How Hydroxyethylcellulose Revolutionizes Cryopreservation Techniques
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Cryopreservation Evolution and HEC Integration
Cryopreservation techniques have undergone significant evolution since their inception in the mid-20th century. Initially, the focus was on preserving simple cell structures, but as research progressed, the field expanded to encompass more complex tissues and organs. The integration of Hydroxyethylcellulose (HEC) marks a pivotal moment in this evolution, revolutionizing the approach to cryoprotection.
Early cryopreservation methods relied heavily on glycerol and dimethyl sulfoxide (DMSO) as cryoprotectants. While effective to some degree, these agents often caused cellular damage due to osmotic stress and toxicity. The introduction of HEC addresses many of these limitations, offering superior protection against ice crystal formation and reducing cellular stress during the freezing and thawing processes.
HEC's unique properties as a non-penetrating cryoprotectant have led to its widespread adoption in various cryopreservation protocols. Its high molecular weight and ability to form a protective gel-like matrix around cells provide enhanced structural support during the freezing process. This characteristic is particularly beneficial for preserving delicate tissues and organs that are susceptible to mechanical damage from ice formation.
The integration of HEC into cryopreservation techniques has also facilitated advancements in vitrification methods. Vitrification, which involves the transformation of a liquid into a glass-like solid without crystallization, has become increasingly important in the preservation of complex biological structures. HEC's role in promoting vitrification has significantly improved the survival rates of cryopreserved samples, particularly in the field of reproductive medicine.
Furthermore, the incorporation of HEC has enabled the development of more sophisticated cryopreservation protocols. These include controlled-rate freezing techniques and the use of HEC in combination with other cryoprotectants to create synergistic effects. Such advancements have expanded the range of biological materials that can be successfully cryopreserved, from individual cells to entire organs.
The evolution of cryopreservation techniques with HEC integration has also led to improvements in long-term storage stability. HEC's ability to maintain cellular integrity over extended periods has increased the viability of cryopreserved samples, a crucial factor in biobanking and regenerative medicine applications. This enhanced stability has opened up new possibilities for the preservation of rare genetic materials and the creation of more diverse and representative biobanks.
Early cryopreservation methods relied heavily on glycerol and dimethyl sulfoxide (DMSO) as cryoprotectants. While effective to some degree, these agents often caused cellular damage due to osmotic stress and toxicity. The introduction of HEC addresses many of these limitations, offering superior protection against ice crystal formation and reducing cellular stress during the freezing and thawing processes.
HEC's unique properties as a non-penetrating cryoprotectant have led to its widespread adoption in various cryopreservation protocols. Its high molecular weight and ability to form a protective gel-like matrix around cells provide enhanced structural support during the freezing process. This characteristic is particularly beneficial for preserving delicate tissues and organs that are susceptible to mechanical damage from ice formation.
The integration of HEC into cryopreservation techniques has also facilitated advancements in vitrification methods. Vitrification, which involves the transformation of a liquid into a glass-like solid without crystallization, has become increasingly important in the preservation of complex biological structures. HEC's role in promoting vitrification has significantly improved the survival rates of cryopreserved samples, particularly in the field of reproductive medicine.
Furthermore, the incorporation of HEC has enabled the development of more sophisticated cryopreservation protocols. These include controlled-rate freezing techniques and the use of HEC in combination with other cryoprotectants to create synergistic effects. Such advancements have expanded the range of biological materials that can be successfully cryopreserved, from individual cells to entire organs.
The evolution of cryopreservation techniques with HEC integration has also led to improvements in long-term storage stability. HEC's ability to maintain cellular integrity over extended periods has increased the viability of cryopreserved samples, a crucial factor in biobanking and regenerative medicine applications. This enhanced stability has opened up new possibilities for the preservation of rare genetic materials and the creation of more diverse and representative biobanks.
