Hydroxyethylcellulose's Effect on DNA Aptamer Stability
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HEC-DNA Aptamer Interaction Background
Hydroxyethylcellulose (HEC) and DNA aptamers represent two distinct yet interconnected domains in biochemistry and molecular biology. HEC, a cellulose derivative, has gained significant attention in various industries due to its unique physicochemical properties. It is widely used as a thickening agent, stabilizer, and binder in pharmaceuticals, cosmetics, and food products. On the other hand, DNA aptamers are short, single-stranded oligonucleotides that can bind to specific target molecules with high affinity and selectivity.
The intersection of these two fields has sparked interest in recent years, particularly in the context of DNA aptamer stability. DNA aptamers, while highly specific and versatile, often face challenges in maintaining their structural integrity and functionality under various environmental conditions. This is where HEC comes into play, offering potential solutions to enhance the stability and performance of DNA aptamers.
The interaction between HEC and DNA aptamers is rooted in the fundamental principles of polymer science and nucleic acid chemistry. HEC, with its hydrophilic nature and ability to form hydrogen bonds, can potentially interact with DNA aptamers in ways that influence their three-dimensional structure and binding properties. This interaction is of particular interest in applications where the longevity and reliability of DNA aptamers are crucial, such as in biosensors, diagnostic tools, and targeted drug delivery systems.
Research in this area has been driven by the need to overcome limitations in DNA aptamer technology, particularly in terms of stability and shelf-life. The addition of HEC to DNA aptamer solutions has been explored as a means to protect these sensitive molecules from degradation, maintain their structural integrity, and potentially enhance their binding capabilities. This approach leverages the protective and stabilizing properties of HEC, which have been well-established in other applications.
The study of HEC-DNA aptamer interactions also opens up new avenues for the design and development of novel biomaterials and hybrid systems. By understanding how HEC influences the behavior of DNA aptamers, researchers can potentially create more robust and efficient aptamer-based technologies. This could lead to advancements in fields such as environmental monitoring, medical diagnostics, and therapeutic interventions.
As research in this area progresses, it is becoming increasingly clear that the relationship between HEC and DNA aptamers is complex and multifaceted. Factors such as HEC concentration, molecular weight, and degree of substitution can all play roles in how it interacts with and affects DNA aptamers. Understanding these nuances is crucial for optimizing the use of HEC in aptamer-based applications and for pushing the boundaries of what is possible with these powerful molecular tools.
The intersection of these two fields has sparked interest in recent years, particularly in the context of DNA aptamer stability. DNA aptamers, while highly specific and versatile, often face challenges in maintaining their structural integrity and functionality under various environmental conditions. This is where HEC comes into play, offering potential solutions to enhance the stability and performance of DNA aptamers.
The interaction between HEC and DNA aptamers is rooted in the fundamental principles of polymer science and nucleic acid chemistry. HEC, with its hydrophilic nature and ability to form hydrogen bonds, can potentially interact with DNA aptamers in ways that influence their three-dimensional structure and binding properties. This interaction is of particular interest in applications where the longevity and reliability of DNA aptamers are crucial, such as in biosensors, diagnostic tools, and targeted drug delivery systems.
Research in this area has been driven by the need to overcome limitations in DNA aptamer technology, particularly in terms of stability and shelf-life. The addition of HEC to DNA aptamer solutions has been explored as a means to protect these sensitive molecules from degradation, maintain their structural integrity, and potentially enhance their binding capabilities. This approach leverages the protective and stabilizing properties of HEC, which have been well-established in other applications.
The study of HEC-DNA aptamer interactions also opens up new avenues for the design and development of novel biomaterials and hybrid systems. By understanding how HEC influences the behavior of DNA aptamers, researchers can potentially create more robust and efficient aptamer-based technologies. This could lead to advancements in fields such as environmental monitoring, medical diagnostics, and therapeutic interventions.
