How Hydroxyethylcellulose Alters Chemical Pathways in Organic Synthesis
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HEC in Organic Synthesis: Background and Objectives
Hydroxyethylcellulose (HEC) has emerged as a significant player in organic synthesis, revolutionizing chemical pathways and offering new possibilities for researchers and industries alike. The journey of HEC in organic chemistry began in the mid-20th century when its unique properties were first recognized. As a cellulose derivative, HEC combines the biodegradability of natural polymers with the versatility of synthetic materials, making it an attractive option for various applications.
The evolution of HEC in organic synthesis has been driven by the increasing demand for sustainable and efficient chemical processes. Its ability to act as a thickening agent, stabilizer, and binder has opened up new avenues for reaction control and product design. The hydrophilic nature of HEC, coupled with its non-ionic character, allows for its use in a wide range of pH conditions, making it a versatile tool in organic synthesis.
One of the primary objectives in exploring HEC's role in organic synthesis is to understand how it alters chemical pathways. This involves investigating its interactions with various reactants, catalysts, and solvents. Researchers aim to elucidate the mechanisms by which HEC influences reaction kinetics, selectivity, and yield. By doing so, they hope to develop more efficient and environmentally friendly synthetic routes for complex organic molecules.
Another key goal is to expand the application scope of HEC in organic synthesis. This includes exploring its potential in asymmetric synthesis, where its chiral nature could be exploited to control stereochemistry. Additionally, there is growing interest in utilizing HEC as a support for catalysts, potentially enhancing their activity and recyclability.
The development of HEC-based materials for organic synthesis also aims to address current challenges in the field. These include improving reaction efficiency, reducing waste generation, and enabling the synthesis of previously inaccessible compounds. By leveraging the unique properties of HEC, researchers hope to create novel reaction media and catalytic systems that can overcome existing limitations in organic synthesis.
As the field progresses, there is an increasing focus on understanding the structure-property relationships of HEC in organic reactions. This involves studying how factors such as molecular weight, degree of substitution, and viscosity affect its performance in different synthetic contexts. Such knowledge is crucial for optimizing HEC-based systems and tailoring them to specific reaction requirements.
In conclusion, the exploration of HEC in organic synthesis represents a convergence of green chemistry principles and innovative synthetic methodologies. By altering chemical pathways, HEC offers the potential to develop more sustainable, efficient, and versatile organic synthesis processes, aligning with the broader goals of modern chemistry research and industrial applications.
The evolution of HEC in organic synthesis has been driven by the increasing demand for sustainable and efficient chemical processes. Its ability to act as a thickening agent, stabilizer, and binder has opened up new avenues for reaction control and product design. The hydrophilic nature of HEC, coupled with its non-ionic character, allows for its use in a wide range of pH conditions, making it a versatile tool in organic synthesis.
One of the primary objectives in exploring HEC's role in organic synthesis is to understand how it alters chemical pathways. This involves investigating its interactions with various reactants, catalysts, and solvents. Researchers aim to elucidate the mechanisms by which HEC influences reaction kinetics, selectivity, and yield. By doing so, they hope to develop more efficient and environmentally friendly synthetic routes for complex organic molecules.
Another key goal is to expand the application scope of HEC in organic synthesis. This includes exploring its potential in asymmetric synthesis, where its chiral nature could be exploited to control stereochemistry. Additionally, there is growing interest in utilizing HEC as a support for catalysts, potentially enhancing their activity and recyclability.
The development of HEC-based materials for organic synthesis also aims to address current challenges in the field. These include improving reaction efficiency, reducing waste generation, and enabling the synthesis of previously inaccessible compounds. By leveraging the unique properties of HEC, researchers hope to create novel reaction media and catalytic systems that can overcome existing limitations in organic synthesis.
As the field progresses, there is an increasing focus on understanding the structure-property relationships of HEC in organic reactions. This involves studying how factors such as molecular weight, degree of substitution, and viscosity affect its performance in different synthetic contexts. Such knowledge is crucial for optimizing HEC-based systems and tailoring them to specific reaction requirements.
