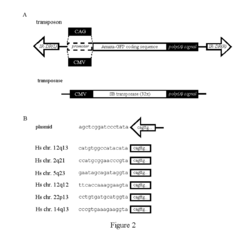
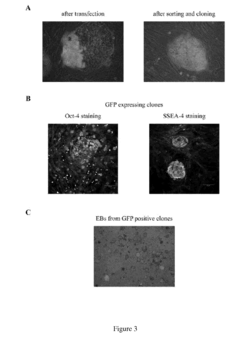
Hydroxyethylcellulose as a Technological Enabler in Gene Therapy
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HEC in Gene Therapy: Background and Objectives
Hydroxyethylcellulose (HEC) has emerged as a promising technological enabler in the rapidly evolving field of gene therapy. This versatile polymer, derived from cellulose, has garnered significant attention due to its unique properties that align well with the requirements of gene delivery systems. The development of HEC as a tool in gene therapy is rooted in the broader context of biomaterial research and the ongoing quest for more effective and safer methods of genetic material delivery.
Gene therapy, a revolutionary approach to treating genetic disorders, has faced numerous challenges since its inception. One of the primary obstacles has been the development of efficient and non-toxic delivery vectors for genetic material. Traditional viral vectors, while effective, have raised safety concerns and faced limitations in terms of payload capacity and production scalability. This has led researchers to explore alternative non-viral delivery systems, where HEC has shown considerable promise.
The journey of HEC in gene therapy began with its well-established use in pharmaceutical and cosmetic industries as a thickening agent and stabilizer. Its biocompatibility, low toxicity, and ability to form hydrogels caught the attention of gene therapy researchers seeking to improve the efficacy and safety of gene delivery systems. The polymer's chemical structure allows for easy modification, enabling the creation of tailored delivery vehicles for various genetic payloads.
The primary objective of incorporating HEC in gene therapy is to overcome the limitations of existing delivery methods while enhancing the overall efficacy of genetic material transfer. Researchers aim to leverage HEC's unique properties to develop delivery systems that can protect genetic material from degradation, facilitate cellular uptake, and ensure controlled release within target cells. Additionally, the use of HEC is expected to address concerns related to immunogenicity and toxicity often associated with viral vectors.
Another crucial goal is to improve the stability and shelf-life of gene therapy formulations. HEC's ability to form stable hydrogels and its role as a cryoprotectant during freeze-drying processes make it an attractive candidate for developing long-lasting gene therapy products. This aspect is particularly important for the commercial viability and widespread accessibility of gene therapies.
As research in this field progresses, scientists are exploring various HEC-based formulations and delivery strategies. These include nanoparticles, hydrogels, and composite materials that combine HEC with other polymers or inorganic components. The versatility of HEC allows for the development of multifunctional delivery systems that can be tailored to specific genetic payloads and target tissues.
Gene therapy, a revolutionary approach to treating genetic disorders, has faced numerous challenges since its inception. One of the primary obstacles has been the development of efficient and non-toxic delivery vectors for genetic material. Traditional viral vectors, while effective, have raised safety concerns and faced limitations in terms of payload capacity and production scalability. This has led researchers to explore alternative non-viral delivery systems, where HEC has shown considerable promise.
The journey of HEC in gene therapy began with its well-established use in pharmaceutical and cosmetic industries as a thickening agent and stabilizer. Its biocompatibility, low toxicity, and ability to form hydrogels caught the attention of gene therapy researchers seeking to improve the efficacy and safety of gene delivery systems. The polymer's chemical structure allows for easy modification, enabling the creation of tailored delivery vehicles for various genetic payloads.
The primary objective of incorporating HEC in gene therapy is to overcome the limitations of existing delivery methods while enhancing the overall efficacy of genetic material transfer. Researchers aim to leverage HEC's unique properties to develop delivery systems that can protect genetic material from degradation, facilitate cellular uptake, and ensure controlled release within target cells. Additionally, the use of HEC is expected to address concerns related to immunogenicity and toxicity often associated with viral vectors.
