Natural Pigment Stabilization Techniques For High-Temperature Dyeing
SEP 2, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Natural Pigment Evolution and Stabilization Goals
Natural pigments have been utilized for coloration since ancient civilizations, with evidence dating back to prehistoric cave paintings and Egyptian artifacts. These pigments, derived from plants, minerals, and animals, were the primary colorants until the mid-19th century when synthetic dyes revolutionized the textile industry. The evolution of natural pigment usage has been characterized by periods of innovation followed by decline, particularly after the introduction of synthetic alternatives that offered greater color consistency, fastness, and economic viability.
In recent decades, however, there has been a resurgence of interest in natural pigments due to increasing environmental concerns and consumer demand for sustainable products. This renewed focus has highlighted the inherent limitations of natural colorants, particularly their instability under high-temperature processing conditions commonly used in modern dyeing operations. The degradation of natural pigments during high-temperature dyeing results in color shifts, reduced intensity, and poor fastness properties, significantly limiting their commercial application.
The primary technical challenge lies in the molecular structure of natural pigments, which often contain conjugated systems and functional groups susceptible to thermal decomposition, oxidation, and hydrolysis. For instance, anthocyanins from berries undergo structural changes above 60°C, while carotenoids isomerize at elevated temperatures, altering their chromophoric properties. Chlorophylls readily degrade through pheophytinization when exposed to heat, resulting in undesirable olive-brown hues.
Current stabilization goals focus on developing techniques that preserve the chromophoric integrity of natural pigments during high-temperature dyeing processes (typically 80-130°C). These goals include enhancing thermal stability through molecular modification, developing protective delivery systems, optimizing process parameters, and creating synergistic formulations that mitigate degradation mechanisms.
The technical objectives extend beyond mere color preservation to address industrial requirements such as reproducibility, scalability, and cost-effectiveness. Researchers aim to achieve color fastness properties comparable to synthetic dyes while maintaining the environmental benefits of natural alternatives. Additionally, there is growing interest in developing universal stabilization approaches that can be applied across diverse pigment classes rather than pigment-specific solutions.
Emerging research directions include biomimetic approaches inspired by natural stabilization mechanisms found in biological systems, the application of nanotechnology for encapsulation and controlled release, and the exploration of enzymatic modifications to enhance pigment resilience. The ultimate goal is to bridge the performance gap between natural and synthetic colorants, enabling broader adoption of sustainable dyeing practices across textile, food, cosmetic, and other industrial sectors.
In recent decades, however, there has been a resurgence of interest in natural pigments due to increasing environmental concerns and consumer demand for sustainable products. This renewed focus has highlighted the inherent limitations of natural colorants, particularly their instability under high-temperature processing conditions commonly used in modern dyeing operations. The degradation of natural pigments during high-temperature dyeing results in color shifts, reduced intensity, and poor fastness properties, significantly limiting their commercial application.
The primary technical challenge lies in the molecular structure of natural pigments, which often contain conjugated systems and functional groups susceptible to thermal decomposition, oxidation, and hydrolysis. For instance, anthocyanins from berries undergo structural changes above 60°C, while carotenoids isomerize at elevated temperatures, altering their chromophoric properties. Chlorophylls readily degrade through pheophytinization when exposed to heat, resulting in undesirable olive-brown hues.
Current stabilization goals focus on developing techniques that preserve the chromophoric integrity of natural pigments during high-temperature dyeing processes (typically 80-130°C). These goals include enhancing thermal stability through molecular modification, developing protective delivery systems, optimizing process parameters, and creating synergistic formulations that mitigate degradation mechanisms.
The technical objectives extend beyond mere color preservation to address industrial requirements such as reproducibility, scalability, and cost-effectiveness. Researchers aim to achieve color fastness properties comparable to synthetic dyes while maintaining the environmental benefits of natural alternatives. Additionally, there is growing interest in developing universal stabilization approaches that can be applied across diverse pigment classes rather than pigment-specific solutions.
Emerging research directions include biomimetic approaches inspired by natural stabilization mechanisms found in biological systems, the application of nanotechnology for encapsulation and controlled release, and the exploration of enzymatic modifications to enhance pigment resilience. The ultimate goal is to bridge the performance gap between natural and synthetic colorants, enabling broader adoption of sustainable dyeing practices across textile, food, cosmetic, and other industrial sectors.
