Hydroxyethylcellulose-Modified Polymers for Tissue Engineering
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HEC-Modified Polymers: Background and Objectives
Hydroxyethylcellulose (HEC) modified polymers have emerged as a promising class of materials in the field of tissue engineering. The development of these polymers stems from the need for biocompatible and versatile scaffolds that can support cell growth and tissue regeneration. HEC, a cellulose derivative, has gained attention due to its excellent biocompatibility, biodegradability, and ability to form hydrogels with tunable properties.
The evolution of HEC-modified polymers can be traced back to the broader field of biomaterials and regenerative medicine. As researchers sought to create more effective scaffolds for tissue engineering, they turned to natural polymers like cellulose and its derivatives. HEC, with its unique properties, became a focal point for modification and enhancement to meet the specific requirements of tissue engineering applications.
The primary objective of research on HEC-modified polymers is to develop advanced biomaterials that can closely mimic the extracellular matrix (ECM) of natural tissues. This involves tailoring the physical, chemical, and biological properties of the polymers to create an optimal environment for cell adhesion, proliferation, and differentiation. Researchers aim to enhance the mechanical strength, porosity, and bioactivity of HEC-based scaffolds while maintaining their biocompatibility and biodegradability.
Another crucial goal is to improve the functionality of HEC-modified polymers by incorporating various bioactive molecules, growth factors, and cell-signaling cues. This approach seeks to create "smart" scaffolds that can actively guide tissue regeneration and promote healing processes. Additionally, researchers are exploring ways to enhance the processability of these polymers, making them suitable for various fabrication techniques such as 3D printing, electrospinning, and microfluidics.
The development of HEC-modified polymers also aligns with the broader trend towards sustainable and eco-friendly materials in biomedical applications. As a cellulose derivative, HEC offers advantages in terms of renewability and reduced environmental impact compared to synthetic polymers. This aspect has further fueled interest in HEC-based materials for tissue engineering and regenerative medicine.
Looking ahead, the research on HEC-modified polymers aims to address several key challenges in tissue engineering. These include improving the mechanical properties of scaffolds for load-bearing applications, enhancing vascularization in engineered tissues, and developing strategies for controlled release of bioactive agents. The ultimate goal is to create multifunctional, biomimetic scaffolds that can effectively support the regeneration of complex tissues and organs, potentially revolutionizing the field of regenerative medicine.
The evolution of HEC-modified polymers can be traced back to the broader field of biomaterials and regenerative medicine. As researchers sought to create more effective scaffolds for tissue engineering, they turned to natural polymers like cellulose and its derivatives. HEC, with its unique properties, became a focal point for modification and enhancement to meet the specific requirements of tissue engineering applications.
The primary objective of research on HEC-modified polymers is to develop advanced biomaterials that can closely mimic the extracellular matrix (ECM) of natural tissues. This involves tailoring the physical, chemical, and biological properties of the polymers to create an optimal environment for cell adhesion, proliferation, and differentiation. Researchers aim to enhance the mechanical strength, porosity, and bioactivity of HEC-based scaffolds while maintaining their biocompatibility and biodegradability.
Another crucial goal is to improve the functionality of HEC-modified polymers by incorporating various bioactive molecules, growth factors, and cell-signaling cues. This approach seeks to create "smart" scaffolds that can actively guide tissue regeneration and promote healing processes. Additionally, researchers are exploring ways to enhance the processability of these polymers, making them suitable for various fabrication techniques such as 3D printing, electrospinning, and microfluidics.
The development of HEC-modified polymers also aligns with the broader trend towards sustainable and eco-friendly materials in biomedical applications. As a cellulose derivative, HEC offers advantages in terms of renewability and reduced environmental impact compared to synthetic polymers. This aspect has further fueled interest in HEC-based materials for tissue engineering and regenerative medicine.