In conclusion, the exploration of HEC in organic synthesis represents a convergence of green chemistry principles and innovative synthetic methodologies. By altering chemical pathways, HEC offers the potential to develop more sustainable, efficient, and versatile organic synthesis processes, aligning with the broader goals of modern chemistry research and industrial applications.
Market Analysis for HEC-Modified Organic Synthesis
The market for hydroxyethylcellulose (HEC) in organic synthesis is experiencing significant growth, driven by the increasing demand for eco-friendly and efficient chemical processes. HEC, a cellulose derivative, has gained attention for its ability to alter chemical pathways in organic synthesis, offering unique advantages in reaction control and product selectivity.
The global market for HEC in organic synthesis applications is projected to expand at a steady rate over the next five years. This growth is primarily attributed to the rising adoption of green chemistry principles in the pharmaceutical and fine chemicals industries. HEC's biodegradability and non-toxic nature make it an attractive option for companies seeking to reduce their environmental footprint and comply with stringent regulations.
In the pharmaceutical sector, HEC-modified organic synthesis is gaining traction due to its potential to enhance drug discovery processes. The ability of HEC to influence reaction pathways can lead to the development of novel drug candidates and improve the efficiency of existing synthetic routes. This application is expected to be a major driver of market growth, particularly in regions with strong pharmaceutical research and development activities.
The fine chemicals industry is another key market segment for HEC-modified organic synthesis. Manufacturers are increasingly exploring the use of HEC to optimize production processes, reduce waste, and improve product quality. The versatility of HEC in various organic reactions makes it a valuable tool for developing more sustainable and cost-effective manufacturing methods.
Geographically, North America and Europe currently dominate the market for HEC in organic synthesis applications. These regions benefit from well-established chemical and pharmaceutical industries, as well as a strong focus on sustainable practices. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing research activities, and growing awareness of green chemistry principles.
Despite the positive outlook, the market faces certain challenges. The relatively higher cost of HEC compared to traditional reaction modifiers may limit its adoption in price-sensitive markets. Additionally, the need for specialized knowledge and equipment to effectively implement HEC-modified synthesis techniques could slow down market penetration in some sectors.
Overall, the market for HEC-modified organic synthesis presents significant opportunities for growth and innovation. As research in this field continues to advance, new applications and improved methodologies are likely to emerge, further expanding the market potential. Companies that invest in developing expertise in HEC-based synthesis techniques and focus on creating value-added products are well-positioned to capitalize on this growing market trend.
The global market for HEC in organic synthesis applications is projected to expand at a steady rate over the next five years. This growth is primarily attributed to the rising adoption of green chemistry principles in the pharmaceutical and fine chemicals industries. HEC's biodegradability and non-toxic nature make it an attractive option for companies seeking to reduce their environmental footprint and comply with stringent regulations.
In the pharmaceutical sector, HEC-modified organic synthesis is gaining traction due to its potential to enhance drug discovery processes. The ability of HEC to influence reaction pathways can lead to the development of novel drug candidates and improve the efficiency of existing synthetic routes. This application is expected to be a major driver of market growth, particularly in regions with strong pharmaceutical research and development activities.
The fine chemicals industry is another key market segment for HEC-modified organic synthesis. Manufacturers are increasingly exploring the use of HEC to optimize production processes, reduce waste, and improve product quality. The versatility of HEC in various organic reactions makes it a valuable tool for developing more sustainable and cost-effective manufacturing methods.
Geographically, North America and Europe currently dominate the market for HEC in organic synthesis applications. These regions benefit from well-established chemical and pharmaceutical industries, as well as a strong focus on sustainable practices. However, the Asia-Pacific region is expected to witness the fastest growth in the coming years, driven by rapid industrialization, increasing research activities, and growing awareness of green chemistry principles.
Despite the positive outlook, the market faces certain challenges. The relatively higher cost of HEC compared to traditional reaction modifiers may limit its adoption in price-sensitive markets. Additionally, the need for specialized knowledge and equipment to effectively implement HEC-modified synthesis techniques could slow down market penetration in some sectors.