Market Demand for Eco-Friendly High-Temperature Dyes
The global market for eco-friendly high-temperature dyes has witnessed significant growth in recent years, driven by increasing consumer awareness about environmental issues and stringent regulations on chemical usage in textile processing. The demand for natural pigment stabilization techniques specifically for high-temperature dyeing processes has emerged as a critical market segment with substantial growth potential.
Consumer preferences have shifted dramatically toward sustainable and environmentally responsible products, with surveys indicating that over 70% of consumers in developed markets are willing to pay premium prices for eco-friendly textiles. This trend is particularly pronounced in fashion and home textile sectors, where end-users increasingly demand transparency regarding production methods and material sourcing.
Regulatory frameworks across major markets including the European Union, North America, and increasingly in Asia-Pacific regions have implemented restrictions on synthetic dyes containing hazardous chemicals. The EU's REACH regulations and similar frameworks in other regions have accelerated the transition toward natural alternatives, creating market opportunities for stabilized natural pigments capable of withstanding high-temperature dyeing processes.
The industrial textile sector represents another significant market driver, with automotive, medical, and technical textile manufacturers seeking natural dyeing solutions that meet their performance requirements without compromising on environmental standards. These industries require color stability under extreme conditions, creating specialized demand for advanced natural pigment stabilization technologies.
Market analysis reveals that the natural dye segment is growing at approximately twice the rate of the overall textile dye market, with particular acceleration in applications requiring high-temperature processing. This growth trajectory is expected to continue as manufacturing facilities invest in equipment and processes compatible with natural pigment applications.
Regional market distribution shows varying adoption rates, with European markets leading in premium eco-friendly textile production, while Asian markets, particularly India and China, leverage their traditional knowledge of natural dyeing techniques to develop industrial-scale applications. North American markets show strong growth in specialized technical textile applications using stabilized natural pigments.
Supply chain considerations have become increasingly important, with brands seeking vertical integration to ensure consistent quality and sustainability credentials. This has created opportunities for technology providers who can deliver stabilization techniques that maintain color integrity throughout complex supply chains and diverse manufacturing environments.
The economic viability of natural pigment stabilization techniques has improved significantly, narrowing the cost gap with synthetic alternatives. This trend, combined with consumer willingness to pay premium prices for sustainable products, has created a favorable market environment for continued innovation and commercialization of advanced natural pigment stabilization technologies for high-temperature dyeing applications.
Consumer preferences have shifted dramatically toward sustainable and environmentally responsible products, with surveys indicating that over 70% of consumers in developed markets are willing to pay premium prices for eco-friendly textiles. This trend is particularly pronounced in fashion and home textile sectors, where end-users increasingly demand transparency regarding production methods and material sourcing.
Regulatory frameworks across major markets including the European Union, North America, and increasingly in Asia-Pacific regions have implemented restrictions on synthetic dyes containing hazardous chemicals. The EU's REACH regulations and similar frameworks in other regions have accelerated the transition toward natural alternatives, creating market opportunities for stabilized natural pigments capable of withstanding high-temperature dyeing processes.
The industrial textile sector represents another significant market driver, with automotive, medical, and technical textile manufacturers seeking natural dyeing solutions that meet their performance requirements without compromising on environmental standards. These industries require color stability under extreme conditions, creating specialized demand for advanced natural pigment stabilization technologies.
Market analysis reveals that the natural dye segment is growing at approximately twice the rate of the overall textile dye market, with particular acceleration in applications requiring high-temperature processing. This growth trajectory is expected to continue as manufacturing facilities invest in equipment and processes compatible with natural pigment applications.
Regional market distribution shows varying adoption rates, with European markets leading in premium eco-friendly textile production, while Asian markets, particularly India and China, leverage their traditional knowledge of natural dyeing techniques to develop industrial-scale applications. North American markets show strong growth in specialized technical textile applications using stabilized natural pigments.
Supply chain considerations have become increasingly important, with brands seeking vertical integration to ensure consistent quality and sustainability credentials. This has created opportunities for technology providers who can deliver stabilization techniques that maintain color integrity throughout complex supply chains and diverse manufacturing environments.
The economic viability of natural pigment stabilization techniques has improved significantly, narrowing the cost gap with synthetic alternatives. This trend, combined with consumer willingness to pay premium prices for sustainable products, has created a favorable market environment for continued innovation and commercialization of advanced natural pigment stabilization technologies for high-temperature dyeing applications.