As research in this area progresses, it is becoming increasingly clear that the relationship between HEC and DNA aptamers is complex and multifaceted. Factors such as HEC concentration, molecular weight, and degree of substitution can all play roles in how it interacts with and affects DNA aptamers. Understanding these nuances is crucial for optimizing the use of HEC in aptamer-based applications and for pushing the boundaries of what is possible with these powerful molecular tools.
Market Analysis for Stabilized Aptamers
The market for stabilized aptamers has shown significant growth potential in recent years, driven by the increasing demand for highly specific and stable molecular recognition elements in various applications. Aptamers, as synthetic single-stranded DNA or RNA molecules, offer several advantages over traditional antibodies, including lower production costs, higher stability, and easier modification. The global aptamer market was valued at approximately $1.5 billion in 2020 and is projected to reach $5.4 billion by 2027, with a compound annual growth rate (CAGR) of 20.1% during the forecast period.
The research on hydroxyethylcellulose's effect on DNA aptamer stability addresses a critical need in the market for improved aptamer performance and longevity. Stabilized aptamers have applications across multiple industries, including diagnostics, therapeutics, and biosensing. In the diagnostics sector, stabilized aptamers are increasingly used in point-of-care testing devices, offering rapid and accurate detection of various biomarkers. The global in vitro diagnostics market, which heavily relies on stable molecular recognition elements, was valued at $83.3 billion in 2020 and is expected to reach $113.1 billion by 2025.
In the therapeutics field, stabilized aptamers are gaining traction as potential drug candidates due to their high specificity and reduced immunogenicity compared to antibodies. The aptamer-based therapeutics market is projected to grow at a CAGR of 18.2% from 2021 to 2028, driven by the increasing prevalence of chronic diseases and the need for targeted drug delivery systems. Notable examples of approved aptamer-based drugs include Macugen for age-related macular degeneration and Fovista for wet AMD, demonstrating the market's acceptance of aptamer technology.
The biosensing industry also presents significant opportunities for stabilized aptamers. With the growing demand for real-time, sensitive, and specific detection methods in environmental monitoring, food safety, and biodefense applications, the global biosensors market is expected to reach $36.7 billion by 2026. Stabilized aptamers offer advantages in terms of reusability and long-term storage stability, making them attractive alternatives to traditional recognition elements in biosensor development.
Geographically, North America dominates the aptamer market, followed by Europe and Asia-Pacific. However, emerging economies in Asia-Pacific and Latin America are expected to witness the highest growth rates in the coming years due to increasing investments in biotechnology research and healthcare infrastructure. The research on hydroxyethylcellulose's effect on DNA aptamer stability has the potential to address market needs across these regions by enhancing the performance and shelf-life of aptamer-based products.
The research on hydroxyethylcellulose's effect on DNA aptamer stability addresses a critical need in the market for improved aptamer performance and longevity. Stabilized aptamers have applications across multiple industries, including diagnostics, therapeutics, and biosensing. In the diagnostics sector, stabilized aptamers are increasingly used in point-of-care testing devices, offering rapid and accurate detection of various biomarkers. The global in vitro diagnostics market, which heavily relies on stable molecular recognition elements, was valued at $83.3 billion in 2020 and is expected to reach $113.1 billion by 2025.
In the therapeutics field, stabilized aptamers are gaining traction as potential drug candidates due to their high specificity and reduced immunogenicity compared to antibodies. The aptamer-based therapeutics market is projected to grow at a CAGR of 18.2% from 2021 to 2028, driven by the increasing prevalence of chronic diseases and the need for targeted drug delivery systems. Notable examples of approved aptamer-based drugs include Macugen for age-related macular degeneration and Fovista for wet AMD, demonstrating the market's acceptance of aptamer technology.
The biosensing industry also presents significant opportunities for stabilized aptamers. With the growing demand for real-time, sensitive, and specific detection methods in environmental monitoring, food safety, and biodefense applications, the global biosensors market is expected to reach $36.7 billion by 2026. Stabilized aptamers offer advantages in terms of reusability and long-term storage stability, making them attractive alternatives to traditional recognition elements in biosensor development.
