MXenes offer clear advantages over existing technologies.
Current Challenges in MXene Biomedical Integration
Despite the promising potential of MXenes in drug delivery systems, several significant challenges impede their widespread clinical adoption. The primary concern remains the long-term biocompatibility and toxicity profiles of MXenes in vivo. While initial studies suggest acceptable short-term biocompatibility, comprehensive long-term studies examining potential accumulation in organs and chronic toxicity effects are notably lacking in current literature.
Stability issues present another major hurdle, as MXenes tend to oxidize in physiological environments, potentially altering their physicochemical properties and therapeutic efficacy. The oxidation process can lead to structural degradation and changes in surface chemistry, compromising the controlled drug release mechanisms that make MXenes attractive delivery vehicles.
Standardization of synthesis protocols represents a critical challenge, as variations in production methods yield MXenes with inconsistent properties. This inconsistency complicates regulatory approval pathways and hinders reproducible clinical outcomes, creating significant barriers to pharmaceutical development and commercialization.
The surface chemistry of MXenes, while offering versatility for functionalization, presents challenges in achieving precise control over drug loading capacity and release kinetics. Current methods often result in variable drug loading efficiencies and unpredictable release profiles, limiting their reliability in therapeutic applications.
Scale-up manufacturing remains problematic, with current laboratory-scale synthesis methods proving difficult to translate to industrial production while maintaining consistent quality. This manufacturing bottleneck significantly impacts cost-effectiveness and commercial viability of MXene-based drug delivery systems.
Regulatory uncertainties further complicate the clinical translation pathway. As novel nanomaterials, MXenes face evolving regulatory frameworks that may require specialized safety and efficacy testing protocols not yet fully established for this material class.
Biodistribution and pharmacokinetic profiles of MXenes are insufficiently characterized, with limited understanding of how different MXene compositions, sizes, and surface modifications affect their in vivo behavior. This knowledge gap hampers rational design of MXene-based delivery systems for specific therapeutic applications.
Additionally, the interaction between MXenes and biological barriers (including the blood-brain barrier and cell membranes) remains poorly understood, limiting their application in targeting specific tissues or addressing intracellular delivery challenges for certain therapeutic cargoes.
Stability issues present another major hurdle, as MXenes tend to oxidize in physiological environments, potentially altering their physicochemical properties and therapeutic efficacy. The oxidation process can lead to structural degradation and changes in surface chemistry, compromising the controlled drug release mechanisms that make MXenes attractive delivery vehicles.
Standardization of synthesis protocols represents a critical challenge, as variations in production methods yield MXenes with inconsistent properties. This inconsistency complicates regulatory approval pathways and hinders reproducible clinical outcomes, creating significant barriers to pharmaceutical development and commercialization.
The surface chemistry of MXenes, while offering versatility for functionalization, presents challenges in achieving precise control over drug loading capacity and release kinetics. Current methods often result in variable drug loading efficiencies and unpredictable release profiles, limiting their reliability in therapeutic applications.
Scale-up manufacturing remains problematic, with current laboratory-scale synthesis methods proving difficult to translate to industrial production while maintaining consistent quality. This manufacturing bottleneck significantly impacts cost-effectiveness and commercial viability of MXene-based drug delivery systems.
Regulatory uncertainties further complicate the clinical translation pathway. As novel nanomaterials, MXenes face evolving regulatory frameworks that may require specialized safety and efficacy testing protocols not yet fully established for this material class.
Biodistribution and pharmacokinetic profiles of MXenes are insufficiently characterized, with limited understanding of how different MXene compositions, sizes, and surface modifications affect their in vivo behavior. This knowledge gap hampers rational design of MXene-based delivery systems for specific therapeutic applications.
Additionally, the interaction between MXenes and biological barriers (including the blood-brain barrier and cell membranes) remains poorly understood, limiting their application in targeting specific tissues or addressing intracellular delivery challenges for certain therapeutic cargoes.