Market Analysis for HEC-based Cryopreservation
The market for HEC-based cryopreservation techniques is experiencing significant growth, driven by increasing demand in various sectors such as biotechnology, healthcare, and research. The global cryopreservation market, which includes HEC-based solutions, is projected to expand at a compound annual growth rate (CAGR) of over 8% from 2021 to 2026. This growth is primarily attributed to the rising need for advanced preservation methods in stem cell research, regenerative medicine, and organ transplantation.
In the healthcare sector, HEC-based cryopreservation techniques are gaining traction due to their superior performance in preserving biological samples, tissues, and organs. The increasing prevalence of chronic diseases and the growing demand for personalized medicine are driving the adoption of these advanced preservation methods. Additionally, the expanding field of regenerative medicine, which relies heavily on the long-term storage of stem cells and tissues, is creating a substantial market opportunity for HEC-based cryopreservation solutions.
The biotechnology and pharmaceutical industries are also significant contributors to the market growth of HEC-based cryopreservation. These sectors require reliable preservation methods for cell lines, proteins, and other biological materials used in drug discovery and development processes. The ability of HEC-based solutions to maintain the viability and functionality of these materials over extended periods is driving their adoption in research and development activities.
Geographically, North America currently holds the largest market share for HEC-based cryopreservation techniques, followed by Europe and Asia-Pacific. The dominance of North America can be attributed to the presence of well-established healthcare and research infrastructure, substantial investments in biotechnology, and favorable government initiatives supporting advanced preservation technologies. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing healthcare expenditure, rising awareness about regenerative medicine, and growing research activities in countries like China, Japan, and India.
The market for HEC-based cryopreservation is characterized by intense competition among key players, including major biotechnology companies and specialized cryopreservation solution providers. These companies are focusing on research and development activities to enhance the efficacy of their products and expand their application areas. Collaborations between academic institutions and industry players are also contributing to the advancement of HEC-based cryopreservation techniques, further fueling market growth.
Despite the positive outlook, the market faces certain challenges, such as the high cost of cryopreservation equipment and solutions, which may limit adoption in developing regions. Additionally, concerns regarding the long-term effects of cryopreservation on biological materials and the need for standardized protocols present opportunities for further innovation and market development in the HEC-based cryopreservation sector.
In the healthcare sector, HEC-based cryopreservation techniques are gaining traction due to their superior performance in preserving biological samples, tissues, and organs. The increasing prevalence of chronic diseases and the growing demand for personalized medicine are driving the adoption of these advanced preservation methods. Additionally, the expanding field of regenerative medicine, which relies heavily on the long-term storage of stem cells and tissues, is creating a substantial market opportunity for HEC-based cryopreservation solutions.
The biotechnology and pharmaceutical industries are also significant contributors to the market growth of HEC-based cryopreservation. These sectors require reliable preservation methods for cell lines, proteins, and other biological materials used in drug discovery and development processes. The ability of HEC-based solutions to maintain the viability and functionality of these materials over extended periods is driving their adoption in research and development activities.
Geographically, North America currently holds the largest market share for HEC-based cryopreservation techniques, followed by Europe and Asia-Pacific. The dominance of North America can be attributed to the presence of well-established healthcare and research infrastructure, substantial investments in biotechnology, and favorable government initiatives supporting advanced preservation technologies. However, the Asia-Pacific region is expected to witness the highest growth rate in the coming years, driven by increasing healthcare expenditure, rising awareness about regenerative medicine, and growing research activities in countries like China, Japan, and India.
The market for HEC-based cryopreservation is characterized by intense competition among key players, including major biotechnology companies and specialized cryopreservation solution providers. These companies are focusing on research and development activities to enhance the efficacy of their products and expand their application areas. Collaborations between academic institutions and industry players are also contributing to the advancement of HEC-based cryopreservation techniques, further fueling market growth.