Geographically, North America dominates the aptamer market, followed by Europe and Asia-Pacific. However, emerging economies in Asia-Pacific and Latin America are expected to witness the highest growth rates in the coming years due to increasing investments in biotechnology research and healthcare infrastructure. The research on hydroxyethylcellulose's effect on DNA aptamer stability has the potential to address market needs across these regions by enhancing the performance and shelf-life of aptamer-based products.
Current Challenges in Aptamer Stability
DNA aptamers have emerged as promising tools in various fields, including diagnostics, therapeutics, and biosensing. However, their widespread application is hindered by several challenges related to their stability. One of the primary concerns is the susceptibility of aptamers to nuclease degradation, which significantly reduces their half-life in biological environments. This vulnerability limits their effectiveness in in vivo applications and necessitates the development of strategies to enhance their stability.
Another critical challenge is the structural instability of aptamers under varying environmental conditions. Factors such as temperature, pH, and ionic strength can significantly affect the three-dimensional structure of aptamers, potentially altering their binding affinity and specificity. This sensitivity to environmental changes poses difficulties in maintaining consistent performance across different experimental or clinical settings.
The issue of chemical stability also presents a significant hurdle. Aptamers are prone to various chemical modifications, including oxidation and hydrolysis, which can compromise their functionality. These modifications can occur during storage, handling, or application, leading to reduced efficacy and reliability of aptamer-based assays or therapies.
Furthermore, the stability of aptamers in complex biological matrices remains a considerable challenge. When introduced into blood, serum, or other biological fluids, aptamers face interactions with numerous biomolecules that can interfere with their target binding or accelerate their degradation. This complexity makes it difficult to predict and control aptamer behavior in real-world applications.
The challenge of long-term storage stability is another critical aspect that requires attention. Aptamers may degrade or lose their functional conformation over time, even under controlled storage conditions. This instability affects the shelf-life of aptamer-based products and poses challenges for their commercial viability and practical implementation in clinical settings.
Additionally, the reproducibility of aptamer synthesis and folding presents a significant challenge. Variations in production processes can lead to inconsistencies in aptamer structure and function, affecting the reliability and comparability of research results and diagnostic outcomes.
Addressing these stability challenges is crucial for advancing aptamer technology. Current research efforts are focused on developing various stabilization strategies, including chemical modifications, conjugation with stabilizing molecules, and the use of protective formulations. The exploration of hydroxyethylcellulose as a potential stabilizing agent for DNA aptamers represents a promising avenue in this ongoing quest to overcome the stability limitations of these versatile molecules.
Another critical challenge is the structural instability of aptamers under varying environmental conditions. Factors such as temperature, pH, and ionic strength can significantly affect the three-dimensional structure of aptamers, potentially altering their binding affinity and specificity. This sensitivity to environmental changes poses difficulties in maintaining consistent performance across different experimental or clinical settings.
The issue of chemical stability also presents a significant hurdle. Aptamers are prone to various chemical modifications, including oxidation and hydrolysis, which can compromise their functionality. These modifications can occur during storage, handling, or application, leading to reduced efficacy and reliability of aptamer-based assays or therapies.
Furthermore, the stability of aptamers in complex biological matrices remains a considerable challenge. When introduced into blood, serum, or other biological fluids, aptamers face interactions with numerous biomolecules that can interfere with their target binding or accelerate their degradation. This complexity makes it difficult to predict and control aptamer behavior in real-world applications.
The challenge of long-term storage stability is another critical aspect that requires attention. Aptamers may degrade or lose their functional conformation over time, even under controlled storage conditions. This instability affects the shelf-life of aptamer-based products and poses challenges for their commercial viability and practical implementation in clinical settings.
Additionally, the reproducibility of aptamer synthesis and folding presents a significant challenge. Variations in production processes can lead to inconsistencies in aptamer structure and function, affecting the reliability and comparability of research results and diagnostic outcomes.
