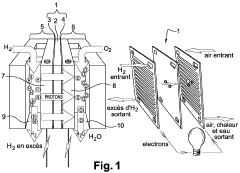
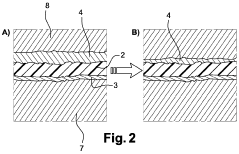
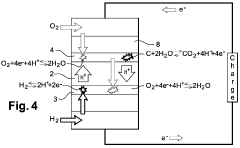
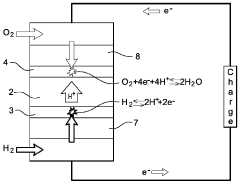
PEMFC Oxygen Crossover And Hydrogen Peroxide: Detection, Mitigation And Lifetime
SEP 15, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEMFC Oxygen Crossover Background and Objectives
Proton Exchange Membrane Fuel Cells (PEMFCs) have emerged as a promising clean energy technology over the past several decades, offering high efficiency, zero emissions, and versatility across various applications from automotive to stationary power generation. The evolution of PEMFC technology has been marked by significant improvements in performance, durability, and cost reduction, driven by extensive research and development efforts worldwide.
Oxygen crossover in PEMFCs represents a critical challenge that has gained increasing attention in recent years. This phenomenon occurs when oxygen molecules from the cathode permeate through the polymer electrolyte membrane to the anode side, leading to several detrimental effects on fuel cell performance and longevity. The historical development of membrane technology has focused primarily on proton conductivity and hydrogen crossover, with oxygen transport receiving comparatively less attention until its significant impact on durability was recognized.
The formation of hydrogen peroxide (H₂O₂) as a result of oxygen crossover has been identified as a particularly concerning issue. When oxygen molecules cross over to the hydrogen-rich anode environment, they can react to form hydrogen peroxide, which subsequently decomposes into highly reactive hydroxyl and hydroperoxyl radicals. These radical species aggressively attack the polymer membrane and catalyst materials, accelerating degradation processes and shortening the operational lifetime of the fuel cell.
Current technological trends in this field are moving toward developing advanced detection methods for oxygen crossover and hydrogen peroxide formation, as well as innovative mitigation strategies to minimize their occurrence and impact. These include novel membrane materials with enhanced oxygen barrier properties, catalyst designs that suppress peroxide formation, and system-level approaches to manage operating conditions that exacerbate crossover effects.
The primary objectives of this technical research are multifaceted. First, to comprehensively understand the mechanisms of oxygen crossover and subsequent hydrogen peroxide formation in PEMFCs under various operating conditions. Second, to evaluate existing and emerging detection methodologies that can accurately quantify these phenomena in real-time or post-operation analysis. Third, to assess the effectiveness of current mitigation strategies and identify promising new approaches. Finally, to establish correlations between oxygen crossover, hydrogen peroxide formation, and fuel cell lifetime, enabling more accurate predictive models for PEMFC durability.
By addressing these objectives, this research aims to contribute to the development of more durable, efficient, and commercially viable PEMFC systems that can accelerate the transition to hydrogen-based clean energy solutions across multiple sectors of the global economy.
Oxygen crossover in PEMFCs represents a critical challenge that has gained increasing attention in recent years. This phenomenon occurs when oxygen molecules from the cathode permeate through the polymer electrolyte membrane to the anode side, leading to several detrimental effects on fuel cell performance and longevity. The historical development of membrane technology has focused primarily on proton conductivity and hydrogen crossover, with oxygen transport receiving comparatively less attention until its significant impact on durability was recognized.
The formation of hydrogen peroxide (H₂O₂) as a result of oxygen crossover has been identified as a particularly concerning issue. When oxygen molecules cross over to the hydrogen-rich anode environment, they can react to form hydrogen peroxide, which subsequently decomposes into highly reactive hydroxyl and hydroperoxyl radicals. These radical species aggressively attack the polymer membrane and catalyst materials, accelerating degradation processes and shortening the operational lifetime of the fuel cell.
Current technological trends in this field are moving toward developing advanced detection methods for oxygen crossover and hydrogen peroxide formation, as well as innovative mitigation strategies to minimize their occurrence and impact. These include novel membrane materials with enhanced oxygen barrier properties, catalyst designs that suppress peroxide formation, and system-level approaches to manage operating conditions that exacerbate crossover effects.