Overall, the market for HEC-modified organic synthesis presents significant opportunities for growth and innovation. As research in this field continues to advance, new applications and improved methodologies are likely to emerge, further expanding the market potential. Companies that invest in developing expertise in HEC-based synthesis techniques and focus on creating value-added products are well-positioned to capitalize on this growing market trend.
Current Applications and Challenges of HEC
Hydroxyethylcellulose (HEC) has gained significant attention in organic synthesis due to its unique properties and versatile applications. As a cellulose derivative, HEC exhibits excellent water solubility, film-forming ability, and thickening properties, making it a valuable additive in various chemical processes. Currently, HEC finds extensive use in pharmaceutical formulations, cosmetics, and personal care products as a stabilizer, thickener, and binder.
In organic synthesis, HEC has emerged as a promising green solvent and reaction medium. Its ability to form hydrogels and act as a template for nanoparticle synthesis has opened up new avenues for catalysis and materials science. Researchers have successfully employed HEC-based hydrogels as supports for metal nanoparticles, enhancing their catalytic activity and recyclability in various organic transformations.
One of the key applications of HEC in organic synthesis is its role as a stabilizer in emulsion polymerization. The polymer's amphiphilic nature allows it to effectively stabilize oil-in-water emulsions, facilitating the synthesis of polymer nanoparticles with controlled size and morphology. This has significant implications for the production of advanced materials with tailored properties.
Despite its promising applications, the use of HEC in organic synthesis faces several challenges. One major hurdle is the limited understanding of how HEC interacts with different reactants and catalysts at the molecular level. The complex structure of HEC, with its hydroxyl and hydroxyethyl groups, can lead to unexpected side reactions or alterations in reaction pathways, necessitating careful optimization of reaction conditions.
Another challenge lies in the control of HEC's molecular weight and degree of substitution, which can significantly impact its performance in organic synthesis. Variations in these parameters can affect the viscosity, solubility, and reactivity of HEC-based systems, leading to inconsistent results across different batches or suppliers. Standardization of HEC properties for specific synthetic applications remains an ongoing challenge for researchers and manufacturers alike.
The scalability of HEC-based organic synthesis processes also presents a significant hurdle. While many laboratory-scale reactions have shown promising results, translating these to industrial-scale production often requires overcoming issues related to mixing, heat transfer, and product separation. The high viscosity of HEC solutions at higher concentrations can pose challenges in large-scale reactors, potentially limiting its applicability in certain industrial processes.
Furthermore, the biodegradability of HEC, while generally considered an advantage from an environmental perspective, can sometimes be a drawback in organic synthesis. The potential for microbial contamination and degradation of HEC-based reaction media over time may limit the shelf life and reproducibility of certain synthetic protocols, particularly in long-term or multi-step reactions.
In organic synthesis, HEC has emerged as a promising green solvent and reaction medium. Its ability to form hydrogels and act as a template for nanoparticle synthesis has opened up new avenues for catalysis and materials science. Researchers have successfully employed HEC-based hydrogels as supports for metal nanoparticles, enhancing their catalytic activity and recyclability in various organic transformations.
One of the key applications of HEC in organic synthesis is its role as a stabilizer in emulsion polymerization. The polymer's amphiphilic nature allows it to effectively stabilize oil-in-water emulsions, facilitating the synthesis of polymer nanoparticles with controlled size and morphology. This has significant implications for the production of advanced materials with tailored properties.
Despite its promising applications, the use of HEC in organic synthesis faces several challenges. One major hurdle is the limited understanding of how HEC interacts with different reactants and catalysts at the molecular level. The complex structure of HEC, with its hydroxyl and hydroxyethyl groups, can lead to unexpected side reactions or alterations in reaction pathways, necessitating careful optimization of reaction conditions.