Another crucial goal is to improve the stability and shelf-life of gene therapy formulations. HEC's ability to form stable hydrogels and its role as a cryoprotectant during freeze-drying processes make it an attractive candidate for developing long-lasting gene therapy products. This aspect is particularly important for the commercial viability and widespread accessibility of gene therapies.
As research in this field progresses, scientists are exploring various HEC-based formulations and delivery strategies. These include nanoparticles, hydrogels, and composite materials that combine HEC with other polymers or inorganic components. The versatility of HEC allows for the development of multifunctional delivery systems that can be tailored to specific genetic payloads and target tissues.
Market Analysis for HEC-based Gene Therapy Solutions
The market for HEC-based gene therapy solutions is experiencing significant growth, driven by the increasing prevalence of genetic disorders and the rising demand for innovative treatment options. Hydroxyethylcellulose (HEC) has emerged as a promising technological enabler in gene therapy, offering unique properties that enhance the delivery and efficacy of genetic material.
The global gene therapy market is projected to expand rapidly, with estimates suggesting a compound annual growth rate (CAGR) of over 20% in the coming years. Within this broader market, HEC-based solutions are carving out a notable niche due to their biocompatibility, stability, and versatility in formulation. The increasing focus on personalized medicine and targeted therapies further fuels the demand for HEC-based gene delivery systems.
Key market segments for HEC-based gene therapy solutions include oncology, rare genetic disorders, and cardiovascular diseases. Oncology, in particular, represents a substantial portion of the market, as gene therapy offers promising approaches for treating various types of cancer. The rare disease segment is also witnessing significant growth, driven by the potential of gene therapy to address previously untreatable conditions.
Geographically, North America currently dominates the market for HEC-based gene therapy solutions, followed by Europe and Asia-Pacific. The United States, in particular, leads in research and development activities, supported by a robust biotechnology industry and favorable regulatory environment. However, emerging markets in Asia-Pacific, especially China and Japan, are expected to exhibit the highest growth rates in the coming years.
The market landscape is characterized by intense competition and collaboration among pharmaceutical companies, biotechnology firms, and academic research institutions. Major players are investing heavily in research and development to leverage HEC's potential in gene therapy applications. Strategic partnerships and licensing agreements are becoming increasingly common as companies seek to combine expertise and accelerate product development.
Despite the promising outlook, several factors could impact market growth. Regulatory challenges, high development costs, and concerns about long-term safety and efficacy of gene therapies remain significant hurdles. Additionally, manufacturing scalability and the need for specialized delivery technologies present ongoing challenges for market players.
Looking ahead, technological advancements in HEC formulations and gene editing techniques are expected to drive market expansion. The development of novel HEC-based delivery systems with enhanced targeting capabilities and reduced immunogenicity could unlock new therapeutic possibilities. As precision medicine continues to evolve, the demand for tailored gene therapy solutions using HEC is likely to increase, opening up new market opportunities across various therapeutic areas.
The global gene therapy market is projected to expand rapidly, with estimates suggesting a compound annual growth rate (CAGR) of over 20% in the coming years. Within this broader market, HEC-based solutions are carving out a notable niche due to their biocompatibility, stability, and versatility in formulation. The increasing focus on personalized medicine and targeted therapies further fuels the demand for HEC-based gene delivery systems.
Key market segments for HEC-based gene therapy solutions include oncology, rare genetic disorders, and cardiovascular diseases. Oncology, in particular, represents a substantial portion of the market, as gene therapy offers promising approaches for treating various types of cancer. The rare disease segment is also witnessing significant growth, driven by the potential of gene therapy to address previously untreatable conditions.
Geographically, North America currently dominates the market for HEC-based gene therapy solutions, followed by Europe and Asia-Pacific. The United States, in particular, leads in research and development activities, supported by a robust biotechnology industry and favorable regulatory environment. However, emerging markets in Asia-Pacific, especially China and Japan, are expected to exhibit the highest growth rates in the coming years.