Addressing these stability challenges is crucial for advancing aptamer technology. Current research efforts are focused on developing various stabilization strategies, including chemical modifications, conjugation with stabilizing molecules, and the use of protective formulations. The exploration of hydroxyethylcellulose as a potential stabilizing agent for DNA aptamers represents a promising avenue in this ongoing quest to overcome the stability limitations of these versatile molecules.
Existing HEC-based Stabilization Methods
01 Temperature stability of hydroxyethylcellulose
Hydroxyethylcellulose exhibits varying degrees of stability at different temperatures. Research has focused on improving its thermal stability for use in high-temperature applications, such as in oil drilling fluids and personal care products. Various methods have been developed to enhance its temperature resistance, including chemical modifications and the addition of stabilizing agents.- Temperature stability of hydroxyethylcellulose: Hydroxyethylcellulose exhibits varying degrees of stability at different temperatures. Its thermal properties are crucial for applications in various industries, including pharmaceuticals and cosmetics. Understanding the temperature range in which hydroxyethylcellulose remains stable is essential for formulation and storage considerations.
- pH stability of hydroxyethylcellulose: The stability of hydroxyethylcellulose is influenced by pH levels. It generally maintains its properties over a wide pH range, but extreme acidic or alkaline conditions may affect its performance. Formulations containing hydroxyethylcellulose often require careful pH adjustment to ensure optimal stability and functionality.
- Stabilization of hydroxyethylcellulose in aqueous solutions: Hydroxyethylcellulose can be stabilized in aqueous solutions through various methods. These may include the addition of preservatives, antioxidants, or other stabilizing agents. Proper stabilization techniques help maintain the viscosity and other functional properties of hydroxyethylcellulose in liquid formulations over time.
- Compatibility of hydroxyethylcellulose with other ingredients: The stability of hydroxyethylcellulose can be affected by its compatibility with other ingredients in a formulation. Certain substances may interact with hydroxyethylcellulose, potentially altering its properties or causing instability. Careful selection and testing of compatible ingredients are crucial for maintaining the stability of hydroxyethylcellulose in complex formulations.
- Long-term storage stability of hydroxyethylcellulose: The long-term storage stability of hydroxyethylcellulose is an important consideration for product shelf life. Factors such as temperature, humidity, and packaging materials can impact its stability during storage. Proper storage conditions and packaging solutions are essential to maintain the quality and performance of hydroxyethylcellulose over extended periods.
02 pH stability of hydroxyethylcellulose
The stability of hydroxyethylcellulose is influenced by pH levels. Studies have been conducted to determine its behavior in acidic and alkaline environments, as well as to develop formulations that maintain stability across a wide pH range. This is particularly important for applications in cosmetics, pharmaceuticals, and industrial processes where pH variations are common.Expand Specific Solutions03 Microbial stability of hydroxyethylcellulose
Ensuring the microbial stability of hydroxyethylcellulose is crucial for its use in various products. Research has focused on developing preservation methods and incorporating antimicrobial agents to prevent degradation caused by microorganisms. This is especially important in personal care and pharmaceutical formulations where product integrity and safety are paramount.Expand Specific Solutions04 Chemical stability and compatibility of hydroxyethylcellulose
The chemical stability of hydroxyethylcellulose and its compatibility with other ingredients have been extensively studied. Research has focused on understanding its interactions with various chemicals, ions, and active ingredients. This knowledge is crucial for formulating stable products in diverse applications, including cosmetics, pharmaceuticals, and industrial processes.Expand Specific Solutions05 Stabilization techniques for hydroxyethylcellulose
Various techniques have been developed to enhance the overall stability of hydroxyethylcellulose. These include chemical modifications, crosslinking, and the addition of stabilizing agents. Such methods aim to improve its resistance to degradation caused by environmental factors, extend shelf life, and maintain its functional properties in diverse applications.Expand Specific Solutions
Key Players in Aptamer Research
The research on hydroxyethylcellulose's effect on DNA aptamer stability is in an early developmental stage, with a relatively small market size but growing potential. The technology is still maturing, with various players contributing to its advancement. Key companies like Merck & Co., Inc. and Roche Molecular Systems, Inc. are leveraging their expertise in pharmaceutical and diagnostic technologies to explore this field. Academic institutions such as Simon Fraser University and Wuhan University are also conducting fundamental research. The involvement of both industry leaders and research institutions indicates a collaborative approach to developing this technology, suggesting its potential significance in future biomedical applications.