Another challenge lies in the control of HEC's molecular weight and degree of substitution, which can significantly impact its performance in organic synthesis. Variations in these parameters can affect the viscosity, solubility, and reactivity of HEC-based systems, leading to inconsistent results across different batches or suppliers. Standardization of HEC properties for specific synthetic applications remains an ongoing challenge for researchers and manufacturers alike.
The scalability of HEC-based organic synthesis processes also presents a significant hurdle. While many laboratory-scale reactions have shown promising results, translating these to industrial-scale production often requires overcoming issues related to mixing, heat transfer, and product separation. The high viscosity of HEC solutions at higher concentrations can pose challenges in large-scale reactors, potentially limiting its applicability in certain industrial processes.
Furthermore, the biodegradability of HEC, while generally considered an advantage from an environmental perspective, can sometimes be a drawback in organic synthesis. The potential for microbial contamination and degradation of HEC-based reaction media over time may limit the shelf life and reproducibility of certain synthetic protocols, particularly in long-term or multi-step reactions.
Mechanisms of HEC in Organic Reactions
01 Synthesis and modification of hydroxyethylcellulose
Hydroxyethylcellulose is synthesized through the reaction of cellulose with ethylene oxide. The chemical pathway involves the substitution of hydroxyl groups on cellulose with hydroxyethyl groups. This process can be modified to control the degree of substitution and molecular weight, which affects the properties of the final product.- Synthesis and modification of hydroxyethylcellulose: Hydroxyethylcellulose is synthesized through the reaction of cellulose with ethylene oxide. The chemical pathway involves the substitution of hydroxyl groups on cellulose with hydroxyethyl groups. This process can be modified to control the degree of substitution and molecular weight, which affects the properties of the final product.
- Applications in pharmaceutical formulations: Hydroxyethylcellulose is widely used in pharmaceutical formulations due to its unique properties. It serves as a thickening agent, stabilizer, and controlled release matrix in various drug delivery systems. The chemical pathways of hydroxyethylcellulose in these applications involve its interaction with active pharmaceutical ingredients and other excipients.
- Use in personal care and cosmetic products: Hydroxyethylcellulose is utilized in personal care and cosmetic products as a rheology modifier and film-forming agent. The chemical pathways in these applications involve the formation of hydrogen bonds with water and other ingredients, creating stable emulsions and gels with desired viscosity and texture.
- Chemical modifications for enhanced properties: Hydroxyethylcellulose can undergo further chemical modifications to enhance its properties for specific applications. These modifications may include crosslinking, grafting with other polymers, or introducing functional groups. The chemical pathways involved in these modifications aim to improve the performance characteristics of hydroxyethylcellulose.
- Environmental and biodegradation aspects: The chemical pathways of hydroxyethylcellulose in environmental contexts are of interest, particularly its biodegradation processes. Understanding these pathways is crucial for assessing the environmental impact and developing eco-friendly applications. Research focuses on the mechanisms of hydroxyethylcellulose breakdown in various environmental conditions.
02 Applications in pharmaceutical formulations
Hydroxyethylcellulose is widely used in pharmaceutical formulations due to its unique properties. It serves as a thickening agent, stabilizer, and controlled release matrix in various drug delivery systems. The chemical pathways of hydroxyethylcellulose in these applications involve its interaction with active pharmaceutical ingredients and other excipients.Expand Specific Solutions03 Use in personal care and cosmetic products
Hydroxyethylcellulose is utilized in personal care and cosmetic products as a rheology modifier and film-forming agent. Its chemical pathways in these applications involve interactions with other ingredients to form stable emulsions, gels, and suspensions. The polymer's ability to form hydrogen bonds with water molecules contributes to its moisturizing properties.Expand Specific Solutions04 Crosslinking and network formation
Hydroxyethylcellulose can undergo crosslinking reactions to form three-dimensional networks. This chemical pathway involves the formation of covalent bonds between polymer chains, often through the use of crosslinking agents. The resulting networks exhibit enhanced mechanical properties and controlled swelling behavior, which are useful in various applications.Expand Specific Solutions05 Biodegradation and environmental impact
The biodegradation of hydroxyethylcellulose involves chemical pathways that break down the polymer into smaller, environmentally friendly components. This process is influenced by factors such as molecular weight, degree of substitution, and environmental conditions. Understanding these pathways is crucial for assessing the environmental impact and developing sustainable applications of hydroxyethylcellulose.Expand Specific Solutions
Key Industry Players and HEC Manufacturers
The competitive landscape for hydroxyethylcellulose in organic synthesis is evolving rapidly, with the market currently in a growth phase. The global market size for this technology is expanding, driven by increasing demand in various industries. While the technology is relatively mature, ongoing research and development efforts by key players such as Hercules Corp., Cargill, Inc., and UPM-Kymmene Oyj are pushing the boundaries of its applications. Academic institutions like Zhejiang University of Technology and Wuhan University are contributing to fundamental research, while companies like Mitsui Chemicals, Inc. and METabolic EXplorer SA are focusing on industrial applications. The collaboration between industry and academia is accelerating innovation in this field, leading to more efficient and sustainable chemical pathways.