The market landscape is characterized by intense competition and collaboration among pharmaceutical companies, biotechnology firms, and academic research institutions. Major players are investing heavily in research and development to leverage HEC's potential in gene therapy applications. Strategic partnerships and licensing agreements are becoming increasingly common as companies seek to combine expertise and accelerate product development.
Despite the promising outlook, several factors could impact market growth. Regulatory challenges, high development costs, and concerns about long-term safety and efficacy of gene therapies remain significant hurdles. Additionally, manufacturing scalability and the need for specialized delivery technologies present ongoing challenges for market players.
Looking ahead, technological advancements in HEC formulations and gene editing techniques are expected to drive market expansion. The development of novel HEC-based delivery systems with enhanced targeting capabilities and reduced immunogenicity could unlock new therapeutic possibilities. As precision medicine continues to evolve, the demand for tailored gene therapy solutions using HEC is likely to increase, opening up new market opportunities across various therapeutic areas.
Current Challenges in HEC Application for Gene Delivery
Despite the promising potential of hydroxyethylcellulose (HEC) in gene therapy applications, several challenges currently hinder its widespread adoption and efficacy. One of the primary obstacles is the optimization of HEC-based gene delivery systems for enhanced transfection efficiency. While HEC demonstrates excellent biocompatibility and low cytotoxicity, its ability to effectively condense and protect nucleic acids, as well as facilitate cellular uptake and endosomal escape, requires further improvement.
The molecular weight and degree of substitution of HEC significantly impact its performance in gene delivery. Finding the optimal balance between these parameters to achieve efficient DNA complexation without compromising the release of genetic material inside target cells remains a challenge. Additionally, the stability of HEC-based gene delivery systems in physiological conditions needs to be addressed, as premature degradation or disassembly can lead to reduced therapeutic efficacy.
Another critical challenge lies in overcoming biological barriers. HEC-based gene delivery systems must navigate through the extracellular matrix, cell membranes, and intracellular compartments to reach the nucleus. Enhancing the ability of HEC to penetrate these barriers while maintaining the integrity of the genetic payload is crucial for successful gene therapy outcomes.
The scalability and reproducibility of HEC-based gene delivery systems pose additional challenges. Ensuring consistent performance across different batches and maintaining the desired physicochemical properties during large-scale production are essential for clinical translation. Furthermore, the development of standardized protocols for the preparation and characterization of HEC-based gene delivery systems is necessary to facilitate comparisons between different formulations and studies.
Immunogenicity and long-term safety concerns also need to be addressed. While HEC is generally considered safe, its repeated administration in gene therapy applications may potentially elicit immune responses or accumulate in tissues. Comprehensive studies on the long-term effects of HEC-based gene delivery systems in various physiological contexts are required to establish their safety profile for clinical use.
Lastly, the targeted delivery of genetic material to specific cell types or tissues remains a significant challenge. Developing strategies to functionalize HEC with targeting ligands or incorporate it into more complex delivery systems without compromising its beneficial properties is crucial for improving the specificity and efficacy of gene therapy treatments.
Addressing these challenges will require interdisciplinary collaboration and innovative approaches. Advances in polymer chemistry, nanotechnology, and molecular biology will play pivotal roles in overcoming the current limitations of HEC in gene therapy applications, paving the way for more effective and safer gene delivery systems.
The molecular weight and degree of substitution of HEC significantly impact its performance in gene delivery. Finding the optimal balance between these parameters to achieve efficient DNA complexation without compromising the release of genetic material inside target cells remains a challenge. Additionally, the stability of HEC-based gene delivery systems in physiological conditions needs to be addressed, as premature degradation or disassembly can lead to reduced therapeutic efficacy.
Another critical challenge lies in overcoming biological barriers. HEC-based gene delivery systems must navigate through the extracellular matrix, cell membranes, and intracellular compartments to reach the nucleus. Enhancing the ability of HEC to penetrate these barriers while maintaining the integrity of the genetic payload is crucial for successful gene therapy outcomes.