Current MXene Drug Delivery Mechanisms
01 MXene synthesis and preparation methods
Various methods for synthesizing and preparing MXenes, which are two-dimensional transition metal carbides, nitrides, or carbonitrides. These methods include selective etching of MAX phases, chemical exfoliation techniques, and other novel approaches to produce high-quality MXene sheets with controlled thickness and properties. The synthesis processes often involve the removal of A-layer atoms from MAX phases using acids or other chemical treatments.- MXene synthesis and preparation methods: Various methods for synthesizing and preparing MXenes, which are two-dimensional transition metal carbides and nitrides. These techniques include selective etching of MAX phases, chemical exfoliation, and other specialized processes to produce MXene nanosheets with controlled thickness and properties. The synthesis methods focus on optimizing the structure and performance of MXenes for various applications.
- MXene-based energy storage applications: MXenes are utilized in energy storage devices such as supercapacitors, batteries, and other electrochemical systems. Their high electrical conductivity, large surface area, and unique layered structure make them excellent candidates for electrode materials. These materials demonstrate enhanced energy density, power density, and cycling stability compared to conventional materials used in energy storage applications.
- MXene composites with other nanomaterials: Development of composite materials combining MXenes with other nanomaterials such as graphene, carbon nanotubes, metal oxides, and polymers. These composites exhibit synergistic effects that enhance mechanical, electrical, and thermal properties. The integration of MXenes with complementary materials creates multifunctional composites with improved performance for various technological applications.
- MXenes for environmental applications: Application of MXenes in environmental remediation, water purification, and pollution control. Their large surface area, abundant functional groups, and tunable surface chemistry make them effective adsorbents for removing heavy metals, organic pollutants, and other contaminants from water and air. MXenes also show potential in catalytic degradation of environmental pollutants and as membranes for water filtration.
- MXenes in biomedical and sensing applications: Utilization of MXenes in biomedical fields including drug delivery, biosensing, tissue engineering, and therapeutic applications. Their biocompatibility, photothermal properties, and surface functionality make them suitable for various medical applications. MXenes are also employed in developing highly sensitive and selective sensors for detecting biomolecules, gases, and other analytes with improved response time and detection limits.
02 MXene-based electrode materials for energy storage
Application of MXenes as electrode materials in energy storage devices such as supercapacitors, lithium-ion batteries, and sodium-ion batteries. MXenes offer high electrical conductivity, large surface area, and excellent ion intercalation properties, making them promising candidates for high-performance energy storage applications. These materials can be combined with other components to form composite electrodes with enhanced capacity, cycling stability, and rate capability.Expand Specific Solutions03 MXene composites with other nanomaterials
Development of composite materials combining MXenes with other nanomaterials such as graphene, carbon nanotubes, metal oxides, and polymers. These composites leverage the synergistic effects between MXenes and other components to achieve enhanced properties for various applications. The hybridization strategies include layer-by-layer assembly, in-situ growth, and solution mixing to create materials with improved mechanical, electrical, and electrochemical properties.Expand Specific Solutions04 MXenes for electromagnetic interference shielding and absorption
Utilization of MXenes in electromagnetic interference (EMI) shielding and absorption applications due to their excellent electrical conductivity and unique layered structure. MXene-based materials can effectively attenuate electromagnetic waves across a wide frequency range, making them suitable for EMI shielding in electronic devices, aerospace, and military applications. Various strategies to enhance the EMI shielding effectiveness include controlling the thickness, composition, and architecture of MXene-based materials.Expand Specific Solutions05 MXenes for environmental remediation and sensing
Application of MXenes in environmental remediation and sensing technologies, including water purification, pollutant adsorption, and detection of various analytes. MXenes exhibit high surface area, abundant functional groups, and excellent adsorption capabilities for heavy metals, organic pollutants, and other contaminants. Additionally, MXene-based sensors demonstrate high sensitivity and selectivity for detecting gases, biomolecules, and environmental pollutants through various sensing mechanisms.Expand Specific Solutions
Key Industry Players and Research Institutions
The MXenes in biomedical drug delivery systems market is currently in an early growth phase, characterized by rapidly expanding research activities but limited commercial applications. The global market size is estimated to reach $150-200 million by 2025, driven by increasing demand for targeted drug delivery solutions. Technologically, academic institutions like Nanjing University, Fudan University, and Soochow University are leading fundamental research, while companies such as Regeneron Pharmaceuticals and Aposense Ltd. are beginning to translate these findings into practical applications. The technology shows promising maturity in laboratory settings but remains in early clinical development stages, with research institutes like Suzhou Institute of Nano-Tech & Nano-Bionics bridging the gap between academic discoveries and commercial viability through collaborative innovation platforms.