Hercules Corp.
Technical Solution: Hercules Corp. has developed a novel approach to utilizing hydroxyethylcellulose (HEC) in organic synthesis. Their method involves modifying HEC to create a biodegradable and renewable catalyst support system. This system enhances the efficiency of various organic reactions, particularly in aqueous media. The modified HEC acts as a stabilizer for metal nanoparticles, allowing for improved catalytic activity and recyclability[1]. The company has also explored the use of HEC as a green solvent in organic transformations, leveraging its unique properties to promote environmentally friendly synthesis routes[2]. Additionally, Hercules Corp. has investigated the potential of HEC-based hydrogels as controlled release matrices for organic reagents, enabling more precise control over reaction kinetics[3].
Strengths: Eco-friendly approach, improved catalytic efficiency, and versatile applications in organic synthesis. Weaknesses: Potential limitations in non-aqueous reaction systems and scalability challenges for industrial processes.
Cargill, Inc.
Technical Solution: Cargill has developed an innovative approach to utilizing hydroxyethylcellulose (HEC) in organic synthesis, focusing on its application in the food and pharmaceutical industries. Their technology involves the use of HEC as a stabilizer and emulsifier in complex organic reactions, particularly those involving sensitive biomolecules. Cargill's method employs HEC to create microenvironments that protect reactive species and control reaction rates[1]. The company has also explored the use of HEC-based hydrogels as a medium for enzymatic organic transformations, enhancing the stability and reusability of biocatalysts[2]. Furthermore, Cargill has investigated the potential of chemically modified HEC derivatives to act as chiral auxiliaries in asymmetric synthesis, opening up new possibilities for the production of enantiopure compounds[3].
Strengths: Expertise in food and pharmaceutical applications, enhanced stability for sensitive reactions, and potential for chiral synthesis. Weaknesses: May be limited to specific industry sectors and potentially higher costs compared to traditional methods.
Innovative HEC-Based Synthetic Methodologies
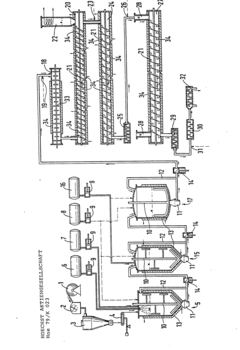
Method for producing cellulose ether derivative
PatentInactiveUS20100311964A1
Innovation
- A process involving low-crystalline powdery cellulose reacted with an organohalide compound in the presence of a base, such as monohaloacetic acid or ethylene chlorohydrin, to produce cellulose ether derivatives with high reaction efficiency and selectivity, reducing by-product formation and simplifying purification.
Continuous process and apparatus for preparing water-soluble hydroxyalkyl cellulose or their mixed ethers
PatentInactiveEP0022923A1
Innovation
- A continuous process involving a suspension of cellulose, an alkalizing agent, and an etherifying agent in the presence of water and an inert organic solvent, with controlled temperature, pressure, and residence time, allowing for high degrees of etherification and reduced solvent usage, utilizing a device with homogenization and tubular reactor stages to ensure uniform reaction and product quality.