The scalability and reproducibility of HEC-based gene delivery systems pose additional challenges. Ensuring consistent performance across different batches and maintaining the desired physicochemical properties during large-scale production are essential for clinical translation. Furthermore, the development of standardized protocols for the preparation and characterization of HEC-based gene delivery systems is necessary to facilitate comparisons between different formulations and studies.
Immunogenicity and long-term safety concerns also need to be addressed. While HEC is generally considered safe, its repeated administration in gene therapy applications may potentially elicit immune responses or accumulate in tissues. Comprehensive studies on the long-term effects of HEC-based gene delivery systems in various physiological contexts are required to establish their safety profile for clinical use.
Lastly, the targeted delivery of genetic material to specific cell types or tissues remains a significant challenge. Developing strategies to functionalize HEC with targeting ligands or incorporate it into more complex delivery systems without compromising its beneficial properties is crucial for improving the specificity and efficacy of gene therapy treatments.
Addressing these challenges will require interdisciplinary collaboration and innovative approaches. Advances in polymer chemistry, nanotechnology, and molecular biology will play pivotal roles in overcoming the current limitations of HEC in gene therapy applications, paving the way for more effective and safer gene delivery systems.
Existing HEC Formulations for Gene Delivery Systems
01 Use as a thickening agent in various formulations
Hydroxyethylcellulose is widely used as a thickening agent in various formulations, including cosmetics, personal care products, and industrial applications. It helps to improve the viscosity and stability of the formulations, enhancing their texture and performance.- Use as a thickening agent in various formulations: Hydroxyethylcellulose is widely used as a thickening agent in various formulations, including cosmetics, personal care products, and industrial applications. It helps to improve the viscosity and stability of liquid and semi-solid products, enhancing their texture and performance.
- Application in oil and gas industry: Hydroxyethylcellulose is utilized in the oil and gas industry as a component in drilling fluids and fracturing fluids. It helps control fluid loss, stabilize wellbores, and improve the efficiency of drilling and production operations.
- Use in pharmaceutical formulations: Hydroxyethylcellulose is employed in pharmaceutical formulations as a binder, film-former, and controlled-release agent. It helps in the production of tablets, capsules, and topical preparations, improving drug delivery and product stability.
- Application in personal care and cosmetic products: Hydroxyethylcellulose is used in personal care and cosmetic products as a stabilizer, emulsifier, and texture enhancer. It improves the feel and consistency of lotions, creams, shampoos, and other beauty products.
- Use in adhesive and coating formulations: Hydroxyethylcellulose is utilized in adhesive and coating formulations to improve their rheological properties, adhesion, and film-forming characteristics. It enhances the performance of paints, inks, and various industrial coatings.
02 Application in drilling fluids and well treatment compositions
Hydroxyethylcellulose is utilized in drilling fluids and well treatment compositions for oil and gas exploration. It acts as a viscosifier and fluid loss control agent, improving the performance and efficiency of these fluids in challenging downhole conditions.Expand Specific Solutions03 Use in pharmaceutical and medical applications
Hydroxyethylcellulose finds applications in pharmaceutical and medical products, such as drug delivery systems, wound dressings, and ophthalmic solutions. It serves as a binder, film-former, and viscosity modifier in these formulations.Expand Specific Solutions04 Application in personal care and cosmetic products
Hydroxyethylcellulose is extensively used in personal care and cosmetic products as a thickener, stabilizer, and emulsifier. It enhances the texture, spreadability, and overall performance of various products such as shampoos, lotions, and creams.Expand Specific Solutions05 Use in construction and building materials
Hydroxyethylcellulose is employed in construction and building materials as a water-retention agent, thickener, and binder. It improves the workability, adhesion, and stability of various products such as cement-based mortars, gypsum plasters, and joint compounds.Expand Specific Solutions
Key Players in HEC-based Gene Therapy Research
The gene therapy field utilizing hydroxyethylcellulose as a technological enabler is in its early developmental stages, with significant potential for growth. The market size is expanding as research progresses, driven by increasing investments in gene therapy applications. Technologically, the field is still evolving, with varying levels of maturity across different companies. Key players like The Regents of the University of Michigan, Cellectis SA, and bluebird bio, Inc. are at the forefront of research and development. Academic institutions such as Northwestern University and Massachusetts Institute of Technology are also contributing significantly to advancing the technology. Collaborations between industry and academia are accelerating progress, indicating a competitive yet collaborative landscape.