Looking ahead, the research on HEC-modified polymers aims to address several key challenges in tissue engineering. These include improving the mechanical properties of scaffolds for load-bearing applications, enhancing vascularization in engineered tissues, and developing strategies for controlled release of bioactive agents. The ultimate goal is to create multifunctional, biomimetic scaffolds that can effectively support the regeneration of complex tissues and organs, potentially revolutionizing the field of regenerative medicine.
Market Analysis for Tissue Engineering Biomaterials
The tissue engineering biomaterials market has experienced significant growth in recent years, driven by increasing demand for regenerative medicine solutions and advancements in material science. The global market for tissue engineering biomaterials is projected to reach substantial value in the coming years, with a compound annual growth rate (CAGR) exceeding industry averages. This growth is primarily attributed to the rising prevalence of chronic diseases, an aging population, and the need for improved healthcare solutions.
Hydroxyethylcellulose-modified polymers have emerged as promising materials in the tissue engineering field, offering unique properties that address several key market demands. These materials provide excellent biocompatibility, tunable mechanical properties, and the ability to support cell growth and differentiation. As a result, they have garnered significant interest from both researchers and industry players seeking to develop innovative tissue engineering products.
The market for hydroxyethylcellulose-modified polymers in tissue engineering applications is expected to grow rapidly, driven by their versatility and potential to address unmet needs in various medical fields. These materials find applications in wound healing, bone regeneration, cartilage repair, and soft tissue reconstruction, among others. The expanding range of applications is likely to contribute to market growth and attract investment from both established companies and startups.
Geographically, North America and Europe currently dominate the tissue engineering biomaterials market, owing to advanced healthcare infrastructure, substantial research and development investments, and favorable regulatory environments. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of regenerative medicine, and improving research capabilities in countries like China, Japan, and South Korea.
The market landscape for tissue engineering biomaterials is characterized by intense competition and ongoing innovation. Key players in the industry are focusing on developing novel biomaterials with enhanced properties, such as improved cell adhesion, controlled degradation rates, and tailored mechanical strength. Collaborations between academic institutions and industry partners are becoming increasingly common, fostering the translation of research findings into commercially viable products.
Despite the promising outlook, several challenges persist in the tissue engineering biomaterials market. These include high development costs, stringent regulatory requirements, and the need for long-term clinical data to demonstrate efficacy and safety. Additionally, concerns regarding the scalability of production processes and the standardization of quality control measures may impact market growth. Addressing these challenges will be crucial for realizing the full potential of hydroxyethylcellulose-modified polymers and other innovative biomaterials in tissue engineering applications.
Hydroxyethylcellulose-modified polymers have emerged as promising materials in the tissue engineering field, offering unique properties that address several key market demands. These materials provide excellent biocompatibility, tunable mechanical properties, and the ability to support cell growth and differentiation. As a result, they have garnered significant interest from both researchers and industry players seeking to develop innovative tissue engineering products.
The market for hydroxyethylcellulose-modified polymers in tissue engineering applications is expected to grow rapidly, driven by their versatility and potential to address unmet needs in various medical fields. These materials find applications in wound healing, bone regeneration, cartilage repair, and soft tissue reconstruction, among others. The expanding range of applications is likely to contribute to market growth and attract investment from both established companies and startups.
Geographically, North America and Europe currently dominate the tissue engineering biomaterials market, owing to advanced healthcare infrastructure, substantial research and development investments, and favorable regulatory environments. However, the Asia-Pacific region is anticipated to witness the fastest growth in the coming years, driven by increasing healthcare expenditure, growing awareness of regenerative medicine, and improving research capabilities in countries like China, Japan, and South Korea.
The market landscape for tissue engineering biomaterials is characterized by intense competition and ongoing innovation. Key players in the industry are focusing on developing novel biomaterials with enhanced properties, such as improved cell adhesion, controlled degradation rates, and tailored mechanical strength. Collaborations between academic institutions and industry partners are becoming increasingly common, fostering the translation of research findings into commercially viable products.