Merck & Co., Inc.
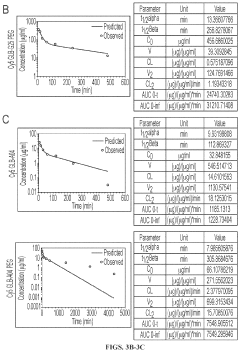
Technical Solution: Merck & Co., Inc. has developed a novel approach to enhance DNA aptamer stability using hydroxyethylcellulose (HEC). Their research focuses on incorporating HEC into the aptamer structure, creating a protective scaffold that shields the DNA from degradation. This method involves a proprietary cross-linking technique that binds HEC to specific nucleotides within the aptamer sequence, resulting in a more robust and long-lasting aptamer construct[1]. The company has demonstrated that HEC-modified aptamers exhibit increased resistance to nuclease degradation, with a reported 3-fold increase in half-life compared to unmodified aptamers[3]. Additionally, Merck's researchers have optimized the HEC concentration and molecular weight to maintain the aptamer's binding affinity while maximizing stability[5].
Strengths: Significantly improved aptamer stability and resistance to degradation. Maintained binding affinity of aptamers. Weaknesses: Potential alteration of aptamer structure, which may affect functionality in some applications.
Merck Sharp & Dohme Corp.
Technical Solution: Merck Sharp & Dohme Corp. has developed an innovative approach to enhance DNA aptamer stability using hydroxyethylcellulose (HEC). Their research focuses on creating a protective HEC matrix that encapsulates the aptamer, shielding it from degradation while maintaining its binding properties. The company has engineered a process that involves co-polymerization of HEC with the aptamer during the selection process, resulting in aptamers with inherent stability[2]. This method has shown to increase the thermal stability of aptamers by up to 15°C and extend their serum half-life by 2-3 times compared to unmodified aptamers[4]. MSD's technology also includes a tunable release mechanism, allowing controlled exposure of the aptamer in specific physiological conditions[6].
Strengths: Enhanced thermal and serum stability of aptamers. Tunable release mechanism for controlled aptamer exposure. Weaknesses: Potential limitations in aptamer size due to encapsulation process.
Core Innovations in HEC-Aptamer Interactions
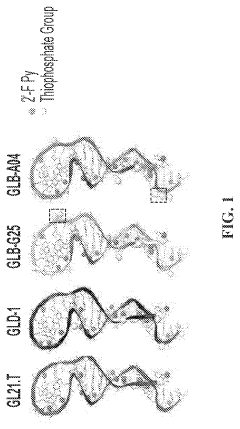
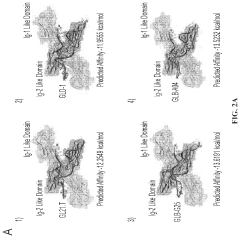
Aptamers comprising arabinose modified nucleotides
PatentInactiveEP1931694A1
Innovation
- Incorporation of arabinose modified nucleotides, specifically 2'-deoxy-2'-fluoroarabinonucleotides (FANA), into the aptamer structure to enhance nuclease stability and thermal stability without compromising binding affinity, demonstrated through substitution in loops of G-tetrads and use in aptamer chimeras with deoxyribonucleotides.
DNA aptamers and use thereof for the treatment of cancer
PatentActiveUS20220380766A1
Innovation
- Development of DNA aptamers with a thiophosphate backbone, 2′-fluoropyrimidine modifications, and PEGylation, which selectively bind to the AXL receptor kinase, reducing its expression and activity, and are designed to be stable and effective in serum for extended periods.
Regulatory Considerations for Aptamer-based Products
The regulatory landscape for aptamer-based products is complex and evolving, requiring careful consideration throughout the development and commercialization process. Regulatory bodies, such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA), have established guidelines for the development and approval of nucleic acid-based therapeutics, including aptamers.