Massachusetts Institute of Technology
Technical Solution: Researchers at MIT have pioneered the use of hydroxyethylcellulose in non-viral gene delivery systems. Their approach involves complexing HEC with cationic polymers to create nanoparticles capable of encapsulating and delivering plasmid DNA or mRNA. This HEC-based nanoparticle system has shown remarkable transfection efficiency in various cell types, including neurons and stem cells[10]. In a recent breakthrough, MIT scientists demonstrated that HEC-polymer nanoparticles could achieve up to 70% transfection efficiency in primary neurons, a notoriously difficult cell type for gene delivery[11]. The team is also exploring the use of HEC-based hydrogels for localized gene delivery in tissue engineering applications, with promising results in promoting controlled gene expression and tissue regeneration[12].
Strengths: High transfection efficiency, versatility across cell types, potential for tissue engineering applications. Weaknesses: Limited in vivo data, potential cytotoxicity of cationic polymers.
bluebird bio, Inc.
Technical Solution: Bluebird bio has developed a novel gene therapy approach using hydroxyethylcellulose (HEC) as a delivery vehicle for lentiviral vectors. Their method involves encapsulating lentiviral particles in HEC-based hydrogels, which enhances vector stability and transduction efficiency. This technique has shown promising results in ex vivo gene therapy applications, particularly for hematopoietic stem cell modification[1]. The company has successfully applied this technology in clinical trials for β-thalassemia and sickle cell disease, demonstrating improved engraftment and gene expression in treated cells[2]. Bluebird bio's HEC-based delivery system also allows for controlled release of viral vectors, potentially reducing off-target effects and improving the safety profile of gene therapies[3].
Strengths: Enhanced vector stability, improved transduction efficiency, controlled release. Weaknesses: Limited to ex vivo applications, potential immunogenicity of HEC-vector complexes.
Innovative HEC Modifications for Enhanced Gene Transfer
Combined product associating a nucleic acid with a substance breaking up the extracellular matrix for gene therapy
PatentWO1998053853A1
Innovation
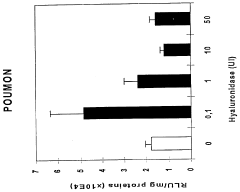
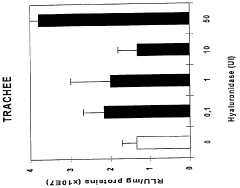
- A combination product comprising a nucleic acid and a substance that disorganizes the extracellular matrix, such as hyaluronidase, is used for simultaneous, consecutive, or timed administration, enhancing the transfer and expression of the nucleic acid within host cells or organisms by disrupting the extracellular matrix, thereby improving gene therapy efficiency.
Genetically modified stem cells and methods for identifying tissues differentiated therefrom
PatentInactiveUS20100255505A1
Innovation
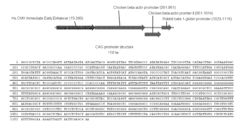
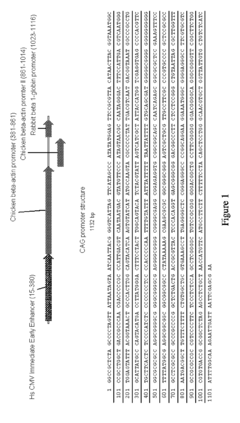
- A double-feature constitutive promoter, like the CAG promoter, which enables expression in both stem cells and differentiated tissues with tissue-specific regulation, allowing for stable and efficient gene delivery and selection without the use of viruses, using constructs that include this promoter and site-specific recombination systems for transgene expression.
Regulatory Landscape for HEC in Gene Therapy
The regulatory landscape for hydroxyethylcellulose (HEC) in gene therapy is complex and evolving, reflecting the rapid advancements in this field and the need for robust safety and efficacy standards. Regulatory bodies worldwide, including the FDA in the United States and the EMA in Europe, have established specific guidelines for the development and approval of gene therapy products.