Despite the positive outlook, the market faces certain challenges, such as the high cost of cryopreservation equipment and solutions, which may limit adoption in developing regions. Additionally, concerns regarding the long-term effects of cryopreservation on biological materials and the need for standardized protocols present opportunities for further innovation and market development in the HEC-based cryopreservation sector.
Current Challenges in Cryopreservation Techniques
Cryopreservation techniques have made significant strides in recent years, yet several challenges persist in ensuring the optimal preservation of biological materials. One of the primary obstacles is the formation of ice crystals during the freezing process, which can cause severe cellular damage. This issue is particularly problematic for complex tissues and organs, where uniform freezing is difficult to achieve.
Another significant challenge is the toxicity of cryoprotectants. While these agents are essential for preventing ice crystal formation, they can be harmful to cells at high concentrations. Balancing the protective effects of cryoprotectants with their potential toxicity remains a delicate task, especially for sensitive biological samples.
The vitrification process, which aims to create a glass-like state without ice crystal formation, faces its own set of challenges. Achieving the rapid cooling rates required for vitrification can be technically demanding and may not be feasible for larger samples or organs. Additionally, the high concentrations of cryoprotectants needed for vitrification can exacerbate toxicity concerns.
Thawing procedures present another critical challenge in cryopreservation. Uneven warming can lead to recrystallization, causing damage that negates the benefits of careful freezing. Developing protocols that ensure uniform and rapid thawing across the entire sample remains an area of active research.
For complex biological systems like organs, maintaining cellular viability and functionality post-thaw is particularly challenging. Issues such as vascular damage, cellular stress responses, and loss of tissue architecture can compromise the integrity and function of preserved organs.
Scale-up and standardization of cryopreservation protocols pose significant hurdles in translating laboratory successes to clinical applications. Variations in sample size, composition, and handling can greatly affect outcomes, making it difficult to establish universally applicable procedures.
Lastly, the long-term effects of cryopreservation on genetic stability and epigenetic modifications are not fully understood. Concerns about potential alterations in gene expression or cellular behavior post-thaw necessitate further investigation to ensure the safety and efficacy of cryopreserved materials, especially in clinical settings.
Addressing these challenges requires interdisciplinary approaches, combining advances in materials science, bioengineering, and molecular biology. Innovations in cryoprotectant formulations, freezing and thawing technologies, and post-thaw recovery strategies are crucial for overcoming the current limitations in cryopreservation techniques.
Another significant challenge is the toxicity of cryoprotectants. While these agents are essential for preventing ice crystal formation, they can be harmful to cells at high concentrations. Balancing the protective effects of cryoprotectants with their potential toxicity remains a delicate task, especially for sensitive biological samples.
The vitrification process, which aims to create a glass-like state without ice crystal formation, faces its own set of challenges. Achieving the rapid cooling rates required for vitrification can be technically demanding and may not be feasible for larger samples or organs. Additionally, the high concentrations of cryoprotectants needed for vitrification can exacerbate toxicity concerns.
Thawing procedures present another critical challenge in cryopreservation. Uneven warming can lead to recrystallization, causing damage that negates the benefits of careful freezing. Developing protocols that ensure uniform and rapid thawing across the entire sample remains an area of active research.
For complex biological systems like organs, maintaining cellular viability and functionality post-thaw is particularly challenging. Issues such as vascular damage, cellular stress responses, and loss of tissue architecture can compromise the integrity and function of preserved organs.
Scale-up and standardization of cryopreservation protocols pose significant hurdles in translating laboratory successes to clinical applications. Variations in sample size, composition, and handling can greatly affect outcomes, making it difficult to establish universally applicable procedures.
Lastly, the long-term effects of cryopreservation on genetic stability and epigenetic modifications are not fully understood. Concerns about potential alterations in gene expression or cellular behavior post-thaw necessitate further investigation to ensure the safety and efficacy of cryopreserved materials, especially in clinical settings.
