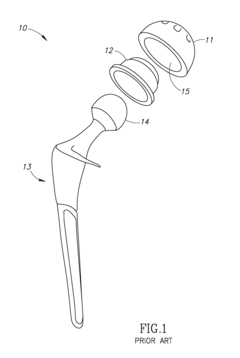
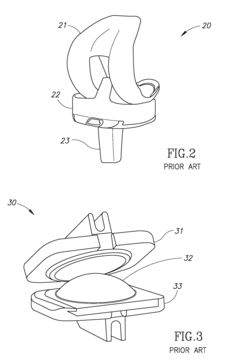
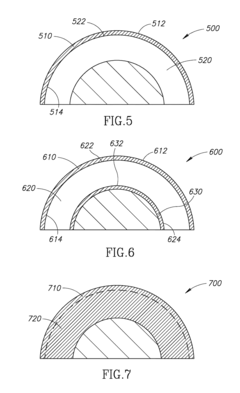
Hydroxyapatite Coatings for Enhancing The Wear Resistance of Joint Implants
JUL 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Hydroxyapatite Coating Background and Objectives
Hydroxyapatite (HA) coatings have emerged as a pivotal technology in the field of orthopedic implants, particularly for enhancing the wear resistance of joint replacements. The development of HA coatings can be traced back to the 1980s when researchers first recognized the potential of this calcium phosphate-based material to improve the biocompatibility and longevity of implants.
The primary objective of HA coating research is to create a durable, bioactive interface between the metallic implant and the surrounding bone tissue. This interface aims to promote osseointegration, reduce wear, and extend the lifespan of joint implants. As the global population ages and the demand for joint replacements increases, the importance of advancing HA coating technology has become more pronounced.
Over the past four decades, significant progress has been made in understanding the properties and behavior of HA coatings. Researchers have explored various deposition techniques, including plasma spraying, sol-gel methods, and electrochemical deposition, each offering unique advantages in terms of coating thickness, crystallinity, and adhesion strength.
The evolution of HA coating technology has been driven by the need to address several key challenges. These include improving the mechanical stability of the coating, enhancing its resistance to dissolution in physiological environments, and optimizing the coating's surface properties to promote rapid and strong bone attachment. Additionally, researchers have focused on developing methods to incorporate bioactive agents and growth factors into the HA coating to further enhance its therapeutic potential.
Recent technological advancements have opened new avenues for HA coating research. The integration of nanotechnology has led to the development of nanostructured HA coatings with improved mechanical properties and biological performance. Furthermore, the advent of 3D printing technologies has enabled the creation of custom-designed implant surfaces with precisely controlled HA coating patterns.
Looking ahead, the objectives of HA coating research are multifaceted. Scientists aim to develop coatings with enhanced wear resistance, improved long-term stability, and the ability to actively promote bone regeneration. There is also a growing interest in creating smart HA coatings that can respond to the local biological environment, releasing therapeutic agents or adapting their properties as needed.
In conclusion, the background of HA coating technology reflects a journey of continuous innovation driven by the need for better-performing joint implants. The current objectives focus on overcoming existing limitations and exploring new frontiers in biomaterial science to ultimately improve patient outcomes and quality of life.
The primary objective of HA coating research is to create a durable, bioactive interface between the metallic implant and the surrounding bone tissue. This interface aims to promote osseointegration, reduce wear, and extend the lifespan of joint implants. As the global population ages and the demand for joint replacements increases, the importance of advancing HA coating technology has become more pronounced.
Over the past four decades, significant progress has been made in understanding the properties and behavior of HA coatings. Researchers have explored various deposition techniques, including plasma spraying, sol-gel methods, and electrochemical deposition, each offering unique advantages in terms of coating thickness, crystallinity, and adhesion strength.
The evolution of HA coating technology has been driven by the need to address several key challenges. These include improving the mechanical stability of the coating, enhancing its resistance to dissolution in physiological environments, and optimizing the coating's surface properties to promote rapid and strong bone attachment. Additionally, researchers have focused on developing methods to incorporate bioactive agents and growth factors into the HA coating to further enhance its therapeutic potential.
Recent technological advancements have opened new avenues for HA coating research. The integration of nanotechnology has led to the development of nanostructured HA coatings with improved mechanical properties and biological performance. Furthermore, the advent of 3D printing technologies has enabled the creation of custom-designed implant surfaces with precisely controlled HA coating patterns.