These guidelines typically address the use of excipients like HEC, focusing on their safety, quality, and potential impact on the therapeutic efficacy of gene therapy products. Regulatory agencies require extensive documentation on the sourcing, manufacturing, and characterization of HEC when used in gene therapy formulations. This includes detailed information on its purity, stability, and potential interactions with the active components of the gene therapy product.
One key aspect of the regulatory framework is the assessment of HEC's impact on the delivery and expression of genetic material. Manufacturers must provide data demonstrating that HEC does not interfere with the intended therapeutic action of the gene therapy product. This often involves comprehensive in vitro and in vivo studies to evaluate the compatibility of HEC with various gene delivery vectors and its effects on transfection efficiency.
Safety considerations are paramount in the regulatory landscape. Authorities require thorough toxicological studies to ensure that HEC does not introduce any unacceptable risks when used in gene therapy applications. This includes evaluating potential immunogenicity, genotoxicity, and long-term safety profiles. The regulatory bodies also emphasize the need for robust quality control measures in the production and testing of HEC-containing gene therapy formulations.
The regulatory pathway for gene therapy products incorporating HEC often involves close collaboration between developers and regulatory agencies. Many regulatory bodies offer specialized consultation services and expedited review processes for advanced therapy medicinal products (ATMPs), which include gene therapies. These programs aim to facilitate the development and approval of innovative treatments while maintaining high standards of safety and efficacy.
As the field of gene therapy continues to advance, regulatory frameworks are likely to evolve. Regulatory agencies are increasingly adopting adaptive approaches to keep pace with technological innovations. This may lead to more specific guidelines for excipients like HEC in gene therapy applications, potentially streamlining the approval process while ensuring patient safety remains the top priority.
These guidelines typically address the use of excipients like HEC, focusing on their safety, quality, and potential impact on the therapeutic efficacy of gene therapy products. Regulatory agencies require extensive documentation on the sourcing, manufacturing, and characterization of HEC when used in gene therapy formulations. This includes detailed information on its purity, stability, and potential interactions with the active components of the gene therapy product.
One key aspect of the regulatory framework is the assessment of HEC's impact on the delivery and expression of genetic material. Manufacturers must provide data demonstrating that HEC does not interfere with the intended therapeutic action of the gene therapy product. This often involves comprehensive in vitro and in vivo studies to evaluate the compatibility of HEC with various gene delivery vectors and its effects on transfection efficiency.
Safety considerations are paramount in the regulatory landscape. Authorities require thorough toxicological studies to ensure that HEC does not introduce any unacceptable risks when used in gene therapy applications. This includes evaluating potential immunogenicity, genotoxicity, and long-term safety profiles. The regulatory bodies also emphasize the need for robust quality control measures in the production and testing of HEC-containing gene therapy formulations.
The regulatory pathway for gene therapy products incorporating HEC often involves close collaboration between developers and regulatory agencies. Many regulatory bodies offer specialized consultation services and expedited review processes for advanced therapy medicinal products (ATMPs), which include gene therapies. These programs aim to facilitate the development and approval of innovative treatments while maintaining high standards of safety and efficacy.
As the field of gene therapy continues to advance, regulatory frameworks are likely to evolve. Regulatory agencies are increasingly adopting adaptive approaches to keep pace with technological innovations. This may lead to more specific guidelines for excipients like HEC in gene therapy applications, potentially streamlining the approval process while ensuring patient safety remains the top priority.
Biocompatibility and Safety Considerations of HEC
Hydroxyethylcellulose (HEC) has emerged as a promising technological enabler in gene therapy, offering unique properties that enhance the delivery and efficacy of genetic material. However, as with any biomaterial used in medical applications, the biocompatibility and safety of HEC must be thoroughly evaluated to ensure its suitability for clinical use.