Addressing these challenges requires interdisciplinary approaches, combining advances in materials science, bioengineering, and molecular biology. Innovations in cryoprotectant formulations, freezing and thawing technologies, and post-thaw recovery strategies are crucial for overcoming the current limitations in cryopreservation techniques.
HEC-based Cryoprotectant Solutions
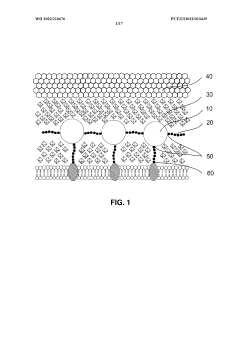
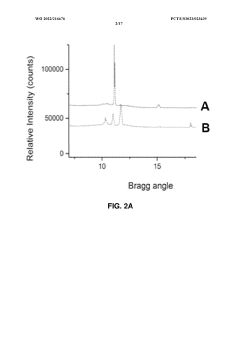
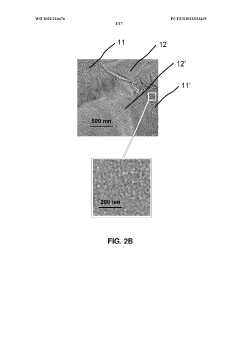
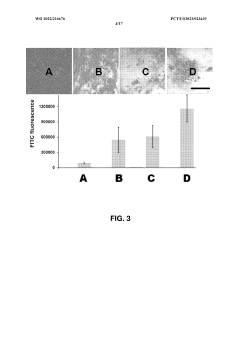
01 Use of hydroxyethylcellulose in cryopreservation solutions
Hydroxyethylcellulose is utilized as a component in cryopreservation solutions to improve the viability of cells and tissues during freezing and thawing processes. It acts as a cryoprotectant and helps maintain cellular integrity by reducing ice crystal formation and osmotic stress.- Use of hydroxyethylcellulose as a cryoprotectant: Hydroxyethylcellulose can be utilized as a cryoprotectant in cryopreservation techniques. It helps to protect cells and tissues from damage during freezing and thawing processes by reducing ice crystal formation and maintaining cellular integrity.
- Combination of hydroxyethylcellulose with other cryoprotectants: Hydroxyethylcellulose can be combined with other cryoprotectants such as dimethyl sulfoxide (DMSO) or glycerol to enhance the overall protective effect during cryopreservation. This combination approach can improve cell viability and recovery rates after thawing.
- Optimization of hydroxyethylcellulose concentration: The concentration of hydroxyethylcellulose in cryopreservation solutions is crucial for effective protection. Optimal concentrations may vary depending on the type of cells or tissues being preserved, and careful optimization can lead to improved cryopreservation outcomes.
- Application in stem cell cryopreservation: Hydroxyethylcellulose-based cryopreservation techniques have shown promise in preserving stem cells, including embryonic and adult stem cells. These methods can help maintain stem cell viability and differentiation potential after long-term storage.
- Development of novel hydroxyethylcellulose derivatives: Research is ongoing to develop novel derivatives of hydroxyethylcellulose with enhanced cryoprotective properties. These modified versions may offer improved performance in cryopreservation applications, potentially leading to better preservation outcomes for various biological materials.