Technical Barriers in Natural Pigment Stability
Natural pigments face significant stability challenges when exposed to high-temperature dyeing processes, which constitute a major technical barrier for their widespread industrial application. The inherent molecular structure of most natural colorants contains conjugated double bonds and chromophore groups that are susceptible to thermal degradation when temperatures exceed 80-100°C, commonly required in commercial dyeing operations. This thermal instability manifests as color fading, hue shifting, or complete chromophore breakdown.
The chemical mechanisms underlying this instability include oxidation reactions, hydrolysis of glycosidic bonds in anthocyanins, and structural rearrangements that alter the pigment's light absorption properties. For instance, betacyanins from beetroot rapidly degrade above 70°C, while curcumin from turmeric undergoes significant molecular transformation at temperatures above 90°C, resulting in diminished tinctorial strength.
pH sensitivity compounds these thermal stability issues, as many natural pigments exhibit altered stability profiles under different pH conditions during high-temperature processing. Anthocyanins, for example, demonstrate optimal stability at acidic pH but undergo irreversible structural changes when heated at neutral or alkaline conditions, presenting a complex matrix of variables that must be controlled simultaneously.
Metal ion interactions present another significant barrier, as trace metals in dyeing equipment or water supplies can catalyze oxidation reactions in natural pigments when heated. These interactions accelerate degradation pathways and can cause unexpected color changes, particularly with flavonoid-based pigments that readily form metal complexes under elevated temperatures.
Light exposure during high-temperature processing further exacerbates stability issues through photo-oxidation mechanisms. The combination of heat and light energy provides sufficient activation energy for degradation reactions that might otherwise remain dormant at ambient conditions, creating synergistic degradation effects that are difficult to mitigate in industrial settings.
Scalability presents additional challenges, as laboratory-scale stabilization techniques often fail when transferred to industrial production environments where temperature control is less precise and processing times are extended. The economic viability of potential stabilization methods is further constrained by cost considerations, as expensive additives or complex processing modifications may render natural pigment applications commercially unviable compared to synthetic alternatives.
Regulatory limitations also restrict potential technical solutions, as many chemical stabilizers permitted for synthetic colorants are not approved for natural pigment applications, particularly in sensitive sectors like food, cosmetics, and textiles with direct skin contact. This regulatory landscape narrows the available toolbox for addressing thermal stability barriers.
The chemical mechanisms underlying this instability include oxidation reactions, hydrolysis of glycosidic bonds in anthocyanins, and structural rearrangements that alter the pigment's light absorption properties. For instance, betacyanins from beetroot rapidly degrade above 70°C, while curcumin from turmeric undergoes significant molecular transformation at temperatures above 90°C, resulting in diminished tinctorial strength.
pH sensitivity compounds these thermal stability issues, as many natural pigments exhibit altered stability profiles under different pH conditions during high-temperature processing. Anthocyanins, for example, demonstrate optimal stability at acidic pH but undergo irreversible structural changes when heated at neutral or alkaline conditions, presenting a complex matrix of variables that must be controlled simultaneously.
Metal ion interactions present another significant barrier, as trace metals in dyeing equipment or water supplies can catalyze oxidation reactions in natural pigments when heated. These interactions accelerate degradation pathways and can cause unexpected color changes, particularly with flavonoid-based pigments that readily form metal complexes under elevated temperatures.
Light exposure during high-temperature processing further exacerbates stability issues through photo-oxidation mechanisms. The combination of heat and light energy provides sufficient activation energy for degradation reactions that might otherwise remain dormant at ambient conditions, creating synergistic degradation effects that are difficult to mitigate in industrial settings.
Scalability presents additional challenges, as laboratory-scale stabilization techniques often fail when transferred to industrial production environments where temperature control is less precise and processing times are extended. The economic viability of potential stabilization methods is further constrained by cost considerations, as expensive additives or complex processing modifications may render natural pigment applications commercially unviable compared to synthetic alternatives.
Regulatory limitations also restrict potential technical solutions, as many chemical stabilizers permitted for synthetic colorants are not approved for natural pigment applications, particularly in sensitive sectors like food, cosmetics, and textiles with direct skin contact. This regulatory landscape narrows the available toolbox for addressing thermal stability barriers.