The primary objectives of this technical research are multifaceted. First, to comprehensively understand the mechanisms of oxygen crossover and subsequent hydrogen peroxide formation in PEMFCs under various operating conditions. Second, to evaluate existing and emerging detection methodologies that can accurately quantify these phenomena in real-time or post-operation analysis. Third, to assess the effectiveness of current mitigation strategies and identify promising new approaches. Finally, to establish correlations between oxygen crossover, hydrogen peroxide formation, and fuel cell lifetime, enabling more accurate predictive models for PEMFC durability.
By addressing these objectives, this research aims to contribute to the development of more durable, efficient, and commercially viable PEMFC systems that can accelerate the transition to hydrogen-based clean energy solutions across multiple sectors of the global economy.
Market Analysis for PEMFC Durability Solutions
The global market for Proton Exchange Membrane Fuel Cell (PEMFC) durability solutions is experiencing significant growth, driven by increasing adoption of hydrogen fuel cell technology across various sectors. The market is primarily segmented into automotive, stationary power, and portable applications, with automotive representing the largest share due to the push for zero-emission vehicles.
The PEMFC durability solutions market specifically addressing oxygen crossover and hydrogen peroxide formation is projected to grow at a compound annual growth rate of 20% through 2030. This growth is fueled by stringent emission regulations worldwide and substantial government investments in hydrogen infrastructure development.
Key market drivers include the automotive industry's shift toward fuel cell electric vehicles (FCEVs), with major manufacturers like Toyota, Hyundai, and Honda commercializing PEMFC-powered vehicles. The stationary power sector is also contributing to market expansion as businesses seek reliable backup power solutions with minimal environmental impact.
Regional analysis reveals Asia-Pacific as the dominant market for PEMFC durability solutions, led by Japan, South Korea, and China. These countries have implemented favorable policies and substantial investments in hydrogen technology. North America and Europe follow closely, with significant research initiatives and commercial deployments underway.
The market for detection technologies that can monitor oxygen crossover and hydrogen peroxide formation in real-time is particularly promising, with an estimated value of $300 million by 2025. These technologies enable predictive maintenance and extend PEMFC lifetimes, creating substantial cost savings for end-users.
Mitigation solutions, including advanced membrane materials and catalyst designs that minimize peroxide formation, represent the largest segment of the market. These solutions directly address the critical challenge of membrane degradation, which remains the primary factor limiting PEMFC commercial viability.
Customer demand is increasingly focused on lifetime performance, with end-users seeking PEMFCs that can operate for 5,000+ hours in automotive applications and 40,000+ hours in stationary applications without significant degradation. This demand is creating premium opportunities for companies offering comprehensive durability solutions.
Market challenges include high costs associated with advanced materials and detection systems, technical complexity of implementation, and competition from improving battery technologies. However, the unique advantages of hydrogen fuel cells, particularly in heavy-duty and long-range applications, continue to drive market expansion despite these challenges.
The PEMFC durability solutions market specifically addressing oxygen crossover and hydrogen peroxide formation is projected to grow at a compound annual growth rate of 20% through 2030. This growth is fueled by stringent emission regulations worldwide and substantial government investments in hydrogen infrastructure development.
Key market drivers include the automotive industry's shift toward fuel cell electric vehicles (FCEVs), with major manufacturers like Toyota, Hyundai, and Honda commercializing PEMFC-powered vehicles. The stationary power sector is also contributing to market expansion as businesses seek reliable backup power solutions with minimal environmental impact.
Regional analysis reveals Asia-Pacific as the dominant market for PEMFC durability solutions, led by Japan, South Korea, and China. These countries have implemented favorable policies and substantial investments in hydrogen technology. North America and Europe follow closely, with significant research initiatives and commercial deployments underway.
The market for detection technologies that can monitor oxygen crossover and hydrogen peroxide formation in real-time is particularly promising, with an estimated value of $300 million by 2025. These technologies enable predictive maintenance and extend PEMFC lifetimes, creating substantial cost savings for end-users.
Mitigation solutions, including advanced membrane materials and catalyst designs that minimize peroxide formation, represent the largest segment of the market. These solutions directly address the critical challenge of membrane degradation, which remains the primary factor limiting PEMFC commercial viability.