Looking ahead, the objectives of HA coating research are multifaceted. Scientists aim to develop coatings with enhanced wear resistance, improved long-term stability, and the ability to actively promote bone regeneration. There is also a growing interest in creating smart HA coatings that can respond to the local biological environment, releasing therapeutic agents or adapting their properties as needed.
In conclusion, the background of HA coating technology reflects a journey of continuous innovation driven by the need for better-performing joint implants. The current objectives focus on overcoming existing limitations and exploring new frontiers in biomaterial science to ultimately improve patient outcomes and quality of life.
Market Analysis for Joint Implant Coatings
The global market for joint implant coatings is experiencing significant growth, driven by an aging population, increasing prevalence of joint disorders, and advancements in medical technology. The demand for joint implants, particularly those with enhanced wear resistance, is on the rise as patients seek longer-lasting and more durable solutions for joint replacement surgeries.
Hydroxyapatite coatings have emerged as a promising technology in the joint implant market, offering improved osseointegration and wear resistance. This has led to a surge in research and development activities focused on enhancing the performance of these coatings. The market for hydroxyapatite-coated implants is expected to grow substantially in the coming years, with a particular focus on hip and knee replacements.
The North American region currently dominates the joint implant coatings market, followed by Europe and Asia-Pacific. This is primarily due to the high prevalence of osteoarthritis and rheumatoid arthritis in these regions, coupled with advanced healthcare infrastructure and higher healthcare expenditure. However, emerging economies in Asia-Pacific and Latin America are showing rapid growth potential, driven by improving healthcare access and rising disposable incomes.
Key market players in the joint implant coatings sector include DePuy Synthes, Zimmer Biomet, Stryker Corporation, and Smith & Nephew. These companies are investing heavily in research and development to improve coating technologies and gain a competitive edge. Smaller, specialized coating technology firms are also making significant contributions to the market through innovative solutions and partnerships with larger implant manufacturers.
The market is witnessing a trend towards personalized joint implants with customized coatings tailored to individual patient needs. This shift is expected to drive further innovation in coating technologies and manufacturing processes. Additionally, there is growing interest in antimicrobial coatings to reduce the risk of post-operative infections, presenting another avenue for market expansion.
Regulatory factors play a crucial role in shaping the joint implant coatings market. Stringent approval processes and quality standards set by regulatory bodies such as the FDA and EMA ensure the safety and efficacy of coated implants. This regulatory landscape influences market dynamics and can impact the speed of innovation and product commercialization.
In conclusion, the market for joint implant coatings, particularly hydroxyapatite coatings for enhancing wear resistance, shows strong growth potential. The combination of demographic trends, technological advancements, and increasing focus on patient outcomes is expected to drive continued expansion and innovation in this sector.
Hydroxyapatite coatings have emerged as a promising technology in the joint implant market, offering improved osseointegration and wear resistance. This has led to a surge in research and development activities focused on enhancing the performance of these coatings. The market for hydroxyapatite-coated implants is expected to grow substantially in the coming years, with a particular focus on hip and knee replacements.
The North American region currently dominates the joint implant coatings market, followed by Europe and Asia-Pacific. This is primarily due to the high prevalence of osteoarthritis and rheumatoid arthritis in these regions, coupled with advanced healthcare infrastructure and higher healthcare expenditure. However, emerging economies in Asia-Pacific and Latin America are showing rapid growth potential, driven by improving healthcare access and rising disposable incomes.
Key market players in the joint implant coatings sector include DePuy Synthes, Zimmer Biomet, Stryker Corporation, and Smith & Nephew. These companies are investing heavily in research and development to improve coating technologies and gain a competitive edge. Smaller, specialized coating technology firms are also making significant contributions to the market through innovative solutions and partnerships with larger implant manufacturers.
The market is witnessing a trend towards personalized joint implants with customized coatings tailored to individual patient needs. This shift is expected to drive further innovation in coating technologies and manufacturing processes. Additionally, there is growing interest in antimicrobial coatings to reduce the risk of post-operative infections, presenting another avenue for market expansion.
Regulatory factors play a crucial role in shaping the joint implant coatings market. Stringent approval processes and quality standards set by regulatory bodies such as the FDA and EMA ensure the safety and efficacy of coated implants. This regulatory landscape influences market dynamics and can impact the speed of innovation and product commercialization.
In conclusion, the market for joint implant coatings, particularly hydroxyapatite coatings for enhancing wear resistance, shows strong growth potential. The combination of demographic trends, technological advancements, and increasing focus on patient outcomes is expected to drive continued expansion and innovation in this sector.