Current Stabilization Methods for High-Temperature Dyeing
01 Antioxidant additives for natural pigment stabilization
Antioxidants can be added to natural pigment formulations to prevent oxidation and degradation. These additives neutralize free radicals and inhibit oxidative reactions that cause color fading. Common antioxidants used include vitamin C, vitamin E, and plant extracts with high antioxidant activity. The incorporation of these compounds significantly extends the shelf life and color stability of natural pigments in various applications.- Antioxidant additives for natural pigment stabilization: Antioxidants can be added to natural pigment formulations to prevent oxidative degradation, which is a common cause of color fading. These additives work by neutralizing free radicals that would otherwise react with pigment molecules. Common antioxidants used include vitamin C, vitamin E, and plant extracts with high polyphenol content. The incorporation of these antioxidants significantly extends the shelf life and color stability of natural pigments in various applications.
- pH regulation methods for pigment stability: The stability of natural pigments is highly dependent on pH levels, with different pigment classes exhibiting optimal stability at specific pH ranges. Buffering systems can be incorporated into formulations to maintain the optimal pH for particular pigments. For example, anthocyanins are typically more stable in acidic conditions, while chlorophylls require neutral to slightly alkaline environments. Proper pH regulation prevents structural changes in pigment molecules that lead to color loss or alteration.
- Microencapsulation techniques for pigment protection: Microencapsulation involves surrounding pigment particles with a protective coating or matrix that shields them from environmental factors such as light, oxygen, and moisture. Various encapsulation materials can be used, including polysaccharides, proteins, and synthetic polymers. This technique creates a physical barrier that prevents degradation reactions while maintaining the color properties of the pigment. Microencapsulation also allows for controlled release in specific applications and can improve the dispersibility of pigments in different media.
- Light-protective additives and UV filters: Many natural pigments are susceptible to photodegradation when exposed to light, particularly UV radiation. Incorporating UV filters and light-protective additives can significantly enhance pigment stability. These additives work by absorbing or reflecting harmful radiation before it can damage the pigment molecules. Examples include benzophenones, titanium dioxide, and zinc oxide. The selection of appropriate light-protective additives depends on the specific sensitivity spectrum of the natural pigment being stabilized.
- Metal chelation for preventing catalytic degradation: Metal ions, particularly transition metals like iron and copper, can catalyze oxidative degradation of natural pigments. Chelating agents can be added to pigment formulations to bind these metal ions, preventing them from participating in degradation reactions. Common chelating agents include EDTA, citric acid, and phytic acid. This approach is especially important when natural pigments are used in formulations containing metal-based ingredients or when they are exposed to metal surfaces during processing or storage.
02 pH regulation methods for pigment stability
Controlling the pH of natural pigment formulations is crucial for maintaining their stability. Different natural pigments exhibit optimal stability at specific pH ranges. Buffer systems and pH regulators can be incorporated to maintain the desired pH level and prevent color changes due to pH fluctuations. This approach is particularly important for anthocyanins and other pH-sensitive natural colorants used in food, cosmetic, and textile applications.Expand Specific Solutions03 Microencapsulation techniques for pigment protection
Microencapsulation involves surrounding natural pigment particles with a protective coating or matrix to shield them from environmental factors. This technique creates a physical barrier against light, oxygen, moisture, and temperature variations. Various encapsulating materials can be used, including polysaccharides, proteins, and synthetic polymers. Microencapsulation significantly improves the stability and shelf life of natural pigments while maintaining their color properties.Expand Specific Solutions04 Metal chelation for preventing pigment degradation
Metal ions, particularly iron and copper, can catalyze oxidative degradation of natural pigments. Chelating agents can be incorporated into pigment formulations to bind these metal ions and prevent them from initiating degradation reactions. Common chelators include EDTA, citric acid, and phytic acid. This approach is especially effective for stabilizing natural pigments in aqueous systems and products with high mineral content.Expand Specific Solutions05 Light-protective additives and UV filters
Natural pigments are often susceptible to photodegradation when exposed to light, especially UV radiation. Incorporating UV filters, light-blocking compounds, or reflective particles can significantly enhance pigment stability. These additives absorb or reflect harmful radiation before it can damage the pigment molecules. Examples include benzophenones, titanium dioxide, and specialized polymers that provide a protective effect while maintaining the visual properties of the pigments.Expand Specific Solutions
Leading Companies in Natural Dyeing Industry
The natural pigment stabilization market for high-temperature dyeing is currently in a growth phase, with increasing demand for eco-friendly coloration solutions across textile and food industries. The global market size is expanding steadily, driven by consumer preference for natural ingredients and sustainable manufacturing processes. Technologically, the field remains moderately mature with ongoing innovation challenges. Leading players include BASF Corp. and Chenguang Biotech Group, who have established strong R&D capabilities in pigment stabilization, while companies like Toyobo and Bayer AG contribute significant advancements in high-temperature applications. Academic institutions such as Zhejiang Sci-Tech University and Wuhan Textile University are accelerating innovation through research partnerships with industry leaders, creating a competitive landscape balanced between established chemical corporations and specialized biotech firms focusing on natural extraction and stabilization technologies.