02 Combination of hydroxyethylcellulose with other cryoprotectants
Hydroxyethylcellulose is often combined with other cryoprotectants such as dimethyl sulfoxide (DMSO) or glycerol to enhance the overall protective effect during cryopreservation. This combination approach can lead to improved cell survival rates and better preservation of cellular functions.Expand Specific Solutions03 Optimization of hydroxyethylcellulose concentration in cryopreservation media
Research focuses on determining the optimal concentration of hydroxyethylcellulose in cryopreservation media for different cell types and tissues. The concentration is crucial for balancing the protective effects against potential toxicity or osmotic imbalances.Expand Specific Solutions04 Application of hydroxyethylcellulose in vitrification techniques
Hydroxyethylcellulose is employed in vitrification techniques, which involve rapid cooling to achieve a glass-like state without ice crystal formation. This approach is particularly useful for preserving delicate biological samples such as embryos or stem cells.Expand Specific Solutions05 Development of novel cryopreservation protocols using hydroxyethylcellulose
Researchers are developing new cryopreservation protocols that incorporate hydroxyethylcellulose to improve the preservation of various biological materials. These protocols aim to enhance long-term storage capabilities and maintain the functional properties of preserved samples upon thawing.Expand Specific Solutions
Key Players in HEC Cryopreservation Industry
The cryopreservation techniques market is in a growth phase, driven by increasing demand in biomedical research and regenerative medicine. The global market size is projected to reach several billion dollars by 2025, with a compound annual growth rate of over 10%. Technological maturity varies across different applications, with some areas like cell preservation being more advanced than complex tissue preservation. Key players such as BioLife Solutions and Sexton Biotechnologies are leading innovation in biopreservation media, while academic institutions like Wuhan University and East Carolina University contribute to fundamental research. The involvement of major chemical companies like Shin-Etsu Chemical and Denka Corp. indicates the growing commercial potential of hydroxyethylcellulose in cryopreservation applications.
Shin-Etsu Chemical Co., Ltd.
Technical Solution: Shin-Etsu Chemical Co., Ltd. has developed a novel cryopreservation technique utilizing hydroxyethylcellulose (HEC) as a key component. Their method involves creating a specialized HEC-based cryoprotectant solution that forms a protective matrix around cells during freezing. This matrix helps prevent ice crystal formation and maintains cellular integrity. The company has optimized the molecular weight and degree of substitution of HEC to enhance its cryoprotective properties[1]. Their technique also incorporates a controlled-rate freezing process, which gradually lowers the temperature to minimize cellular stress. Additionally, Shin-Etsu has developed a proprietary thawing protocol that ensures gentle and uniform warming of the preserved samples[2].
Strengths: Superior ice crystal prevention, enhanced cellular viability post-thaw, and scalability for industrial applications. Weaknesses: Potential cost implications due to specialized HEC formulations and equipment requirements.
BioLife Solutions, Inc.
Technical Solution: BioLife Solutions has integrated hydroxyethylcellulose into their CryoStor® cryopreservation media line. Their approach combines HEC with other cryoprotective agents to create a synergistic effect. The HEC component acts as a viscosity modifier and helps to reduce extracellular ice formation. BioLife's formulation also includes antioxidants and energy precursors to support cellular metabolism during the freezing and thawing processes[3]. The company has conducted extensive research on the optimal concentration of HEC in their media, balancing cryoprotection with ease of handling. Their technology has shown particular success in preserving stem cells and other sensitive cell types, with reported post-thaw viabilities exceeding 90% in some applications[4].
Strengths: Proven effectiveness for a wide range of cell types, ready-to-use formulations, and extensive validation data. Weaknesses: May require optimization for specific cell types or tissues.
Innovations in HEC Cryopreservation Methods
Tool for cryopreservation of cell or tissue
PatentInactiveJP2019187244A
Innovation
- A frozen storage jig with a hydroxypropyl cellulose layer on its surface allows easy peeling and recovery of cells or tissues during thawing, combined with a storage liquid absorber to minimize the amount of preservation solution and reduce chemical toxicity.
Efficient biocompatible cryopreservation medium that eliminates the need for cell permeating cryoprotectants
PatentWO2022216676A1
Innovation
- A cryopreservation medium comprising a hydrophilic, spherical first cryoprotective particle or macromolecule, such as Ficoll 70, and a second cryoprotective particle or macromolecule with affinity for the first and cell membranes, forming nano-scale cubic ice to prevent hexagonal ice formation and recrystallization, eliminating the need for cell permeating cryoprotectants.