Environmental Impact of HEC in Chemical Industry
The environmental impact of hydroxyethylcellulose (HEC) in the chemical industry is a critical consideration as its usage continues to grow. HEC, a cellulose derivative, has found widespread applications in various sectors, including pharmaceuticals, personal care products, and construction materials. However, its increasing presence in industrial processes raises concerns about potential ecological consequences.
One of the primary environmental benefits of HEC is its biodegradability. As a modified natural polymer, HEC can be broken down by microorganisms in the environment, reducing long-term accumulation in ecosystems. This characteristic makes it an attractive alternative to synthetic polymers in many applications, potentially lowering the overall environmental footprint of chemical processes.
Despite its biodegradability, the production of HEC does have some environmental implications. The manufacturing process involves chemical modifications of cellulose, which requires energy and resources. Additionally, the use of ethylene oxide in HEC production raises concerns about potential emissions and worker safety. However, advancements in green chemistry techniques are gradually improving the sustainability of HEC production.
In aquatic environments, HEC's high solubility and thickening properties can affect water quality if released in large quantities. While not directly toxic to aquatic life, high concentrations of HEC may alter water viscosity and potentially impact oxygen transfer in water bodies. This underscores the importance of proper waste management and treatment in industries utilizing HEC.
The use of HEC in organic synthesis can lead to reduced solvent usage in certain reactions, contributing to greener chemistry practices. By modifying reaction pathways, HEC can enhance the efficiency of some processes, potentially reducing energy consumption and waste generation. This aspect of HEC usage aligns with the principles of green chemistry and sustainable industrial practices.
However, the increasing demand for HEC may lead to expanded cellulose harvesting, potentially impacting forest ecosystems if not managed sustainably. This indirect environmental effect highlights the need for responsible sourcing practices and the exploration of alternative cellulose sources, such as agricultural waste or fast-growing crops.
In conclusion, while HEC offers several environmental advantages over synthetic alternatives, its growing industrial use necessitates careful consideration of its full lifecycle impact. Balancing the benefits of biodegradability and process efficiency with the challenges of production emissions and resource management is crucial for ensuring the sustainable integration of HEC in the chemical industry.
One of the primary environmental benefits of HEC is its biodegradability. As a modified natural polymer, HEC can be broken down by microorganisms in the environment, reducing long-term accumulation in ecosystems. This characteristic makes it an attractive alternative to synthetic polymers in many applications, potentially lowering the overall environmental footprint of chemical processes.
Despite its biodegradability, the production of HEC does have some environmental implications. The manufacturing process involves chemical modifications of cellulose, which requires energy and resources. Additionally, the use of ethylene oxide in HEC production raises concerns about potential emissions and worker safety. However, advancements in green chemistry techniques are gradually improving the sustainability of HEC production.
In aquatic environments, HEC's high solubility and thickening properties can affect water quality if released in large quantities. While not directly toxic to aquatic life, high concentrations of HEC may alter water viscosity and potentially impact oxygen transfer in water bodies. This underscores the importance of proper waste management and treatment in industries utilizing HEC.
The use of HEC in organic synthesis can lead to reduced solvent usage in certain reactions, contributing to greener chemistry practices. By modifying reaction pathways, HEC can enhance the efficiency of some processes, potentially reducing energy consumption and waste generation. This aspect of HEC usage aligns with the principles of green chemistry and sustainable industrial practices.
However, the increasing demand for HEC may lead to expanded cellulose harvesting, potentially impacting forest ecosystems if not managed sustainably. This indirect environmental effect highlights the need for responsible sourcing practices and the exploration of alternative cellulose sources, such as agricultural waste or fast-growing crops.
In conclusion, while HEC offers several environmental advantages over synthetic alternatives, its growing industrial use necessitates careful consideration of its full lifecycle impact. Balancing the benefits of biodegradability and process efficiency with the challenges of production emissions and resource management is crucial for ensuring the sustainable integration of HEC in the chemical industry.