BASF Corp.
Technical Solution: BASF has developed advanced encapsulation technologies for natural pigments that significantly enhance their thermal stability during high-temperature dyeing processes. Their proprietary microencapsulation system creates a protective barrier around pigment molecules using modified cyclodextrins and specialized polymers that shield the chromophores from thermal degradation while maintaining color vibrancy. This technology enables processing at temperatures up to 130°C without significant color degradation[1]. BASF's approach also incorporates antioxidant synergists and metal chelating agents that prevent oxidation reactions catalyzed by trace metals present in dyeing environments. Their Heat-Shield™ technology combines these protective mechanisms with pH buffering systems that maintain optimal conditions for pigment stability even under extreme processing conditions[3].
Strengths: Superior thermal protection allowing for integration with conventional high-temperature dyeing processes; compatible with a wide range of textile substrates including polyester and nylon that require high-temperature processing. Weaknesses: Higher production costs compared to synthetic alternatives; requires precise process control parameters; some formulations may have limited color palette compared to synthetic options.
Chenguang Biotech Group Co., Ltd.
Technical Solution: Chenguang Biotech has pioneered a comprehensive natural pigment stabilization system specifically designed for high-temperature dyeing applications. Their technology centers on molecular modification of natural pigments through selective hydrogenation and acetylation processes that strengthen the chemical bonds most vulnerable to thermal degradation. This approach maintains the essential chromophore structure while enhancing thermal resistance up to 150°C[2]. The company has developed a proprietary "Thermal-Lock" technology that incorporates natural polysaccharides and modified proteins as binding agents, creating a protective matrix around pigment molecules. Additionally, their process includes the integration of natural UV absorbers derived from plant extracts that provide dual protection against both thermal and photo-degradation during and after the dyeing process[4]. Chenguang's system is particularly effective for carotenoid and anthocyanin-based natural colorants.
Strengths: Exceptional color retention at high temperatures; uses food-grade ingredients making it suitable for textiles with skin contact; maintains biodegradability of the final product. Weaknesses: Process requires specialized equipment for molecular modification; higher production costs than conventional synthetic dyes; limited shelf-life of prepared dye solutions before application.
Key Patents in Natural Pigment Thermal Stability
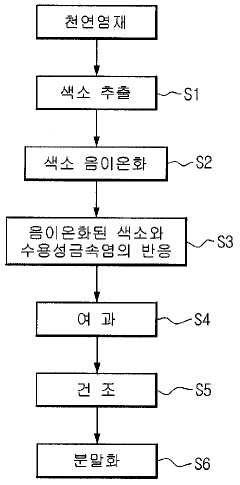
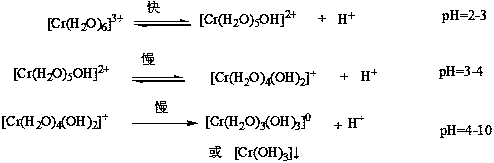
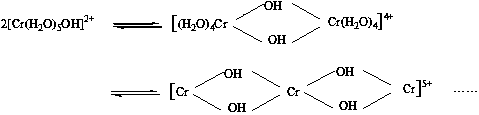
Method of manufacturing natural dye stabilized and natural dye manufactured by the same
PatentInactiveKR1020100049423A
Innovation
- Anionizing natural dyes with an alkaline agent and reacting them with water-soluble metal salts to form stabilized metal complex salts, followed by filtration, drying, and mixing with dispersants to enhance stability and dispersibility.