Regulatory Framework for Cryopreservation
The regulatory framework for cryopreservation is a complex and evolving landscape that governs the use of cryogenic techniques in various fields, including medicine, research, and biotechnology. As hydroxyethylcellulose (HEC) emerges as a revolutionary agent in cryopreservation, it is crucial to understand the existing regulations and how they may adapt to accommodate this innovation.
In the United States, the Food and Drug Administration (FDA) plays a central role in regulating cryopreservation techniques, particularly in the context of human cells, tissues, and cellular and tissue-based products (HCT/Ps). The FDA's regulatory approach is based on the level of risk associated with the product and its intended use. For cryopreservation involving HEC, the FDA may classify it under the 361 HCT/P category if it meets specific criteria, or as a 351 HCT/P if it requires more stringent oversight.
The European Union has established a comprehensive regulatory framework for cryopreservation through the European Union Tissue and Cells Directives (EUTCD). These directives set standards for the quality and safety of human tissues and cells intended for human applications. As HEC introduces new possibilities in cryopreservation, the European Medicines Agency (EMA) may need to review and potentially update these directives to ensure they adequately address the use of this novel cryoprotectant.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees the regulation of cryopreservation techniques. The country has been proactive in developing guidelines for regenerative medicine and cell therapy, which often involve cryopreservation. The introduction of HEC may prompt the PMDA to issue specific guidance on its use in cryopreservation protocols.
International organizations, such as the International Society for Biological and Environmental Repositories (ISBER), provide best practice guidelines for biobanking and cryopreservation. While not regulatory bodies, their recommendations often inform regulatory decisions and industry standards. ISBER may need to update its guidelines to incorporate the use of HEC in cryopreservation protocols.
As HEC demonstrates its potential to revolutionize cryopreservation techniques, regulatory bodies worldwide will likely need to reassess their frameworks. This may involve conducting safety assessments, evaluating long-term effects, and establishing new quality control measures specific to HEC-based cryopreservation. Regulatory agencies may also need to consider the ethical implications of improved cryopreservation techniques, particularly in areas such as fertility preservation and organ banking.
Harmonization of regulations across different countries and regions will be crucial as HEC-based cryopreservation techniques gain global traction. Initiatives like the International Conference on Harmonisation (ICH) may play a role in developing consistent guidelines for the use of HEC in cryopreservation, ensuring that safety and efficacy standards are universally applied.
In the United States, the Food and Drug Administration (FDA) plays a central role in regulating cryopreservation techniques, particularly in the context of human cells, tissues, and cellular and tissue-based products (HCT/Ps). The FDA's regulatory approach is based on the level of risk associated with the product and its intended use. For cryopreservation involving HEC, the FDA may classify it under the 361 HCT/P category if it meets specific criteria, or as a 351 HCT/P if it requires more stringent oversight.
The European Union has established a comprehensive regulatory framework for cryopreservation through the European Union Tissue and Cells Directives (EUTCD). These directives set standards for the quality and safety of human tissues and cells intended for human applications. As HEC introduces new possibilities in cryopreservation, the European Medicines Agency (EMA) may need to review and potentially update these directives to ensure they adequately address the use of this novel cryoprotectant.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) oversees the regulation of cryopreservation techniques. The country has been proactive in developing guidelines for regenerative medicine and cell therapy, which often involve cryopreservation. The introduction of HEC may prompt the PMDA to issue specific guidance on its use in cryopreservation protocols.
International organizations, such as the International Society for Biological and Environmental Repositories (ISBER), provide best practice guidelines for biobanking and cryopreservation. While not regulatory bodies, their recommendations often inform regulatory decisions and industry standards. ISBER may need to update its guidelines to incorporate the use of HEC in cryopreservation protocols.
As HEC demonstrates its potential to revolutionize cryopreservation techniques, regulatory bodies worldwide will likely need to reassess their frameworks. This may involve conducting safety assessments, evaluating long-term effects, and establishing new quality control measures specific to HEC-based cryopreservation. Regulatory agencies may also need to consider the ethical implications of improved cryopreservation techniques, particularly in areas such as fertility preservation and organ banking.