Regulatory Framework for HEC Usage in Synthesis
The regulatory framework for hydroxyethylcellulose (HEC) usage in organic synthesis is a complex and evolving landscape that requires careful consideration by researchers and manufacturers. At the global level, organizations such as the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use (ICH) provide guidelines that influence the use of HEC in pharmaceutical synthesis. These guidelines emphasize the importance of quality control, safety assessments, and environmental impact considerations.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating HEC usage, particularly in pharmaceutical applications. The FDA's guidance on process analytical technology (PAT) and quality by design (QbD) principles directly impacts how HEC is incorporated into synthesis processes. Manufacturers must demonstrate a thorough understanding of how HEC affects chemical pathways and final product quality to gain regulatory approval.
The European Union's regulatory framework, governed by the European Medicines Agency (EMA), imposes stringent requirements on the use of excipients like HEC in drug synthesis. The EU's REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) regulation also applies to HEC, mandating comprehensive safety data and risk assessments for its use in various applications, including organic synthesis.
In Asia, countries like Japan and China have their own regulatory bodies that oversee the use of HEC in synthesis processes. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established guidelines that manufacturers must adhere to when utilizing HEC in their production processes.
Environmental regulations also play a significant role in shaping the use of HEC in organic synthesis. Many countries have implemented strict waste management and disposal protocols for chemicals used in industrial processes. Manufacturers must consider these regulations when designing synthesis pathways that incorporate HEC, ensuring compliance with local and international environmental standards.
Occupational health and safety regulations further influence the use of HEC in synthesis. Organizations such as the Occupational Safety and Health Administration (OSHA) in the US and the European Agency for Safety and Health at Work (EU-OSHA) set standards for worker protection when handling chemicals like HEC. These regulations mandate proper training, personal protective equipment, and workplace safety measures.
As the field of organic synthesis continues to evolve, regulatory frameworks are likely to adapt to new technologies and methodologies. Researchers and manufacturers must stay informed about changes in regulations and proactively engage with regulatory bodies to ensure compliance and optimize their use of HEC in chemical pathways.
In the United States, the Food and Drug Administration (FDA) plays a crucial role in regulating HEC usage, particularly in pharmaceutical applications. The FDA's guidance on process analytical technology (PAT) and quality by design (QbD) principles directly impacts how HEC is incorporated into synthesis processes. Manufacturers must demonstrate a thorough understanding of how HEC affects chemical pathways and final product quality to gain regulatory approval.
The European Union's regulatory framework, governed by the European Medicines Agency (EMA), imposes stringent requirements on the use of excipients like HEC in drug synthesis. The EU's REACH (Registration, Evaluation, Authorisation and Restriction of Chemicals) regulation also applies to HEC, mandating comprehensive safety data and risk assessments for its use in various applications, including organic synthesis.
In Asia, countries like Japan and China have their own regulatory bodies that oversee the use of HEC in synthesis processes. Japan's Pharmaceuticals and Medical Devices Agency (PMDA) and China's National Medical Products Administration (NMPA) have established guidelines that manufacturers must adhere to when utilizing HEC in their production processes.
Environmental regulations also play a significant role in shaping the use of HEC in organic synthesis. Many countries have implemented strict waste management and disposal protocols for chemicals used in industrial processes. Manufacturers must consider these regulations when designing synthesis pathways that incorporate HEC, ensuring compliance with local and international environmental standards.
Occupational health and safety regulations further influence the use of HEC in synthesis. Organizations such as the Occupational Safety and Health Administration (OSHA) in the US and the European Agency for Safety and Health at Work (EU-OSHA) set standards for worker protection when handling chemicals like HEC. These regulations mandate proper training, personal protective equipment, and workplace safety measures.
As the field of organic synthesis continues to evolve, regulatory frameworks are likely to adapt to new technologies and methodologies. Researchers and manufacturers must stay informed about changes in regulations and proactively engage with regulatory bodies to ensure compliance and optimize their use of HEC in chemical pathways.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!