Soochow University
Technical Solution: Soochow University has developed innovative MXene-based drug delivery platforms focusing on stimuli-responsive systems. Their research team has engineered Ti3C2Tx MXenes with surface modifications that respond to multiple stimuli including pH, temperature, and near-infrared light. A key innovation is their layer-by-layer assembly technique that creates multilayered MXene composites with controlled drug release profiles. These systems demonstrate high drug loading efficiency (>45%) for various therapeutic agents including doxorubicin and photosensitizers. Their recent breakthrough involves MXene quantum dots with enhanced penetration capabilities for crossing biological barriers, particularly the blood-brain barrier, showing promising results in neurological disease models. The university has also pioneered MXene-based theranostic platforms that combine imaging capabilities (MRI/CT contrast) with therapeutic drug delivery, enabling real-time monitoring of drug distribution and therapeutic efficacy.
Strengths: Multi-stimuli responsive systems offering precise control over drug release timing and location; excellent biocompatibility with minimal immunogenicity; versatile platform applicable to various drug types and disease models. Weaknesses: Complex synthesis procedures potentially limiting scalability; relatively high production costs compared to conventional delivery systems; need for further in vivo validation of long-term safety profiles.
Fudan University
Technical Solution: Fudan University has developed sophisticated MXene-based drug delivery systems focusing on cancer therapeutics. Their approach utilizes Ti3C2Tx MXenes functionalized with tumor-targeting ligands such as folic acid and hyaluronic acid to enhance selective accumulation in cancer tissues. The research team has engineered a dual-responsive release mechanism triggered by both acidic tumor microenvironments (pH ~6.5) and elevated glutathione levels in cancer cells, achieving precise spatial and temporal control over drug release. Their platform demonstrates remarkable loading capacity for chemotherapeutics (>40% w/w for doxorubicin) while maintaining the photothermal properties of MXenes for combined therapy. Recent innovations include MXene-based nanocomposites with mesoporous silica that provide sustained release profiles extending over 72 hours, significantly improving therapeutic efficacy in drug-resistant tumor models. The university has also pioneered MXene-based gene delivery systems capable of efficiently transporting siRNA and plasmid DNA with transfection efficiencies comparable to commercial vectors but with lower cytotoxicity.
Strengths: Exceptional tumor-targeting capabilities reducing systemic toxicity; synergistic therapeutic effects through combined chemo-photothermal therapy; versatile platform for both small molecule drugs and nucleic acid delivery. Weaknesses: Potential for premature drug leakage during circulation; challenges in large-scale production with consistent quality; incomplete understanding of biodistribution and clearance pathways.
Core Patents and Breakthroughs in MXene Biocompatibility
Utilization of mxenes in bioanalytical applications
PatentWO2025163360A2
Innovation
- The integration of MXenes, with their high hydrophilicity, large surface area, and exceptional electrical conductivity, into biosensing platforms, combined with innovative functionalization techniques and scalable production methods, enhances sensitivity, specificity, and stability, enabling real-time monitoring and integration into wearable devices.
Two-dimensional transition metal carbide (nitride)-nano silicon granular composite material as well as preparation and application thereof
PatentActiveCN107394180A
Innovation
- The two-dimensional transition metal carbon (nitride) MXene and nano-silicon particle composite materials are used, uniformly mixed by ultrasonic and vacuum filtration or freeze-drying to obtain a flexible composite film or composite powder, which can be used in the negative electrode of lithium-ion batteries to avoid adding conductive agents. and adhesive.