Despite the promising outlook, several challenges persist in the tissue engineering biomaterials market. These include high development costs, stringent regulatory requirements, and the need for long-term clinical data to demonstrate efficacy and safety. Additionally, concerns regarding the scalability of production processes and the standardization of quality control measures may impact market growth. Addressing these challenges will be crucial for realizing the full potential of hydroxyethylcellulose-modified polymers and other innovative biomaterials in tissue engineering applications.
Current Challenges in HEC-Modified Polymer Development
Despite the promising potential of hydroxyethylcellulose (HEC)-modified polymers in tissue engineering, several significant challenges persist in their development and application. These obstacles span across various aspects of material design, synthesis, and performance optimization.
One of the primary challenges lies in achieving precise control over the degree of HEC modification. The extent of HEC incorporation significantly influences the polymer's physical and chemical properties, which in turn affects its suitability for specific tissue engineering applications. Researchers struggle to develop reproducible methods that allow for fine-tuning of HEC content while maintaining consistent polymer characteristics.
Another critical issue is the optimization of mechanical properties. HEC-modified polymers must possess adequate strength and elasticity to support cell growth and tissue formation. However, balancing these mechanical attributes with the desired biodegradability and biocompatibility remains a complex task. Engineers face difficulties in creating scaffolds that can withstand physiological stresses while gradually degrading at a rate that matches tissue regeneration.
Biocompatibility and cell adhesion present additional hurdles. While HEC modification can enhance the hydrophilicity of polymers, promoting cell attachment, excessive hydrophilicity may lead to reduced protein adsorption and consequently impaired cell adhesion. Striking the right balance between hydrophilicity and cell-friendly surface properties is an ongoing challenge in the field.
The controlled release of bioactive molecules from HEC-modified polymers poses another significant obstacle. Researchers aim to develop systems that can deliver growth factors, drugs, or other therapeutic agents in a sustained and targeted manner. However, achieving precise control over release kinetics while maintaining the structural integrity of the polymer scaffold remains a complex endeavor.
Scalability and manufacturing consistency are also major concerns. As research progresses towards clinical applications, the ability to produce HEC-modified polymers on a larger scale with consistent quality becomes crucial. Current laboratory-scale synthesis methods often face challenges in scaling up without compromising material properties or increasing production costs.
Lastly, the long-term stability and degradation behavior of HEC-modified polymers in physiological environments require further investigation. Understanding how these materials interact with surrounding tissues over extended periods and ensuring their degradation products are non-toxic and easily metabolized by the body are critical aspects that demand ongoing research efforts.
Addressing these challenges requires interdisciplinary collaboration among material scientists, bioengineers, and clinicians. As the field progresses, innovative approaches in polymer chemistry, advanced characterization techniques, and in vivo studies will be essential to overcome these hurdles and realize the full potential of HEC-modified polymers in tissue engineering applications.
One of the primary challenges lies in achieving precise control over the degree of HEC modification. The extent of HEC incorporation significantly influences the polymer's physical and chemical properties, which in turn affects its suitability for specific tissue engineering applications. Researchers struggle to develop reproducible methods that allow for fine-tuning of HEC content while maintaining consistent polymer characteristics.
Another critical issue is the optimization of mechanical properties. HEC-modified polymers must possess adequate strength and elasticity to support cell growth and tissue formation. However, balancing these mechanical attributes with the desired biodegradability and biocompatibility remains a complex task. Engineers face difficulties in creating scaffolds that can withstand physiological stresses while gradually degrading at a rate that matches tissue regeneration.
Biocompatibility and cell adhesion present additional hurdles. While HEC modification can enhance the hydrophilicity of polymers, promoting cell attachment, excessive hydrophilicity may lead to reduced protein adsorption and consequently impaired cell adhesion. Striking the right balance between hydrophilicity and cell-friendly surface properties is an ongoing challenge in the field.
The controlled release of bioactive molecules from HEC-modified polymers poses another significant obstacle. Researchers aim to develop systems that can deliver growth factors, drugs, or other therapeutic agents in a sustained and targeted manner. However, achieving precise control over release kinetics while maintaining the structural integrity of the polymer scaffold remains a complex endeavor.