Current Challenges in Implant Wear Resistance
Despite significant advancements in joint implant technology, wear resistance remains a critical challenge in the field of orthopedic biomaterials. The primary issue stems from the continuous friction between the implant surface and surrounding tissues or other implant components, leading to material degradation and potential implant failure.
One of the most pressing challenges is the generation of wear particles. As implants undergo repeated loading and movement, microscopic particles can detach from the surface, triggering inflammatory responses in the surrounding tissues. This inflammation can lead to osteolysis, a condition where bone tissue around the implant begins to break down, ultimately compromising the implant's stability and necessitating revision surgery.
Another significant challenge lies in balancing wear resistance with biocompatibility. While some materials exhibit excellent wear properties, they may not integrate well with the body's natural tissues or may release potentially harmful ions as they degrade. This trade-off between durability and biological compatibility continues to be a major focus of research and development efforts.
The diversity of patient populations also presents a challenge in implant wear resistance. Factors such as age, activity level, body weight, and underlying health conditions can significantly impact the wear rates of implants. Developing a one-size-fits-all solution that can withstand the varied demands of different patient groups remains elusive.
Furthermore, the complex biomechanical environment of joints poses additional challenges. The multidirectional forces, varying load distributions, and presence of biological fluids create a unique and demanding setting for implant materials. Replicating these conditions accurately in laboratory testing to predict long-term wear performance is an ongoing challenge for researchers and manufacturers.
The issue of long-term performance and durability is particularly crucial. While many implants show promising results in short-term studies, predicting and ensuring their performance over decades of use remains challenging. This is especially important given the increasing life expectancy and the growing number of younger patients receiving joint implants.
Lastly, the regulatory landscape presents its own set of challenges. Stringent approval processes for new materials and coatings, while necessary for patient safety, can slow the introduction of innovative wear-resistant technologies into clinical practice. Balancing innovation with regulatory compliance continues to be a delicate act in the field of implant development.
One of the most pressing challenges is the generation of wear particles. As implants undergo repeated loading and movement, microscopic particles can detach from the surface, triggering inflammatory responses in the surrounding tissues. This inflammation can lead to osteolysis, a condition where bone tissue around the implant begins to break down, ultimately compromising the implant's stability and necessitating revision surgery.
Another significant challenge lies in balancing wear resistance with biocompatibility. While some materials exhibit excellent wear properties, they may not integrate well with the body's natural tissues or may release potentially harmful ions as they degrade. This trade-off between durability and biological compatibility continues to be a major focus of research and development efforts.
The diversity of patient populations also presents a challenge in implant wear resistance. Factors such as age, activity level, body weight, and underlying health conditions can significantly impact the wear rates of implants. Developing a one-size-fits-all solution that can withstand the varied demands of different patient groups remains elusive.
Furthermore, the complex biomechanical environment of joints poses additional challenges. The multidirectional forces, varying load distributions, and presence of biological fluids create a unique and demanding setting for implant materials. Replicating these conditions accurately in laboratory testing to predict long-term wear performance is an ongoing challenge for researchers and manufacturers.
The issue of long-term performance and durability is particularly crucial. While many implants show promising results in short-term studies, predicting and ensuring their performance over decades of use remains challenging. This is especially important given the increasing life expectancy and the growing number of younger patients receiving joint implants.
Lastly, the regulatory landscape presents its own set of challenges. Stringent approval processes for new materials and coatings, while necessary for patient safety, can slow the introduction of innovative wear-resistant technologies into clinical practice. Balancing innovation with regulatory compliance continues to be a delicate act in the field of implant development.
Existing Hydroxyapatite Coating Solutions
01 Composition modification for improved wear resistance
Enhancing the wear resistance of hydroxyapatite coatings by modifying their composition. This can involve incorporating additional elements or compounds into the hydroxyapatite structure to improve its mechanical properties and durability. Such modifications can lead to increased hardness, toughness, and overall wear resistance of the coating.- Composition modification for improved wear resistance: Enhancing the wear resistance of hydroxyapatite coatings by modifying their composition. This can involve incorporating additional elements or compounds into the hydroxyapatite structure to improve its mechanical properties and durability. Such modifications can lead to increased hardness, toughness, and overall wear resistance of the coating.
- Surface treatment techniques: Applying various surface treatment techniques to hydroxyapatite coatings to enhance their wear resistance. These methods may include laser treatment, ion implantation, or chemical treatments that alter the surface properties of the coating. Such treatments can improve the coating's adhesion to the substrate and increase its resistance to wear and abrasion.