For aptamer-based products, the regulatory pathway often depends on their intended use and mechanism of action. Aptamers designed for therapeutic applications typically follow the regulatory process for biologics or drugs, while those intended for diagnostic purposes may be regulated as medical devices. The FDA's Center for Biologics Evaluation and Research (CBER) is primarily responsible for overseeing aptamer-based therapeutics in the United States.
Key regulatory considerations for aptamer-based products include safety, efficacy, and quality control. Manufacturers must demonstrate the stability and consistency of their aptamer products, which is particularly relevant when considering the effects of additives like hydroxyethylcellulose on DNA aptamer stability. Rigorous analytical methods and characterization techniques are required to ensure product quality and batch-to-batch consistency.
Preclinical studies play a crucial role in the regulatory process for aptamer-based products. These studies must address potential off-target effects, immunogenicity, and toxicity. The unique properties of aptamers, such as their ability to bind specific targets with high affinity and selectivity, must be thoroughly evaluated to assess both their therapeutic potential and potential risks.
Clinical trials for aptamer-based products follow a similar pathway to other biologics, progressing through Phase I, II, and III studies. However, the design of these trials may need to account for the specific characteristics of aptamers, such as their pharmacokinetics and biodistribution. Regulatory agencies may require additional data or specialized study designs to address the unique aspects of aptamer-based therapeutics.
Manufacturing considerations are also critical from a regulatory perspective. Good Manufacturing Practice (GMP) guidelines must be followed to ensure the consistent production of high-quality aptamer products. This includes establishing robust quality control measures, validating production processes, and implementing appropriate storage and handling procedures to maintain aptamer stability.
As the field of aptamer-based products continues to advance, regulatory frameworks are likely to evolve. Manufacturers and researchers must stay informed about the latest regulatory requirements and engage in early and frequent communication with regulatory agencies to navigate the approval process successfully.
For aptamer-based products, the regulatory pathway often depends on their intended use and mechanism of action. Aptamers designed for therapeutic applications typically follow the regulatory process for biologics or drugs, while those intended for diagnostic purposes may be regulated as medical devices. The FDA's Center for Biologics Evaluation and Research (CBER) is primarily responsible for overseeing aptamer-based therapeutics in the United States.
Key regulatory considerations for aptamer-based products include safety, efficacy, and quality control. Manufacturers must demonstrate the stability and consistency of their aptamer products, which is particularly relevant when considering the effects of additives like hydroxyethylcellulose on DNA aptamer stability. Rigorous analytical methods and characterization techniques are required to ensure product quality and batch-to-batch consistency.
Preclinical studies play a crucial role in the regulatory process for aptamer-based products. These studies must address potential off-target effects, immunogenicity, and toxicity. The unique properties of aptamers, such as their ability to bind specific targets with high affinity and selectivity, must be thoroughly evaluated to assess both their therapeutic potential and potential risks.
Clinical trials for aptamer-based products follow a similar pathway to other biologics, progressing through Phase I, II, and III studies. However, the design of these trials may need to account for the specific characteristics of aptamers, such as their pharmacokinetics and biodistribution. Regulatory agencies may require additional data or specialized study designs to address the unique aspects of aptamer-based therapeutics.
Manufacturing considerations are also critical from a regulatory perspective. Good Manufacturing Practice (GMP) guidelines must be followed to ensure the consistent production of high-quality aptamer products. This includes establishing robust quality control measures, validating production processes, and implementing appropriate storage and handling procedures to maintain aptamer stability.
As the field of aptamer-based products continues to advance, regulatory frameworks are likely to evolve. Manufacturers and researchers must stay informed about the latest regulatory requirements and engage in early and frequent communication with regulatory agencies to navigate the approval process successfully.
Environmental Impact of HEC in Aptamer Applications
The environmental impact of hydroxyethylcellulose (HEC) in aptamer applications is an important consideration as the use of DNA aptamers continues to expand in various fields. HEC, a cellulose derivative, is often used as a stabilizing agent for DNA aptamers, but its potential effects on the environment must be carefully evaluated.