Scalability and manufacturing consistency are also major concerns. As research progresses towards clinical applications, the ability to produce HEC-modified polymers on a larger scale with consistent quality becomes crucial. Current laboratory-scale synthesis methods often face challenges in scaling up without compromising material properties or increasing production costs.
Lastly, the long-term stability and degradation behavior of HEC-modified polymers in physiological environments require further investigation. Understanding how these materials interact with surrounding tissues over extended periods and ensuring their degradation products are non-toxic and easily metabolized by the body are critical aspects that demand ongoing research efforts.
Addressing these challenges requires interdisciplinary collaboration among material scientists, bioengineers, and clinicians. As the field progresses, innovative approaches in polymer chemistry, advanced characterization techniques, and in vivo studies will be essential to overcome these hurdles and realize the full potential of HEC-modified polymers in tissue engineering applications.
Existing HEC-Modified Polymer Solutions
01 Hydroxyethylcellulose-modified polymers in drilling fluids
Hydroxyethylcellulose-modified polymers are used in drilling fluids to improve viscosity and fluid loss control. These polymers can be combined with other additives to enhance the performance of drilling muds in various geological formations.- Hydroxyethylcellulose-modified polymers in drilling fluids: Hydroxyethylcellulose-modified polymers are used in drilling fluids to improve rheological properties and fluid loss control. These polymers enhance the stability and performance of drilling fluids in various temperature and pressure conditions, making them suitable for challenging drilling environments.
- Hydroxyethylcellulose-modified polymers in personal care products: These modified polymers are utilized in personal care formulations such as hair care and skin care products. They provide improved texture, stability, and moisturizing properties to the formulations, enhancing the overall performance and user experience of the products.
- Hydroxyethylcellulose-modified polymers in coatings and adhesives: Modified hydroxyethylcellulose polymers are employed in coating and adhesive applications. They contribute to improved film-forming properties, adhesion, and durability of the coatings. These polymers also enhance the rheological properties and stability of adhesive formulations.
- Hydroxyethylcellulose-modified polymers in controlled release systems: These polymers are used in controlled release systems for pharmaceuticals and agrochemicals. They provide a matrix for sustained release of active ingredients, allowing for improved efficacy and reduced frequency of application or dosing.
- Synthesis and modification of hydroxyethylcellulose polymers: Various methods for synthesizing and modifying hydroxyethylcellulose polymers are developed to tailor their properties for specific applications. These include chemical modifications, graft copolymerization, and crosslinking techniques to enhance performance characteristics such as water solubility, viscosity, and thermal stability.
02 Hydroxyethylcellulose-modified polymers in personal care products
These modified polymers are utilized in personal care formulations such as shampoos, conditioners, and skincare products. They provide improved rheology, stability, and sensory properties to the formulations.Expand Specific Solutions03 Hydroxyethylcellulose-modified polymers in coating applications
Modified hydroxyethylcellulose polymers are employed in various coating formulations to enhance properties such as film formation, adhesion, and water resistance. They are particularly useful in paints and industrial coatings.Expand Specific Solutions04 Hydroxyethylcellulose-modified polymers in controlled release systems
These polymers are used in the development of controlled release systems for pharmaceuticals and agrochemicals. They can modulate the release rate of active ingredients and improve the overall efficacy of the formulations.Expand Specific Solutions05 Synthesis and modification of hydroxyethylcellulose polymers
Various methods for synthesizing and modifying hydroxyethylcellulose polymers are described. These include chemical modifications, grafting techniques, and crosslinking processes to tailor the properties of the polymers for specific applications.Expand Specific Solutions
Key Players in HEC-Modified Polymer Research
The research on hydroxyethylcellulose-modified polymers for tissue engineering is in a growth phase, with increasing market potential and technological advancements. The global tissue engineering market is expanding rapidly, driven by rising demand for regenerative medicine and innovative biomaterials. While the technology is progressing, it is not yet fully mature, with ongoing research to optimize polymer properties and biocompatibility. Key players in this field include academic institutions like Massachusetts Institute of Technology and University of Washington, as well as industry leaders such as 3M Innovative Properties Co. and L'Oréal SA. These organizations are actively developing and patenting novel polymer formulations and applications, indicating a competitive and dynamic landscape in this emerging field.