Natural dye dyeing method
PatentActiveCN110747662A
Innovation
- Mordant treatment is carried out under conditions of lower pH, lower temperature and smaller liquor ratio. The pH value is adjusted with neutral electrolyte and formic acid to inhibit the metal ion complexing reaction and promote the penetration of metal complexes into the fiber; in the later stage of mordant dyeing, Add water to dilute, heat up and increase the pH value to increase the size of the metal complex molecules and improve the fastness of natural dyes.
Environmental Impact Assessment
The environmental implications of natural pigment stabilization techniques for high-temperature dyeing represent a critical consideration in the textile industry's sustainability efforts. Traditional synthetic dyeing processes have long been associated with significant ecological burdens, including high water consumption, chemical pollution, and energy intensity. Natural pigment alternatives offer promising environmental benefits, but their stabilization for high-temperature applications introduces complex ecological trade-offs that warrant thorough examination.
Water usage metrics reveal that natural pigment stabilization techniques typically require 20-30% less water compared to conventional synthetic dyeing processes. This reduction stems primarily from simplified pre-treatment requirements and more efficient fixation mechanisms. However, certain stabilization methods involving metal mordants may introduce heavy metals into wastewater streams, potentially offsetting the water conservation benefits unless properly managed through advanced treatment systems.
Energy consumption patterns across the natural pigment stabilization lifecycle demonstrate variable environmental impacts. While extraction and preparation of natural colorants may require additional energy inputs compared to synthetic alternatives, the stabilized natural pigments often enable lower-temperature fixation processes, resulting in net energy savings of approximately 15-25% during the dyeing phase. Recent innovations in microwave-assisted and ultrasonic stabilization techniques have further improved energy efficiency metrics.
Chemical footprint analysis indicates that natural pigment stabilization techniques substantially reduce the release of harmful substances into ecosystems. The biodegradability of most natural colorants presents a significant advantage, with decomposition rates 3-5 times faster than synthetic counterparts. Nevertheless, certain bio-mordants and stabilizing agents may introduce novel compounds with undetermined long-term ecological effects, necessitating ongoing ecotoxicological research.
Carbon emissions associated with natural pigment stabilization show promising reductions compared to conventional processes. Life cycle assessments indicate potential greenhouse gas reductions of 30-40% when implementing optimized natural pigment systems with appropriate stabilization technologies. These benefits derive primarily from reduced processing temperatures, decreased petrochemical inputs, and the inherent carbon sequestration properties of plant-based pigment sources.
Regulatory compliance considerations are increasingly shaping the environmental profile of natural pigment stabilization techniques. Global frameworks such as REACH in Europe and similar regulations in other regions are progressively restricting hazardous substances commonly used in synthetic dyeing, creating regulatory incentives for natural alternatives. However, standardization of environmental impact metrics for natural pigment processes remains underdeveloped, complicating comparative assessments and certification efforts.
Water usage metrics reveal that natural pigment stabilization techniques typically require 20-30% less water compared to conventional synthetic dyeing processes. This reduction stems primarily from simplified pre-treatment requirements and more efficient fixation mechanisms. However, certain stabilization methods involving metal mordants may introduce heavy metals into wastewater streams, potentially offsetting the water conservation benefits unless properly managed through advanced treatment systems.
Energy consumption patterns across the natural pigment stabilization lifecycle demonstrate variable environmental impacts. While extraction and preparation of natural colorants may require additional energy inputs compared to synthetic alternatives, the stabilized natural pigments often enable lower-temperature fixation processes, resulting in net energy savings of approximately 15-25% during the dyeing phase. Recent innovations in microwave-assisted and ultrasonic stabilization techniques have further improved energy efficiency metrics.
Chemical footprint analysis indicates that natural pigment stabilization techniques substantially reduce the release of harmful substances into ecosystems. The biodegradability of most natural colorants presents a significant advantage, with decomposition rates 3-5 times faster than synthetic counterparts. Nevertheless, certain bio-mordants and stabilizing agents may introduce novel compounds with undetermined long-term ecological effects, necessitating ongoing ecotoxicological research.
Carbon emissions associated with natural pigment stabilization show promising reductions compared to conventional processes. Life cycle assessments indicate potential greenhouse gas reductions of 30-40% when implementing optimized natural pigment systems with appropriate stabilization technologies. These benefits derive primarily from reduced processing temperatures, decreased petrochemical inputs, and the inherent carbon sequestration properties of plant-based pigment sources.