Customer demand is increasingly focused on lifetime performance, with end-users seeking PEMFCs that can operate for 5,000+ hours in automotive applications and 40,000+ hours in stationary applications without significant degradation. This demand is creating premium opportunities for companies offering comprehensive durability solutions.
Market challenges include high costs associated with advanced materials and detection systems, technical complexity of implementation, and competition from improving battery technologies. However, the unique advantages of hydrogen fuel cells, particularly in heavy-duty and long-range applications, continue to drive market expansion despite these challenges.
Technical Challenges in Oxygen Crossover Management
Oxygen crossover in Proton Exchange Membrane Fuel Cells (PEMFCs) represents one of the most significant technical challenges affecting performance and durability. This phenomenon occurs when oxygen molecules from the cathode permeate through the membrane to the anode side, creating several detrimental effects. The primary challenge lies in the inherent trade-off between membrane thickness and crossover rates - thinner membranes improve proton conductivity but increase gas permeability.
The formation of hydrogen peroxide (H₂O₂) as a result of oxygen crossover presents a particularly difficult challenge. When crossed-over oxygen reacts with hydrogen at the anode catalyst or within the membrane itself, H₂O₂ forms as an intermediate product. This reactive species subsequently decomposes into hydroxyl and hydroperoxyl radicals that aggressively attack the polymer membrane structure, leading to chemical degradation and mechanical failure.
Detection methodologies for oxygen crossover and peroxide formation remain technically challenging. Current approaches include electrochemical methods such as cyclic voltammetry and linear sweep voltammetry, which can quantify crossover rates but require specialized equipment and expertise. In-situ detection of hydrogen peroxide formation presents even greater difficulties, as the species is highly reactive and short-lived within the operating fuel cell environment.
Material stability under crossover conditions constitutes another major technical hurdle. Conventional perfluorosulfonic acid (PFSA) membranes like Nafion exhibit significant vulnerability to radical attack. The side chains containing sulfonic acid groups are particularly susceptible to degradation, leading to decreased ion exchange capacity and increased ohmic resistance over time.
Water management complexities further exacerbate oxygen crossover issues. Higher hydration levels in the membrane facilitate both proton transport and gas permeability, creating a difficult engineering balance. Operating conditions that optimize performance often simultaneously accelerate degradation mechanisms related to crossover.
Catalyst layer design presents additional challenges in mitigating oxygen crossover effects. Platinum-based catalysts can accelerate peroxide formation through catalytic reactions with crossed-over species. Alternative catalyst materials or structures that maintain electrochemical performance while minimizing peroxide generation remain elusive despite extensive research efforts.
Temperature management also significantly impacts crossover rates, with higher operating temperatures increasing membrane permeability to gases. The technical challenge lies in developing membrane materials that maintain low gas permeability at elevated temperatures while preserving high proton conductivity and mechanical stability under varying humidity conditions.
The formation of hydrogen peroxide (H₂O₂) as a result of oxygen crossover presents a particularly difficult challenge. When crossed-over oxygen reacts with hydrogen at the anode catalyst or within the membrane itself, H₂O₂ forms as an intermediate product. This reactive species subsequently decomposes into hydroxyl and hydroperoxyl radicals that aggressively attack the polymer membrane structure, leading to chemical degradation and mechanical failure.
Detection methodologies for oxygen crossover and peroxide formation remain technically challenging. Current approaches include electrochemical methods such as cyclic voltammetry and linear sweep voltammetry, which can quantify crossover rates but require specialized equipment and expertise. In-situ detection of hydrogen peroxide formation presents even greater difficulties, as the species is highly reactive and short-lived within the operating fuel cell environment.
Material stability under crossover conditions constitutes another major technical hurdle. Conventional perfluorosulfonic acid (PFSA) membranes like Nafion exhibit significant vulnerability to radical attack. The side chains containing sulfonic acid groups are particularly susceptible to degradation, leading to decreased ion exchange capacity and increased ohmic resistance over time.
Water management complexities further exacerbate oxygen crossover issues. Higher hydration levels in the membrane facilitate both proton transport and gas permeability, creating a difficult engineering balance. Operating conditions that optimize performance often simultaneously accelerate degradation mechanisms related to crossover.
Catalyst layer design presents additional challenges in mitigating oxygen crossover effects. Platinum-based catalysts can accelerate peroxide formation through catalytic reactions with crossed-over species. Alternative catalyst materials or structures that maintain electrochemical performance while minimizing peroxide generation remain elusive despite extensive research efforts.