Massachusetts Institute of Technology
Technical Solution: MIT has developed a novel approach to hydroxyethylcellulose (HEC) modification for tissue engineering applications. Their research focuses on creating HEC-based hydrogels with tunable mechanical properties and biocompatibility. The team has successfully incorporated cell-adhesive peptides and growth factors into the HEC backbone, enhancing cell attachment and proliferation[1]. They have also developed a photocrosslinking method that allows for precise control over the hydrogel's stiffness and degradation rate[2]. This technology has shown promising results in supporting the growth of various cell types, including stem cells and endothelial cells, making it suitable for a wide range of tissue engineering applications[3].
Strengths: Highly customizable hydrogels, excellent biocompatibility, and versatility for various cell types. Weaknesses: Potential challenges in scaling up production and ensuring long-term stability of the modified polymers.
University of Massachusetts
Technical Solution: The University of Massachusetts has made significant strides in developing HEC-modified polymers for tissue engineering. Their approach involves the creation of nanocomposite hydrogels by combining HEC with nanoparticles, such as silica or hydroxyapatite[4]. This combination results in improved mechanical strength and bioactivity of the scaffolds. The research team has also explored the use of HEC-based hydrogels for controlled drug delivery within tissue engineering constructs[5]. Their innovative method of functionalizing HEC with cell-specific ligands has shown enhanced cell adhesion and differentiation, particularly in bone and cartilage tissue engineering applications[6].
Strengths: Enhanced mechanical properties, controlled drug delivery capabilities, and specific tissue targeting. Weaknesses: Potential concerns regarding the long-term effects of nanoparticles in the body and the complexity of the manufacturing process.
Core Innovations in HEC-Modified Polymer Technology
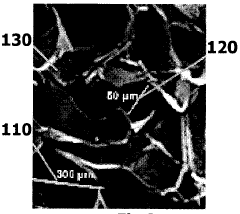
A cross-linked structure for tissue regeneration and engineering and the method for synthesising the same.
PatentActiveMX2016012389A
Innovation
- A reticular structure formed by hemicellulose, carboxymethyl cellulose (CMC), and hydroxyethyl cellulose (HEC) is synthesized through a process involving cold solubilization, crosslinking with a biocompatible agent, and controlled pore formation via freezing and thawing, resulting in a lattice structure with interconnected pores suitable for tissue regeneration.
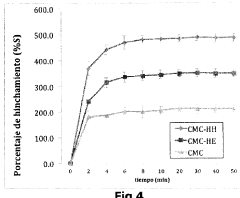
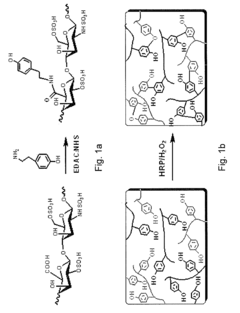
Hydrogels based on polymers of dextran tyramine and tyramine conjugates of natural polymers
PatentActiveEP3067069A1
Innovation
- A composition comprising a dextran-tyramine conjugate and a glycosaminoglycan-tyramine conjugate, such as heparin-tyramine, with a suitable weight ratio and hydrogen peroxide, forming an injectable hydrogel that enhances mechanical properties and maintains chondrocyte phenotype, along with the inclusion of growth factors like BMP or TGF-β for improved tissue regeneration.
Biocompatibility and Safety Considerations
Biocompatibility and safety considerations are paramount in the development of hydroxyethylcellulose-modified polymers for tissue engineering applications. These materials must not only support cell growth and tissue regeneration but also ensure minimal adverse reactions within the host organism. The evaluation of biocompatibility begins at the molecular level, assessing the potential for cytotoxicity, genotoxicity, and immunogenicity of the modified polymers.