Harmonization of regulations across different countries and regions will be crucial as HEC-based cryopreservation techniques gain global traction. Initiatives like the International Conference on Harmonisation (ICH) may play a role in developing consistent guidelines for the use of HEC in cryopreservation, ensuring that safety and efficacy standards are universally applied.
Bioethical Implications of HEC Cryopreservation
The bioethical implications of using Hydroxyethylcellulose (HEC) in cryopreservation techniques are multifaceted and require careful consideration. As this technology advances, it raises important questions about the long-term effects on biological materials and the potential consequences for human and animal subjects.
One primary concern is the safety and efficacy of HEC-based cryopreservation methods. While initial studies show promising results, the long-term effects of HEC on cellular structures and genetic material remain uncertain. This uncertainty necessitates extensive research and rigorous testing protocols to ensure that the use of HEC does not inadvertently cause harm or unintended mutations in preserved specimens.
The accessibility and cost of HEC cryopreservation techniques also present ethical challenges. If this technology proves superior to current methods, it may create disparities in access to high-quality preservation services. This could potentially exacerbate existing inequalities in healthcare and research, particularly in developing countries or underfunded institutions.
Another significant ethical consideration is the potential for misuse or abuse of advanced cryopreservation techniques. Improved preservation methods could enable the long-term storage of biological materials, raising questions about ownership, consent, and the appropriate use of preserved specimens. Clear guidelines and regulations must be established to protect individual rights and prevent exploitation.
The use of HEC in cryopreservation also intersects with broader debates surrounding life extension and the manipulation of natural processes. As preservation techniques improve, they may challenge our understanding of death and the boundaries between life and non-life. This could have profound implications for medical, legal, and philosophical frameworks.
Furthermore, the environmental impact of widespread HEC use in cryopreservation must be considered. The production and disposal of HEC and associated materials should be evaluated to ensure that the technology does not contribute significantly to environmental degradation or resource depletion.
Lastly, the ethical implications extend to animal research and welfare. While improved cryopreservation techniques may reduce the need for live animal subjects in some areas of research, they could also lead to increased preservation and use of animal tissues and specimens. Balancing scientific progress with animal welfare concerns will require ongoing ethical scrutiny and guidance.
One primary concern is the safety and efficacy of HEC-based cryopreservation methods. While initial studies show promising results, the long-term effects of HEC on cellular structures and genetic material remain uncertain. This uncertainty necessitates extensive research and rigorous testing protocols to ensure that the use of HEC does not inadvertently cause harm or unintended mutations in preserved specimens.
The accessibility and cost of HEC cryopreservation techniques also present ethical challenges. If this technology proves superior to current methods, it may create disparities in access to high-quality preservation services. This could potentially exacerbate existing inequalities in healthcare and research, particularly in developing countries or underfunded institutions.
Another significant ethical consideration is the potential for misuse or abuse of advanced cryopreservation techniques. Improved preservation methods could enable the long-term storage of biological materials, raising questions about ownership, consent, and the appropriate use of preserved specimens. Clear guidelines and regulations must be established to protect individual rights and prevent exploitation.
The use of HEC in cryopreservation also intersects with broader debates surrounding life extension and the manipulation of natural processes. As preservation techniques improve, they may challenge our understanding of death and the boundaries between life and non-life. This could have profound implications for medical, legal, and philosophical frameworks.
Furthermore, the environmental impact of widespread HEC use in cryopreservation must be considered. The production and disposal of HEC and associated materials should be evaluated to ensure that the technology does not contribute significantly to environmental degradation or resource depletion.
Lastly, the ethical implications extend to animal research and welfare. While improved cryopreservation techniques may reduce the need for live animal subjects in some areas of research, they could also lead to increased preservation and use of animal tissues and specimens. Balancing scientific progress with animal welfare concerns will require ongoing ethical scrutiny and guidance.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!