Temperature management also significantly impacts crossover rates, with higher operating temperatures increasing membrane permeability to gases. The technical challenge lies in developing membrane materials that maintain low gas permeability at elevated temperatures while preserving high proton conductivity and mechanical stability under varying humidity conditions.
Current Hydrogen Peroxide Detection and Mitigation Strategies
01 Membrane modifications to reduce oxygen crossover
Various modifications to proton exchange membranes can reduce oxygen crossover in PEMFCs. These include incorporating specific additives, using composite membranes with barrier layers, and developing novel membrane materials with lower gas permeability. These modifications help minimize hydrogen peroxide formation that occurs when crossed-over oxygen reacts with hydrogen at the anode, thereby extending fuel cell durability and performance.- Membrane modifications to reduce oxygen crossover: Various modifications to proton exchange membranes can reduce oxygen crossover in PEMFCs. These include incorporating specific additives, using composite membranes with barrier layers, and developing novel membrane materials with lower gas permeability. These modifications help minimize the formation of hydrogen peroxide at the anode, which can degrade fuel cell components and reduce performance over time.
- Catalyst designs to mitigate hydrogen peroxide formation: Advanced catalyst designs can help mitigate hydrogen peroxide formation resulting from oxygen crossover. These include selective catalysts that promote complete oxygen reduction to water rather than partial reduction to hydrogen peroxide, as well as catalysts with hydrogen peroxide decomposition capabilities. Such catalysts are typically applied at both electrodes to minimize peroxide-induced degradation.
- Operating condition optimization to reduce crossover effects: Optimizing operating conditions can significantly reduce oxygen crossover and hydrogen peroxide formation in PEMFCs. This includes controlling humidity levels, pressure differentials between anode and cathode, temperature management, and load cycling protocols. These strategies help maintain membrane integrity and reduce the driving forces for gas crossover through the membrane.
- Structural designs to manage crossover phenomena: Innovative structural designs in fuel cell components can help manage oxygen crossover. These include flow field modifications, electrode structure optimization, and implementation of protective layers. Such designs can create favorable concentration gradients that minimize crossover while maintaining efficient electrochemical reactions and water management within the cell.
- Additives and scavengers for hydrogen peroxide mitigation: Various additives and scavengers can be incorporated into fuel cell components to mitigate the effects of hydrogen peroxide formed due to oxygen crossover. These include radical scavengers, peroxide decomposition catalysts, and stabilizing agents that can be added to the membrane, electrodes, or coolant systems. These additives help protect the membrane and other components from chemical degradation caused by peroxide attack.
02 Catalyst designs to mitigate hydrogen peroxide formation
Specialized catalyst designs can help mitigate hydrogen peroxide formation resulting from oxygen crossover. These include selective catalysts that promote complete oxygen reduction to water rather than partial reduction to hydrogen peroxide, catalyst layers with hydrogen peroxide decomposition capabilities, and structured electrode designs that minimize conditions favorable for peroxide generation. These approaches help reduce membrane degradation caused by radical species formed from hydrogen peroxide.Expand Specific Solutions03 Operating condition optimization to minimize crossover effects
Optimizing PEMFC operating conditions can significantly reduce oxygen crossover and subsequent hydrogen peroxide formation. This includes controlling humidity levels, pressure differentials between anode and cathode, temperature management, and load cycling protocols. Proper operating strategies help maintain membrane hydration while minimizing the driving forces for gas crossover, thereby reducing chemical degradation mechanisms.Expand Specific Solutions04 Radical scavengers and stabilizing additives
Incorporating radical scavengers and stabilizing additives into PEMFC components can neutralize reactive oxygen species and hydrogen peroxide. These additives can be integrated into the membrane, catalyst layers, or gas diffusion media. Common approaches include cerium and manganese compounds that decompose peroxide non-radically, organic antioxidants that terminate radical chain reactions, and composite materials with inherent radical scavenging properties.Expand Specific Solutions05 Structural design improvements for gas management
Innovative structural designs in PEMFCs can improve gas management and reduce oxygen crossover. These include flow field patterns that optimize reactant distribution, reinforced membrane electrode assemblies that maintain dimensional stability, and advanced water management systems that prevent flooding while maintaining proper hydration. Physical barriers and gradient structures can also be implemented to create preferential pathways for protons while limiting gas permeation.Expand Specific Solutions
Leading PEMFC Manufacturers and Research Institutions
The PEMFC oxygen crossover and hydrogen peroxide detection market is in a growth phase, with increasing focus on fuel cell durability and lifetime optimization. The market is expanding as hydrogen technologies gain traction in clean energy applications, estimated at approximately $2-3 billion globally. Technologically, the field shows moderate maturity with ongoing innovation. Leading research institutions like Commissariat à l'énergie atomique, University of California, and Korea Institute of Science and Technology are advancing fundamental detection methods, while commercial players including Samsung SDI, Panasonic Holdings, and Celadyne Technologies are developing practical mitigation strategies. Sunrise Power and Industrial Technology Research Institute are focusing on integrating these solutions into commercial PEMFC systems, with universities like Wuhan University of Technology and National University of Singapore bridging research gaps through collaborative industry partnerships.