In vitro studies are typically the first step in evaluating biocompatibility. These involve exposing cultured cells to the hydroxyethylcellulose-modified polymers and observing cell viability, proliferation, and morphology. Advanced techniques such as live/dead staining, metabolic activity assays, and gene expression analysis provide comprehensive insights into cellular responses. It is crucial to test the materials with relevant cell types that would interact with the engineered tissue in vivo.
The degradation profile of hydroxyethylcellulose-modified polymers is a critical safety consideration. The breakdown products must be non-toxic and easily metabolized or excreted by the body. Researchers must carefully characterize the degradation kinetics and identify all potential byproducts. This information helps predict long-term safety and guides the design of polymers with controlled degradation rates suitable for specific tissue engineering applications.
Immunological responses to the modified polymers are another key aspect of biocompatibility. While a mild initial inflammatory response may be acceptable or even beneficial for tissue integration, chronic inflammation or severe immune reactions can lead to implant rejection or tissue damage. Assessing the activation of immune cells, cytokine production, and complement system interactions is essential for understanding the potential immunological impact of these materials.
In vivo studies in animal models represent a critical phase in evaluating the safety and biocompatibility of hydroxyethylcellulose-modified polymers. These studies allow for the assessment of tissue integration, vascularization, and long-term performance of the engineered constructs. Histological analysis, immunohistochemistry, and functional tests provide valuable data on the host response and the ability of the modified polymers to support tissue regeneration without causing adverse effects.
Regulatory considerations play a significant role in the development of these materials for clinical applications. Adherence to guidelines set by regulatory bodies such as the FDA and EMA is essential. This includes conducting standardized biocompatibility tests, such as those outlined in ISO 10993, and providing comprehensive documentation on material characterization, manufacturing processes, and quality control measures.
In vitro studies are typically the first step in evaluating biocompatibility. These involve exposing cultured cells to the hydroxyethylcellulose-modified polymers and observing cell viability, proliferation, and morphology. Advanced techniques such as live/dead staining, metabolic activity assays, and gene expression analysis provide comprehensive insights into cellular responses. It is crucial to test the materials with relevant cell types that would interact with the engineered tissue in vivo.
The degradation profile of hydroxyethylcellulose-modified polymers is a critical safety consideration. The breakdown products must be non-toxic and easily metabolized or excreted by the body. Researchers must carefully characterize the degradation kinetics and identify all potential byproducts. This information helps predict long-term safety and guides the design of polymers with controlled degradation rates suitable for specific tissue engineering applications.
Immunological responses to the modified polymers are another key aspect of biocompatibility. While a mild initial inflammatory response may be acceptable or even beneficial for tissue integration, chronic inflammation or severe immune reactions can lead to implant rejection or tissue damage. Assessing the activation of immune cells, cytokine production, and complement system interactions is essential for understanding the potential immunological impact of these materials.
In vivo studies in animal models represent a critical phase in evaluating the safety and biocompatibility of hydroxyethylcellulose-modified polymers. These studies allow for the assessment of tissue integration, vascularization, and long-term performance of the engineered constructs. Histological analysis, immunohistochemistry, and functional tests provide valuable data on the host response and the ability of the modified polymers to support tissue regeneration without causing adverse effects.
Regulatory considerations play a significant role in the development of these materials for clinical applications. Adherence to guidelines set by regulatory bodies such as the FDA and EMA is essential. This includes conducting standardized biocompatibility tests, such as those outlined in ISO 10993, and providing comprehensive documentation on material characterization, manufacturing processes, and quality control measures.
Scalability and Manufacturing Processes
The scalability and manufacturing processes of hydroxyethylcellulose-modified polymers for tissue engineering applications are critical factors in their successful translation from laboratory research to clinical use. These processes must be carefully designed to ensure consistent quality, cost-effectiveness, and the ability to meet increasing demand as the technology advances.