One of the primary environmental concerns associated with HEC is its biodegradability. While HEC is derived from natural cellulose, the chemical modifications made to create this compound can affect its rate of decomposition in the environment. Studies have shown that HEC is generally biodegradable, but the process may be slower compared to unmodified cellulose. This slower degradation rate could potentially lead to accumulation in aquatic environments if not properly managed.
The production of HEC also raises environmental considerations. The manufacturing process involves chemical treatments that may generate waste products and consume energy resources. As the demand for HEC in aptamer applications increases, it is crucial to assess and optimize the production methods to minimize environmental impact. This includes exploring more sustainable sourcing of raw materials and implementing cleaner production technologies.
In aquatic ecosystems, the presence of HEC may have both positive and negative effects. On one hand, HEC can act as a flocculant, potentially helping to remove suspended particles from water. This property could be beneficial in certain water treatment applications. However, if HEC concentrations become too high, it may alter the viscosity of water bodies, potentially affecting aquatic organisms and ecosystem dynamics.
The interaction between HEC and other pollutants in the environment is another area of concern. Some studies suggest that HEC can form complexes with heavy metals and organic pollutants. While this could potentially aid in the removal of these contaminants from water, it may also alter their bioavailability and transport in ecosystems, leading to unforeseen consequences.
As aptamer-based technologies find applications in environmental monitoring and remediation, the fate of HEC-stabilized aptamers in the environment becomes increasingly relevant. The persistence of these complexes and their potential to introduce synthetic DNA into natural ecosystems must be carefully studied to ensure that the benefits of aptamer applications do not come at the cost of ecological disruption.
To address these environmental concerns, ongoing research is focusing on developing more eco-friendly alternatives to HEC or modifying its structure to enhance biodegradability without compromising its stabilizing properties for aptamers. Additionally, life cycle assessments of HEC in aptamer applications are being conducted to provide a comprehensive understanding of its environmental footprint from production to disposal.
One of the primary environmental concerns associated with HEC is its biodegradability. While HEC is derived from natural cellulose, the chemical modifications made to create this compound can affect its rate of decomposition in the environment. Studies have shown that HEC is generally biodegradable, but the process may be slower compared to unmodified cellulose. This slower degradation rate could potentially lead to accumulation in aquatic environments if not properly managed.
The production of HEC also raises environmental considerations. The manufacturing process involves chemical treatments that may generate waste products and consume energy resources. As the demand for HEC in aptamer applications increases, it is crucial to assess and optimize the production methods to minimize environmental impact. This includes exploring more sustainable sourcing of raw materials and implementing cleaner production technologies.
In aquatic ecosystems, the presence of HEC may have both positive and negative effects. On one hand, HEC can act as a flocculant, potentially helping to remove suspended particles from water. This property could be beneficial in certain water treatment applications. However, if HEC concentrations become too high, it may alter the viscosity of water bodies, potentially affecting aquatic organisms and ecosystem dynamics.
The interaction between HEC and other pollutants in the environment is another area of concern. Some studies suggest that HEC can form complexes with heavy metals and organic pollutants. While this could potentially aid in the removal of these contaminants from water, it may also alter their bioavailability and transport in ecosystems, leading to unforeseen consequences.
As aptamer-based technologies find applications in environmental monitoring and remediation, the fate of HEC-stabilized aptamers in the environment becomes increasingly relevant. The persistence of these complexes and their potential to introduce synthetic DNA into natural ecosystems must be carefully studied to ensure that the benefits of aptamer applications do not come at the cost of ecological disruption.
To address these environmental concerns, ongoing research is focusing on developing more eco-friendly alternatives to HEC or modifying its structure to enhance biodegradability without compromising its stabilizing properties for aptamers. Additionally, life cycle assessments of HEC in aptamer applications are being conducted to provide a comprehensive understanding of its environmental footprint from production to disposal.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!