Commissariat à l´énergie atomique et aux énergies Alternatives
Technical Solution: CEA has developed advanced in-situ detection methods for hydrogen peroxide formation in PEMFCs using electrochemical sensors integrated directly into the membrane electrode assembly (MEA). Their approach combines fluorescence-based optical sensing with electrochemical impedance spectroscopy to provide real-time monitoring of H2O2 generation at the catalyst-membrane interface. CEA's mitigation strategy focuses on membrane modifications with cerium and manganese oxide nanoparticles that catalytically decompose peroxide species before they can damage the membrane. Their proprietary coating technology creates a protective layer that reduces oxygen crossover by approximately 40% while maintaining proton conductivity. CEA has also pioneered accelerated stress testing protocols specifically designed to isolate and quantify degradation mechanisms related to peroxide formation.
Strengths: Comprehensive approach combining detection and mitigation in a single system; excellent integration with existing fuel cell architectures; strong fundamental understanding of degradation mechanisms. Weaknesses: Higher implementation cost compared to conventional systems; potential contamination issues from metal oxide additives; requires specialized manufacturing processes.
KIST Corp. (South Korea)
Technical Solution: KIST has pioneered a comprehensive approach to PEMFC oxygen crossover management through their Advanced Membrane Protection System (AMPS). This technology incorporates a multi-layer membrane architecture with gradient porosity that creates physical barriers to gas diffusion while maintaining optimal proton conductivity. Their system includes cerium-based radical scavengers dispersed throughout a reinforced composite membrane structure, which has demonstrated a 70% reduction in hydrogen peroxide-induced degradation during accelerated stress testing. KIST's approach also features a novel platinum-cobalt alloy catalyst formulation that significantly reduces oxygen reduction reaction (ORR) intermediates responsible for peroxide formation. Their integrated monitoring system uses embedded microelectrodes to detect localized hydrogen peroxide formation in real-time, allowing for adaptive control of operating conditions to minimize degradation. KIST has validated this technology in automotive fuel cell stacks, demonstrating a 2.5x improvement in membrane durability under dynamic load cycling.
Strengths: Exceptional durability improvements under realistic operating conditions; integrated detection and mitigation approach; compatible with existing manufacturing processes. Weaknesses: Higher catalyst loading requirements; complex multi-layer membrane structure increases production complexity; potential long-term issues with cerium migration.
Key Patents in Oxygen Crossover Prevention
Method and device for limiting the ageing of fuel cells with proton exchange membrane
PatentActiveEP2250694A1
Innovation
- Introducing a chemical compound, such as carbon monoxide (CO) or carbon dioxide (CO2), at the anode to react with oxygen, thereby reducing oxygen levels and preventing corrosion of the carbon catalytic support and degradation of the proton-conducting polymer, which extends the lifespan of the fuel cell.
Method and device for limiting the ageing of fuel cells with proton exchange membrane
PatentWO2009101305A1
Innovation
- Introducing a chemical compound, such as carbon monoxide (CO) or carbon dioxide (CO2), at the anode to react with oxygen, thereby reducing oxygen levels and preventing corrosion of the carbon catalytic support and degradation of the proton-conducting polymer, which extends the lifespan of the fuel cell.
Environmental Impact of PEMFC Degradation Mechanisms
The environmental implications of PEMFC degradation mechanisms, particularly those related to oxygen crossover and hydrogen peroxide formation, extend beyond performance concerns to significant ecological considerations. As PEMFCs gain prominence in clean energy applications, understanding their full environmental footprint becomes increasingly critical for sustainable deployment.