- Multilayer coating systems: Developing multilayer coating systems that incorporate hydroxyapatite as one of the layers. This approach can combine the biocompatibility of hydroxyapatite with the wear-resistant properties of other materials. The multilayer structure can provide enhanced mechanical properties and improved wear resistance compared to single-layer hydroxyapatite coatings.
- Nanostructured hydroxyapatite coatings: Utilizing nanostructured hydroxyapatite coatings to improve wear resistance. By controlling the grain size and structure at the nanoscale, these coatings can exhibit superior mechanical properties and wear resistance compared to conventional hydroxyapatite coatings. Nanostructured coatings can also provide better adhesion to the substrate and improved overall performance.
- Composite coatings with hydroxyapatite: Developing composite coatings that combine hydroxyapatite with other wear-resistant materials. These composites can leverage the biocompatibility of hydroxyapatite while incorporating materials with superior mechanical properties to enhance overall wear resistance. The synergistic effect of different materials in the composite can result in coatings with improved durability and performance.
02 Surface treatment techniques
Applying various surface treatment techniques to hydroxyapatite coatings to enhance their wear resistance. These methods may include laser treatment, ion implantation, or chemical treatments that alter the surface properties of the coating. Such treatments can improve the coating's adhesion to the substrate and increase its resistance to wear and abrasion.Expand Specific Solutions03 Multilayer coating systems
Developing multilayer coating systems that incorporate hydroxyapatite as one of the layers. This approach can combine the biocompatibility of hydroxyapatite with the wear-resistant properties of other materials. The multilayer structure can provide enhanced mechanical properties and improved wear resistance compared to single-layer hydroxyapatite coatings.Expand Specific Solutions04 Nanostructured hydroxyapatite coatings
Utilizing nanostructured hydroxyapatite coatings to improve wear resistance. By controlling the grain size and structure at the nanoscale, these coatings can exhibit superior mechanical properties and wear resistance compared to conventional hydroxyapatite coatings. Nanostructured coatings can also provide better adhesion to the substrate and enhanced durability.Expand Specific Solutions05 Composite coatings with hydroxyapatite
Developing composite coatings that combine hydroxyapatite with other wear-resistant materials. These composites can leverage the biocompatibility of hydroxyapatite while incorporating materials with superior mechanical properties to enhance overall wear resistance. The synergistic effect of different materials in the composite can result in improved durability and performance of the coating.Expand Specific Solutions
Key Players in Joint Implant Coating Industry
The research on hydroxyapatite coatings for enhancing wear resistance of joint implants is in a mature development stage, with a significant market size driven by the growing demand for orthopedic implants. The global market for these coatings is expected to continue expanding due to an aging population and increasing prevalence of joint disorders. Technologically, the field is well-established but still evolving, with companies like Promimic AB, DePuy Synthes, and Stryker European Operations leading innovation. Academic institutions such as Zhejiang University, MIT, and Sichuan University contribute significantly to research advancements. The competitive landscape is characterized by a mix of established medical device manufacturers and specialized coating technology firms, with ongoing efforts to improve coating performance, durability, and biocompatibility.
Promimic AB
Technical Solution: Promimic AB has developed a unique HAnano Surface technology for enhancing the wear resistance of joint implants. This technology involves applying an ultra-thin layer of synthetic hydroxyapatite (HA) to the implant surface using a wet-chemical process. The nano-sized HA crystals (20-50 nm) closely mimic the natural bone mineral, promoting rapid osseointegration[1]. The coating thickness is typically around 20-80 nm, which is significantly thinner than traditional plasma-sprayed HA coatings (50-200 μm)[2]. This ultra-thin layer maintains the underlying implant surface topography while providing the bioactive benefits of HA. The process can be applied to various materials, including titanium, PEEK, and ceramics, making it versatile for different types of joint implants[3].
Strengths: Excellent biocompatibility, rapid osseointegration, and versatility across different implant materials. The ultra-thin coating preserves the original implant surface properties. Weaknesses: May have limited long-term durability compared to thicker coatings, and the technology is relatively new with limited long-term clinical data.
DePuy Synthes Products, Inc.