One of the primary challenges in scaling up production is maintaining the precise chemical modifications and physical properties of the polymers that are crucial for their performance in tissue engineering. This requires the development of robust and reproducible synthesis protocols that can be implemented on an industrial scale. Continuous flow reactors and automated synthesis systems have shown promise in achieving this goal, allowing for better control over reaction conditions and reducing batch-to-batch variability.
The purification of hydroxyethylcellulose-modified polymers is another key consideration in the manufacturing process. Traditional methods such as dialysis and precipitation may not be feasible for large-scale production due to time and resource constraints. Advanced separation techniques, including tangential flow filtration and chromatography, are being explored to improve efficiency and maintain product purity during scale-up.
Sterilization is a critical step in the production of materials for tissue engineering. While conventional methods like autoclaving may compromise the integrity of the modified polymers, alternative approaches such as gamma irradiation or ethylene oxide treatment are being investigated. These methods must be optimized to ensure complete sterilization without altering the polymers' bioactive properties or degradation profiles.
The development of standardized quality control protocols is essential for consistent manufacturing. This includes the implementation of in-process controls and final product testing to verify chemical composition, molecular weight distribution, and functional properties. Advanced analytical techniques, such as high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) spectroscopy, are being integrated into production lines for real-time monitoring and adjustment of manufacturing parameters.
As the demand for hydroxyethylcellulose-modified polymers grows, there is an increasing focus on sustainable and eco-friendly manufacturing processes. This includes the exploration of green chemistry principles, such as the use of environmentally benign solvents and catalysts, as well as the development of closed-loop systems for reagent recycling and waste minimization.
The successful scale-up and manufacturing of these polymers also depend on addressing regulatory requirements for medical-grade materials. This involves establishing Good Manufacturing Practice (GMP) compliant facilities and processes, as well as developing comprehensive documentation and traceability systems to meet stringent quality and safety standards for clinical applications.
One of the primary challenges in scaling up production is maintaining the precise chemical modifications and physical properties of the polymers that are crucial for their performance in tissue engineering. This requires the development of robust and reproducible synthesis protocols that can be implemented on an industrial scale. Continuous flow reactors and automated synthesis systems have shown promise in achieving this goal, allowing for better control over reaction conditions and reducing batch-to-batch variability.
The purification of hydroxyethylcellulose-modified polymers is another key consideration in the manufacturing process. Traditional methods such as dialysis and precipitation may not be feasible for large-scale production due to time and resource constraints. Advanced separation techniques, including tangential flow filtration and chromatography, are being explored to improve efficiency and maintain product purity during scale-up.
Sterilization is a critical step in the production of materials for tissue engineering. While conventional methods like autoclaving may compromise the integrity of the modified polymers, alternative approaches such as gamma irradiation or ethylene oxide treatment are being investigated. These methods must be optimized to ensure complete sterilization without altering the polymers' bioactive properties or degradation profiles.
The development of standardized quality control protocols is essential for consistent manufacturing. This includes the implementation of in-process controls and final product testing to verify chemical composition, molecular weight distribution, and functional properties. Advanced analytical techniques, such as high-performance liquid chromatography (HPLC) and nuclear magnetic resonance (NMR) spectroscopy, are being integrated into production lines for real-time monitoring and adjustment of manufacturing parameters.
As the demand for hydroxyethylcellulose-modified polymers grows, there is an increasing focus on sustainable and eco-friendly manufacturing processes. This includes the exploration of green chemistry principles, such as the use of environmentally benign solvents and catalysts, as well as the development of closed-loop systems for reagent recycling and waste minimization.
The successful scale-up and manufacturing of these polymers also depend on addressing regulatory requirements for medical-grade materials. This involves establishing Good Manufacturing Practice (GMP) compliant facilities and processes, as well as developing comprehensive documentation and traceability systems to meet stringent quality and safety standards for clinical applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!