Membrane degradation resulting from oxygen crossover and peroxide attack leads to the release of fluoride ions from the perfluorosulfonic acid membranes commonly used in PEMFCs. These fluorinated compounds, when released into the environment, present potential ecotoxicological concerns due to their persistence and bioaccumulative properties. Studies indicate that membrane degradation can release between 0.1-5 μg of fluoride per kWh generated, depending on operating conditions and degradation severity.
Water discharge from degraded fuel cells contains trace amounts of catalyst metals, primarily platinum and ruthenium. These precious metals, while present in minute quantities (typically 0.01-0.5 ppm), may accumulate in aquatic ecosystems. Research suggests that platinum nanoparticles can interact with aquatic organisms, potentially disrupting cellular functions in exposed species, though concentration thresholds for ecological harm remain under investigation.
The carbon footprint implications of accelerated PEMFC degradation are substantial when viewed from a lifecycle perspective. Premature stack replacement due to membrane failure effectively multiplies the embodied carbon cost of fuel cell systems. Analysis indicates that extending PEMFC lifetime from 5,000 to 20,000 hours through improved oxygen crossover mitigation could reduce lifetime carbon emissions by approximately 60-75% per kWh delivered.
Manufacturing replacement components for degraded PEMFCs involves energy-intensive processes and rare materials extraction. The platinum group metals used in catalysts require approximately 40,000 kWh of energy per kilogram produced, with significant associated emissions. Reducing degradation rates could substantially decrease this environmental burden by extending component lifespans.
Hydrogen peroxide formation during PEMFC operation can potentially impact local air quality if vented. While concentrations are typically low (5-50 ppm in exhaust water), continuous release in enclosed spaces may contribute to localized oxidative stress in surrounding ecosystems, particularly in aquatic environments where discharge water may accumulate.
Mitigation strategies for oxygen crossover themselves carry environmental considerations. Chemical additives used as peroxide decomposition catalysts must be evaluated for their own environmental persistence and toxicity. Similarly, modified membrane materials designed to resist peroxide attack must be assessed for end-of-life recyclability and biodegradation potential to ensure comprehensive environmental benefits.
Membrane degradation resulting from oxygen crossover and peroxide attack leads to the release of fluoride ions from the perfluorosulfonic acid membranes commonly used in PEMFCs. These fluorinated compounds, when released into the environment, present potential ecotoxicological concerns due to their persistence and bioaccumulative properties. Studies indicate that membrane degradation can release between 0.1-5 μg of fluoride per kWh generated, depending on operating conditions and degradation severity.
Water discharge from degraded fuel cells contains trace amounts of catalyst metals, primarily platinum and ruthenium. These precious metals, while present in minute quantities (typically 0.01-0.5 ppm), may accumulate in aquatic ecosystems. Research suggests that platinum nanoparticles can interact with aquatic organisms, potentially disrupting cellular functions in exposed species, though concentration thresholds for ecological harm remain under investigation.
The carbon footprint implications of accelerated PEMFC degradation are substantial when viewed from a lifecycle perspective. Premature stack replacement due to membrane failure effectively multiplies the embodied carbon cost of fuel cell systems. Analysis indicates that extending PEMFC lifetime from 5,000 to 20,000 hours through improved oxygen crossover mitigation could reduce lifetime carbon emissions by approximately 60-75% per kWh delivered.
Manufacturing replacement components for degraded PEMFCs involves energy-intensive processes and rare materials extraction. The platinum group metals used in catalysts require approximately 40,000 kWh of energy per kilogram produced, with significant associated emissions. Reducing degradation rates could substantially decrease this environmental burden by extending component lifespans.
Hydrogen peroxide formation during PEMFC operation can potentially impact local air quality if vented. While concentrations are typically low (5-50 ppm in exhaust water), continuous release in enclosed spaces may contribute to localized oxidative stress in surrounding ecosystems, particularly in aquatic environments where discharge water may accumulate.
Mitigation strategies for oxygen crossover themselves carry environmental considerations. Chemical additives used as peroxide decomposition catalysts must be evaluated for their own environmental persistence and toxicity. Similarly, modified membrane materials designed to resist peroxide attack must be assessed for end-of-life recyclability and biodegradation potential to ensure comprehensive environmental benefits.