Technical Solution: DePuy Synthes, a Johnson & Johnson company, has developed the POROCOAT® Porous Coating technology for enhancing wear resistance in joint implants. This technology involves applying a titanium plasma spray coating to create a rough, porous surface that promotes bone ingrowth. The coating thickness ranges from 150 to 300 μm, with an average pore size of 200-400 μm[4]. To further enhance wear resistance, DePuy Synthes has combined this technology with a hydroxyapatite (HA) coating in some of their implants. The HA coating is applied using a plasma spray technique, resulting in a layer thickness of 50-150 μm[5]. This dual-layer approach aims to provide both mechanical interlocking through the porous titanium layer and biological fixation through the bioactive HA layer, potentially improving long-term implant stability and wear resistance[6].
Strengths: Combines mechanical and biological fixation, potentially leading to improved long-term stability and wear resistance. Established technology with extensive clinical data. Weaknesses: Thicker coating may alter implant dimensions and affect fit. Plasma-sprayed HA coatings may have issues with long-term stability and delamination.
Innovations in Wear-Resistant Hydroxyapatite Coatings
Liners for medical joint implants with improved wear-resistance
PatentInactiveUS20170224872A1
Innovation
- A polymeric liner for medical joint implants is developed, composed of a polymeric matrix with a volume concentration of 95%-99.9% and metal chalcogenides or dichalcogenides nanotube nanoparticles, such as TiS2 or WS2, distributed within the matrix to enhance wear resistance and reduce friction.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the development and application of hydroxyapatite (HA) coatings for joint implants. The primary goal of these coatings is to enhance wear resistance while maintaining optimal biocompatibility and ensuring patient safety.
HA coatings have demonstrated excellent biocompatibility due to their chemical similarity to natural bone mineral. This similarity promotes osseointegration, allowing for direct bonding between the implant and surrounding bone tissue. The bioactive nature of HA coatings stimulates bone growth and accelerates the healing process, leading to improved implant stability and longevity.
However, the safety of HA coatings must be thoroughly evaluated to mitigate potential risks. One key consideration is the stability of the coating under physiological conditions. Degradation or delamination of the coating could lead to the release of particles, potentially causing adverse reactions or compromising the implant's performance.
The purity and composition of the HA coating material are critical factors affecting biocompatibility and safety. Impurities or variations in the calcium-to-phosphate ratio can influence the coating's biological response and long-term stability. Rigorous quality control measures must be implemented to ensure consistent and high-quality HA coatings.
The coating process itself must be carefully controlled to avoid introducing contaminants or altering the substrate material's properties. Techniques such as plasma spraying or sol-gel deposition require optimization to achieve uniform coating thickness and adherence without compromising the implant's mechanical integrity.
Long-term studies are essential to assess the in vivo behavior of HA-coated implants. Potential concerns include the effects of coating degradation on local tissue response, the risk of particle-induced inflammation, and the impact on overall implant performance over time.
Regulatory compliance is a crucial aspect of ensuring the safety of HA-coated joint implants. Adherence to standards set by organizations such as the FDA and ISO is necessary for market approval and patient safety. This includes comprehensive testing for biocompatibility, toxicity, and mechanical properties.
In conclusion, while HA coatings offer significant benefits for enhancing wear resistance and promoting osseointegration in joint implants, careful consideration of biocompatibility and safety aspects is essential. Ongoing research and development efforts should focus on optimizing coating properties, improving long-term stability, and addressing potential safety concerns to ensure the best possible outcomes for patients receiving these implants.
HA coatings have demonstrated excellent biocompatibility due to their chemical similarity to natural bone mineral. This similarity promotes osseointegration, allowing for direct bonding between the implant and surrounding bone tissue. The bioactive nature of HA coatings stimulates bone growth and accelerates the healing process, leading to improved implant stability and longevity.
However, the safety of HA coatings must be thoroughly evaluated to mitigate potential risks. One key consideration is the stability of the coating under physiological conditions. Degradation or delamination of the coating could lead to the release of particles, potentially causing adverse reactions or compromising the implant's performance.
The purity and composition of the HA coating material are critical factors affecting biocompatibility and safety. Impurities or variations in the calcium-to-phosphate ratio can influence the coating's biological response and long-term stability. Rigorous quality control measures must be implemented to ensure consistent and high-quality HA coatings.
The coating process itself must be carefully controlled to avoid introducing contaminants or altering the substrate material's properties. Techniques such as plasma spraying or sol-gel deposition require optimization to achieve uniform coating thickness and adherence without compromising the implant's mechanical integrity.
Long-term studies are essential to assess the in vivo behavior of HA-coated implants. Potential concerns include the effects of coating degradation on local tissue response, the risk of particle-induced inflammation, and the impact on overall implant performance over time.