Regulatory compliance considerations are increasingly shaping the environmental profile of natural pigment stabilization techniques. Global frameworks such as REACH in Europe and similar regulations in other regions are progressively restricting hazardous substances commonly used in synthetic dyeing, creating regulatory incentives for natural alternatives. However, standardization of environmental impact metrics for natural pigment processes remains underdeveloped, complicating comparative assessments and certification efforts.
Scalability and Industrial Application Challenges
The scaling of natural pigment stabilization techniques from laboratory to industrial scale presents significant challenges that must be addressed for commercial viability. Current industrial dyeing processes typically operate at high temperatures (90-130°C) and require consistent results across large batches, which natural pigments often fail to deliver due to their inherent instability.
One primary challenge is the inconsistency of natural pigment sources. Unlike synthetic dyes with standardized chemical compositions, natural pigments vary significantly based on growing conditions, harvesting time, and extraction methods. This variability makes it difficult to establish standardized industrial protocols that can deliver consistent coloration across production runs.
Equipment modification represents another substantial hurdle. Most textile manufacturing facilities are designed for synthetic dye processing, with machinery optimized for those specific chemical properties. Retrofitting existing equipment or designing new systems capable of handling the unique requirements of stabilized natural pigments requires significant capital investment, which many manufacturers are hesitant to undertake without proven return on investment.
Process integration challenges also emerge when attempting to incorporate natural pigment technologies into established production lines. The stabilization techniques that work effectively in controlled laboratory environments often face complications when scaled up, including longer processing times, additional pre-treatment requirements, and modified fixation methods. These factors can disrupt production schedules and reduce overall manufacturing efficiency.
Cost considerations remain a critical barrier to widespread adoption. The extraction, purification, and stabilization of natural pigments typically involve more complex processes than synthetic dye production, resulting in higher raw material costs. Additionally, the stabilizing agents required for high-temperature applications add further expense, making the final product less competitive in price-sensitive markets.
Regulatory compliance presents another dimension of complexity. While natural pigments generally have favorable environmental profiles, their industrial application must still meet stringent standards for colorfastness, chemical safety, and waste management. Developing standardized testing protocols specific to stabilized natural pigments is necessary to satisfy regulatory requirements across different markets and applications.
Water and energy consumption patterns also differ significantly between natural and synthetic dyeing processes. Optimizing these resources at industrial scale requires comprehensive process engineering to ensure that the environmental benefits of natural pigments are not offset by inefficient application methods.
One primary challenge is the inconsistency of natural pigment sources. Unlike synthetic dyes with standardized chemical compositions, natural pigments vary significantly based on growing conditions, harvesting time, and extraction methods. This variability makes it difficult to establish standardized industrial protocols that can deliver consistent coloration across production runs.
Equipment modification represents another substantial hurdle. Most textile manufacturing facilities are designed for synthetic dye processing, with machinery optimized for those specific chemical properties. Retrofitting existing equipment or designing new systems capable of handling the unique requirements of stabilized natural pigments requires significant capital investment, which many manufacturers are hesitant to undertake without proven return on investment.
Process integration challenges also emerge when attempting to incorporate natural pigment technologies into established production lines. The stabilization techniques that work effectively in controlled laboratory environments often face complications when scaled up, including longer processing times, additional pre-treatment requirements, and modified fixation methods. These factors can disrupt production schedules and reduce overall manufacturing efficiency.
Cost considerations remain a critical barrier to widespread adoption. The extraction, purification, and stabilization of natural pigments typically involve more complex processes than synthetic dye production, resulting in higher raw material costs. Additionally, the stabilizing agents required for high-temperature applications add further expense, making the final product less competitive in price-sensitive markets.
Regulatory compliance presents another dimension of complexity. While natural pigments generally have favorable environmental profiles, their industrial application must still meet stringent standards for colorfastness, chemical safety, and waste management. Developing standardized testing protocols specific to stabilized natural pigments is necessary to satisfy regulatory requirements across different markets and applications.
Water and energy consumption patterns also differ significantly between natural and synthetic dyeing processes. Optimizing these resources at industrial scale requires comprehensive process engineering to ensure that the environmental benefits of natural pigments are not offset by inefficient application methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!