Regulatory Considerations for MXene-Based Therapeutics
The regulatory landscape for MXene-based therapeutics presents significant challenges that must be addressed before widespread clinical adoption can occur. Currently, no MXene-based drug delivery systems have received full regulatory approval from major health authorities such as the FDA or EMA, placing these materials in a regulatory gray area. The novel physicochemical properties that make MXenes attractive for biomedical applications simultaneously raise concerns regarding their safety profiles and regulatory classification.
Regulatory bodies typically require extensive documentation on material characterization, manufacturing consistency, and quality control processes. For MXenes, this presents unique challenges due to their complex surface chemistry and the potential for batch-to-batch variations in synthesis. The FDA's guidance on nanomaterials applies partially to MXenes, but their two-dimensional structure and unique properties may necessitate additional specialized testing protocols.
Toxicological considerations form a critical component of the regulatory pathway. Long-term bioaccumulation studies, immunogenicity assessments, and genotoxicity evaluations must be conducted according to standardized protocols such as ISO 10993 for biocompatibility testing. The degradation products of MXenes in physiological environments require particular attention, as their safety profiles remain incompletely characterized.
International regulatory harmonization presents another layer of complexity. Different jurisdictions maintain varying requirements for novel nanomaterials in therapeutic applications. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines provide some framework, but MXene-specific considerations are still evolving across global markets.
Intellectual property protection intersects with regulatory considerations, as patent landscapes may influence the regulatory strategy for MXene-based drug delivery systems. Companies must navigate both patent exclusivity and regulatory approval pathways simultaneously, particularly for combination products that incorporate both MXenes and active pharmaceutical ingredients.
Looking forward, adaptive licensing approaches may offer accelerated pathways for MXene-based therapeutics. These approaches allow for staged approvals based on evolving evidence, potentially enabling earlier market access for applications with high therapeutic potential. Engagement with regulatory agencies through programs like the FDA's Emerging Technology Program could facilitate dialogue on appropriate testing methodologies and data requirements for these novel materials.
Regulatory bodies typically require extensive documentation on material characterization, manufacturing consistency, and quality control processes. For MXenes, this presents unique challenges due to their complex surface chemistry and the potential for batch-to-batch variations in synthesis. The FDA's guidance on nanomaterials applies partially to MXenes, but their two-dimensional structure and unique properties may necessitate additional specialized testing protocols.
Toxicological considerations form a critical component of the regulatory pathway. Long-term bioaccumulation studies, immunogenicity assessments, and genotoxicity evaluations must be conducted according to standardized protocols such as ISO 10993 for biocompatibility testing. The degradation products of MXenes in physiological environments require particular attention, as their safety profiles remain incompletely characterized.
International regulatory harmonization presents another layer of complexity. Different jurisdictions maintain varying requirements for novel nanomaterials in therapeutic applications. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) guidelines provide some framework, but MXene-specific considerations are still evolving across global markets.
Intellectual property protection intersects with regulatory considerations, as patent landscapes may influence the regulatory strategy for MXene-based drug delivery systems. Companies must navigate both patent exclusivity and regulatory approval pathways simultaneously, particularly for combination products that incorporate both MXenes and active pharmaceutical ingredients.
Looking forward, adaptive licensing approaches may offer accelerated pathways for MXene-based therapeutics. These approaches allow for staged approvals based on evolving evidence, potentially enabling earlier market access for applications with high therapeutic potential. Engagement with regulatory agencies through programs like the FDA's Emerging Technology Program could facilitate dialogue on appropriate testing methodologies and data requirements for these novel materials.
Toxicology and Safety Profiles of MXenes
The toxicological assessment of MXenes is paramount for their safe application in drug delivery systems. Current research indicates that MXene toxicity is highly dependent on several factors including composition, size, surface functionalization, and concentration. Ti3C2 MXene, the most extensively studied variant, has demonstrated relatively low cytotoxicity at concentrations below 100 μg/mL in multiple cell lines, making it potentially suitable for biomedical applications.