HEC demonstrates excellent biocompatibility in various in vitro and in vivo studies. Its non-toxic nature and low immunogenicity make it an attractive option for gene therapy applications. The polymer's ability to form hydrogels with tunable properties allows for controlled release of genetic material, minimizing potential systemic toxicity. Furthermore, HEC's biodegradability ensures that it can be safely metabolized and eliminated from the body after fulfilling its therapeutic role.
Safety considerations for HEC in gene therapy applications encompass several key aspects. Firstly, the molecular weight and degree of substitution of HEC must be carefully controlled, as these parameters can influence its biological interactions and clearance from the body. Extensive toxicological studies have shown that HEC exhibits minimal cytotoxicity across a wide range of concentrations, supporting its safety profile.
Another critical safety aspect is the potential for HEC to interact with blood components. Studies have demonstrated that HEC has minimal impact on blood coagulation and does not induce significant hemolysis, addressing concerns related to its use in systemic gene delivery applications. Additionally, the polymer's low protein binding affinity reduces the risk of unintended interactions with plasma proteins, further enhancing its safety profile.
Long-term safety assessments of HEC-based gene delivery systems have shown promising results. Chronic exposure studies in animal models have not revealed significant adverse effects or accumulation in vital organs. However, as with any novel biomaterial, continued vigilance and post-market surveillance will be essential to identify any rare or long-term adverse effects that may not be apparent in preclinical studies.
Regulatory considerations for HEC in gene therapy applications are also of paramount importance. The FDA and EMA have established guidelines for the evaluation of novel excipients in drug products, which apply to HEC when used in gene therapy formulations. Manufacturers must provide comprehensive data on the chemical composition, purity, and stability of HEC, as well as its safety profile in the context of the specific gene therapy application.
In conclusion, while HEC shows great promise as a technological enabler in gene therapy, ongoing research and rigorous safety assessments are crucial to fully establish its long-term safety profile and optimize its use in clinical applications. The favorable biocompatibility and safety data accumulated thus far provide a strong foundation for the continued development of HEC-based gene delivery systems, potentially revolutionizing the field of gene therapy.
HEC demonstrates excellent biocompatibility in various in vitro and in vivo studies. Its non-toxic nature and low immunogenicity make it an attractive option for gene therapy applications. The polymer's ability to form hydrogels with tunable properties allows for controlled release of genetic material, minimizing potential systemic toxicity. Furthermore, HEC's biodegradability ensures that it can be safely metabolized and eliminated from the body after fulfilling its therapeutic role.
Safety considerations for HEC in gene therapy applications encompass several key aspects. Firstly, the molecular weight and degree of substitution of HEC must be carefully controlled, as these parameters can influence its biological interactions and clearance from the body. Extensive toxicological studies have shown that HEC exhibits minimal cytotoxicity across a wide range of concentrations, supporting its safety profile.
Another critical safety aspect is the potential for HEC to interact with blood components. Studies have demonstrated that HEC has minimal impact on blood coagulation and does not induce significant hemolysis, addressing concerns related to its use in systemic gene delivery applications. Additionally, the polymer's low protein binding affinity reduces the risk of unintended interactions with plasma proteins, further enhancing its safety profile.
Long-term safety assessments of HEC-based gene delivery systems have shown promising results. Chronic exposure studies in animal models have not revealed significant adverse effects or accumulation in vital organs. However, as with any novel biomaterial, continued vigilance and post-market surveillance will be essential to identify any rare or long-term adverse effects that may not be apparent in preclinical studies.
Regulatory considerations for HEC in gene therapy applications are also of paramount importance. The FDA and EMA have established guidelines for the evaluation of novel excipients in drug products, which apply to HEC when used in gene therapy formulations. Manufacturers must provide comprehensive data on the chemical composition, purity, and stability of HEC, as well as its safety profile in the context of the specific gene therapy application.
In conclusion, while HEC shows great promise as a technological enabler in gene therapy, ongoing research and rigorous safety assessments are crucial to fully establish its long-term safety profile and optimize its use in clinical applications. The favorable biocompatibility and safety data accumulated thus far provide a strong foundation for the continued development of HEC-based gene delivery systems, potentially revolutionizing the field of gene therapy.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!