Regulatory compliance is a crucial aspect of ensuring the safety of HA-coated joint implants. Adherence to standards set by organizations such as the FDA and ISO is necessary for market approval and patient safety. This includes comprehensive testing for biocompatibility, toxicity, and mechanical properties.
In conclusion, while HA coatings offer significant benefits for enhancing wear resistance and promoting osseointegration in joint implants, careful consideration of biocompatibility and safety aspects is essential. Ongoing research and development efforts should focus on optimizing coating properties, improving long-term stability, and addressing potential safety concerns to ensure the best possible outcomes for patients receiving these implants.
Regulatory Framework for Medical Implant Coatings
The regulatory framework for medical implant coatings, particularly hydroxyapatite coatings for joint implants, is a complex and evolving landscape designed to ensure patient safety and product efficacy. In the United States, the Food and Drug Administration (FDA) plays a pivotal role in regulating these coatings under the Medical Device Regulations.
The FDA classifies joint implants with hydroxyapatite coatings as Class III medical devices, requiring the most stringent regulatory controls. Manufacturers must submit a Premarket Approval (PMA) application, which includes comprehensive clinical data demonstrating the safety and effectiveness of the coating.
In the European Union, the Medical Device Regulation (MDR) governs the approval process for such implants. The MDR requires manufacturers to obtain CE marking, which involves a conformity assessment procedure and clinical evaluation reports. The European Medicines Agency (EMA) also provides guidelines on the development and evaluation of coatings for medical devices.
International standards play a crucial role in harmonizing regulatory requirements across different regions. ISO 13779 specifically addresses hydroxyapatite coatings for surgical implants, outlining requirements for chemical composition, crystallinity, and other physical properties. ASTM F1185 provides standard specifications for the composition of hydroxyapatite for surgical implants.
Regulatory bodies also focus on the manufacturing processes of these coatings. Good Manufacturing Practice (GMP) guidelines must be followed to ensure consistent quality and safety. This includes validated cleaning and sterilization procedures, as well as rigorous quality control measures throughout the production process.
Post-market surveillance is another critical aspect of the regulatory framework. Manufacturers are required to monitor the performance of their coated implants in real-world settings and report any adverse events or product failures to the relevant authorities.
As research in this field progresses, regulatory frameworks are continuously updated to address new findings and technologies. For instance, recent developments in nanotechnology-enhanced hydroxyapatite coatings have prompted regulatory bodies to consider additional guidelines for evaluating the safety and efficacy of these novel materials.
Compliance with these regulatory requirements is essential for manufacturers seeking to bring hydroxyapatite-coated joint implants to market. The framework not only ensures patient safety but also promotes innovation by providing clear guidelines for the development and approval of new coating technologies.
The FDA classifies joint implants with hydroxyapatite coatings as Class III medical devices, requiring the most stringent regulatory controls. Manufacturers must submit a Premarket Approval (PMA) application, which includes comprehensive clinical data demonstrating the safety and effectiveness of the coating.
In the European Union, the Medical Device Regulation (MDR) governs the approval process for such implants. The MDR requires manufacturers to obtain CE marking, which involves a conformity assessment procedure and clinical evaluation reports. The European Medicines Agency (EMA) also provides guidelines on the development and evaluation of coatings for medical devices.
International standards play a crucial role in harmonizing regulatory requirements across different regions. ISO 13779 specifically addresses hydroxyapatite coatings for surgical implants, outlining requirements for chemical composition, crystallinity, and other physical properties. ASTM F1185 provides standard specifications for the composition of hydroxyapatite for surgical implants.
Regulatory bodies also focus on the manufacturing processes of these coatings. Good Manufacturing Practice (GMP) guidelines must be followed to ensure consistent quality and safety. This includes validated cleaning and sterilization procedures, as well as rigorous quality control measures throughout the production process.
Post-market surveillance is another critical aspect of the regulatory framework. Manufacturers are required to monitor the performance of their coated implants in real-world settings and report any adverse events or product failures to the relevant authorities.
As research in this field progresses, regulatory frameworks are continuously updated to address new findings and technologies. For instance, recent developments in nanotechnology-enhanced hydroxyapatite coatings have prompted regulatory bodies to consider additional guidelines for evaluating the safety and efficacy of these novel materials.
Compliance with these regulatory requirements is essential for manufacturers seeking to bring hydroxyapatite-coated joint implants to market. The framework not only ensures patient safety but also promotes innovation by providing clear guidelines for the development and approval of new coating technologies.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!