In vitro studies have revealed that MXenes can induce dose-dependent cytotoxicity, with higher concentrations leading to increased reactive oxygen species (ROS) generation, membrane damage, and potential DNA disruption. However, surface functionalization with biocompatible polymers such as polyethylene glycol (PEG) or chitosan significantly reduces these toxic effects by modifying surface properties and decreasing cellular uptake rates.
The biodistribution and clearance pathways of MXenes represent critical safety considerations. Research indicates that smaller MXene nanosheets (<50 nm) are primarily cleared through renal excretion, while larger sheets tend to accumulate in the reticuloendothelial system, particularly in the liver and spleen. This size-dependent biodistribution pattern necessitates careful engineering of MXene dimensions for specific biomedical applications.
Hemocompatibility studies have shown that properly functionalized MXenes exhibit minimal hemolytic activity and negligible effects on blood coagulation at therapeutic concentrations. Nevertheless, pristine MXenes may cause some hemolysis due to their sharp edges and surface charge interactions with erythrocyte membranes, highlighting the importance of appropriate surface modifications.
Long-term in vivo toxicity studies remain limited but are essential for clinical translation. Preliminary investigations in rodent models suggest that MXenes at therapeutic doses do not cause significant organ damage or systemic toxicity over observation periods of up to 30 days. However, comprehensive chronic toxicity studies spanning months to years are still lacking in the scientific literature.
Environmental considerations also factor into MXene safety profiles. The potential degradation of MXenes in biological environments may lead to the release of constituent metals, particularly titanium, which warrants careful monitoring. Recent studies indicate that MXenes undergo gradual oxidative degradation in physiological conditions, potentially mitigating long-term accumulation concerns but raising questions about degradation product toxicity.
Standardization of toxicological assessment protocols specifically for MXenes remains an urgent need in the field. Current variations in testing methodologies make direct comparisons between studies challenging, highlighting the necessity for established guidelines to systematically evaluate MXene biocompatibility across different formulations and applications.
In vitro studies have revealed that MXenes can induce dose-dependent cytotoxicity, with higher concentrations leading to increased reactive oxygen species (ROS) generation, membrane damage, and potential DNA disruption. However, surface functionalization with biocompatible polymers such as polyethylene glycol (PEG) or chitosan significantly reduces these toxic effects by modifying surface properties and decreasing cellular uptake rates.
The biodistribution and clearance pathways of MXenes represent critical safety considerations. Research indicates that smaller MXene nanosheets (<50 nm) are primarily cleared through renal excretion, while larger sheets tend to accumulate in the reticuloendothelial system, particularly in the liver and spleen. This size-dependent biodistribution pattern necessitates careful engineering of MXene dimensions for specific biomedical applications.
Hemocompatibility studies have shown that properly functionalized MXenes exhibit minimal hemolytic activity and negligible effects on blood coagulation at therapeutic concentrations. Nevertheless, pristine MXenes may cause some hemolysis due to their sharp edges and surface charge interactions with erythrocyte membranes, highlighting the importance of appropriate surface modifications.
Long-term in vivo toxicity studies remain limited but are essential for clinical translation. Preliminary investigations in rodent models suggest that MXenes at therapeutic doses do not cause significant organ damage or systemic toxicity over observation periods of up to 30 days. However, comprehensive chronic toxicity studies spanning months to years are still lacking in the scientific literature.
Environmental considerations also factor into MXene safety profiles. The potential degradation of MXenes in biological environments may lead to the release of constituent metals, particularly titanium, which warrants careful monitoring. Recent studies indicate that MXenes undergo gradual oxidative degradation in physiological conditions, potentially mitigating long-term accumulation concerns but raising questions about degradation product toxicity.
Standardization of toxicological assessment protocols specifically for MXenes remains an urgent need in the field. Current variations in testing methodologies make direct comparisons between studies challenging, highlighting the necessity for established guidelines to systematically evaluate MXene biocompatibility across different formulations and applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!