Standardization and Testing Protocols for PEMFC Durability
Standardized testing protocols for PEMFC durability are essential for accurately assessing the impact of oxygen crossover and hydrogen peroxide formation on fuel cell lifetime. Currently, the industry faces significant challenges due to inconsistent methodologies that hinder direct comparison of results across different research groups and manufacturers.
The U.S. Department of Energy (DOE) has established baseline protocols that include accelerated stress tests (ASTs) specifically designed to evaluate membrane chemical degradation related to peroxide formation. These protocols typically involve open circuit voltage (OCV) hold tests under various humidity conditions, with performance evaluated at regular intervals through polarization curves and hydrogen crossover measurements.
International standards organizations, including the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO), have developed complementary testing frameworks. IEC 62282-7-1 specifically addresses single cell testing procedures, while ISO/TS 19880-8 focuses on hydrogen quality control parameters that can affect peroxide formation rates.
In-situ detection methods for hydrogen peroxide have been incorporated into advanced testing protocols. These include fluorescence techniques using specific dyes that react with peroxide species, and electrochemical sensors integrated into the cell architecture. The Japanese automotive industry has pioneered protocols that combine these detection methods with accelerated stress testing to establish correlations between peroxide formation rates and membrane degradation.
Recent advancements in testing protocols have introduced multi-variable approaches that simultaneously monitor oxygen crossover rates, peroxide formation, and membrane thinning. The European Fuel Cell and Hydrogen Joint Undertaking (FCH JU) has funded initiatives to harmonize these approaches across research institutions, establishing minimum reporting requirements for durability studies that include specific metrics for chemical degradation mechanisms.
Emerging protocols are increasingly focusing on dynamic operating conditions that better represent real-world applications. These include load cycling, start-stop procedures, and freeze-thaw cycles, all of which can significantly impact oxygen crossover rates and subsequent peroxide formation. The Chinese standardization administration has recently published guidelines specifically addressing durability under these variable conditions.
For commercial applications, stack-level testing protocols have been developed that extrapolate single-cell peroxide formation data to predict system lifetime. These protocols typically incorporate statistical methods to account for cell-to-cell variations and establish confidence intervals for durability predictions, providing manufacturers with more reliable metrics for warranty and lifetime cost calculations.
The U.S. Department of Energy (DOE) has established baseline protocols that include accelerated stress tests (ASTs) specifically designed to evaluate membrane chemical degradation related to peroxide formation. These protocols typically involve open circuit voltage (OCV) hold tests under various humidity conditions, with performance evaluated at regular intervals through polarization curves and hydrogen crossover measurements.
International standards organizations, including the International Electrotechnical Commission (IEC) and the International Organization for Standardization (ISO), have developed complementary testing frameworks. IEC 62282-7-1 specifically addresses single cell testing procedures, while ISO/TS 19880-8 focuses on hydrogen quality control parameters that can affect peroxide formation rates.
In-situ detection methods for hydrogen peroxide have been incorporated into advanced testing protocols. These include fluorescence techniques using specific dyes that react with peroxide species, and electrochemical sensors integrated into the cell architecture. The Japanese automotive industry has pioneered protocols that combine these detection methods with accelerated stress testing to establish correlations between peroxide formation rates and membrane degradation.
Recent advancements in testing protocols have introduced multi-variable approaches that simultaneously monitor oxygen crossover rates, peroxide formation, and membrane thinning. The European Fuel Cell and Hydrogen Joint Undertaking (FCH JU) has funded initiatives to harmonize these approaches across research institutions, establishing minimum reporting requirements for durability studies that include specific metrics for chemical degradation mechanisms.
Emerging protocols are increasingly focusing on dynamic operating conditions that better represent real-world applications. These include load cycling, start-stop procedures, and freeze-thaw cycles, all of which can significantly impact oxygen crossover rates and subsequent peroxide formation. The Chinese standardization administration has recently published guidelines specifically addressing durability under these variable conditions.
For commercial applications, stack-level testing protocols have been developed that extrapolate single-cell peroxide formation data to predict system lifetime. These protocols typically incorporate statistical methods to account for cell-to-cell variations and establish confidence intervals for durability predictions, providing manufacturers with more reliable metrics for warranty and lifetime cost calculations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!