Role of Hydroxyethylcellulose in Aiding Immune Oncology Therapies
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
HEC in Oncology: Background and Objectives
Hydroxyethylcellulose (HEC) has emerged as a promising component in the field of immune oncology therapies, offering potential to enhance the efficacy of cancer treatments. This polysaccharide derivative, traditionally used in various industries for its thickening and stabilizing properties, is now being explored for its unique characteristics that may aid in the delivery and effectiveness of immunotherapeutic agents.
The evolution of cancer treatment has seen a significant shift towards immunotherapy in recent years. This approach harnesses the power of the body's immune system to recognize and combat cancer cells. However, the success of these therapies often faces challenges related to drug delivery, bioavailability, and maintaining the stability of therapeutic agents within the tumor microenvironment.
HEC's role in this context stems from its ability to form hydrogels and its biocompatibility. These properties make it an excellent candidate for creating advanced drug delivery systems that can potentially overcome some of the limitations faced by current immunotherapeutic approaches. The polymer's capacity to encapsulate and gradually release therapeutic agents offers a promising avenue for improving the sustained delivery of immunomodulatory drugs to tumor sites.
The primary objective of investigating HEC in immune oncology is to develop more effective and targeted delivery systems for immunotherapeutic agents. This includes exploring its potential to enhance the penetration of drugs into solid tumors, prolong the release of active compounds, and protect sensitive biomolecules from degradation. Additionally, researchers aim to leverage HEC's properties to create scaffolds that can support and modulate immune cell functions within the tumor microenvironment.
Another critical goal is to assess HEC's ability to improve the pharmacokinetics and pharmacodynamics of immunotherapeutic drugs. This involves studying how HEC-based formulations can alter drug distribution, metabolism, and excretion, potentially leading to enhanced therapeutic efficacy and reduced side effects. The polymer's non-toxic nature and biodegradability make it an attractive option for developing safer and more tolerable treatment modalities.
Furthermore, the research into HEC in oncology aims to explore its synergistic effects with various immunotherapeutic strategies, including checkpoint inhibitors, cancer vaccines, and adoptive cell therapies. Understanding how HEC can be optimized to complement these treatments could pave the way for more comprehensive and effective cancer management approaches.
As the field of immune oncology continues to advance, the exploration of HEC represents a convergence of material science and immunotherapy. This interdisciplinary approach holds the promise of addressing some of the persistent challenges in cancer treatment, potentially leading to improved patient outcomes and quality of life.
The evolution of cancer treatment has seen a significant shift towards immunotherapy in recent years. This approach harnesses the power of the body's immune system to recognize and combat cancer cells. However, the success of these therapies often faces challenges related to drug delivery, bioavailability, and maintaining the stability of therapeutic agents within the tumor microenvironment.
HEC's role in this context stems from its ability to form hydrogels and its biocompatibility. These properties make it an excellent candidate for creating advanced drug delivery systems that can potentially overcome some of the limitations faced by current immunotherapeutic approaches. The polymer's capacity to encapsulate and gradually release therapeutic agents offers a promising avenue for improving the sustained delivery of immunomodulatory drugs to tumor sites.
The primary objective of investigating HEC in immune oncology is to develop more effective and targeted delivery systems for immunotherapeutic agents. This includes exploring its potential to enhance the penetration of drugs into solid tumors, prolong the release of active compounds, and protect sensitive biomolecules from degradation. Additionally, researchers aim to leverage HEC's properties to create scaffolds that can support and modulate immune cell functions within the tumor microenvironment.
Another critical goal is to assess HEC's ability to improve the pharmacokinetics and pharmacodynamics of immunotherapeutic drugs. This involves studying how HEC-based formulations can alter drug distribution, metabolism, and excretion, potentially leading to enhanced therapeutic efficacy and reduced side effects. The polymer's non-toxic nature and biodegradability make it an attractive option for developing safer and more tolerable treatment modalities.
Furthermore, the research into HEC in oncology aims to explore its synergistic effects with various immunotherapeutic strategies, including checkpoint inhibitors, cancer vaccines, and adoptive cell therapies. Understanding how HEC can be optimized to complement these treatments could pave the way for more comprehensive and effective cancer management approaches.
As the field of immune oncology continues to advance, the exploration of HEC represents a convergence of material science and immunotherapy. This interdisciplinary approach holds the promise of addressing some of the persistent challenges in cancer treatment, potentially leading to improved patient outcomes and quality of life.
Market Analysis: Immune Oncology Therapies
The immune oncology therapies market has experienced significant growth in recent years, driven by the increasing prevalence of cancer and the promising outcomes of immunotherapy treatments. This market segment is expected to continue its upward trajectory, with a compound annual growth rate (CAGR) projected to remain in the double digits over the next five years.
The global immune oncology therapies market is characterized by a diverse range of products, including checkpoint inhibitors, CAR-T cell therapies, cancer vaccines, and cytokine-based therapies. Among these, checkpoint inhibitors have emerged as the dominant category, accounting for a substantial portion of market revenue. The success of drugs like pembrolizumab and nivolumab has fueled further research and development in this area.
Geographically, North America leads the market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to its advanced healthcare infrastructure, high healthcare expenditure, and favorable reimbursement policies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years, driven by increasing healthcare investments and rising cancer incidence rates.
The market is highly competitive, with key players including Merck & Co., Bristol-Myers Squibb, Roche, AstraZeneca, and Pfizer. These companies are continuously investing in research and development to expand their product portfolios and maintain their market positions. Additionally, numerous smaller biotech firms and startups are entering the market with innovative approaches, contributing to the overall dynamism of the sector.
One of the key trends shaping the immune oncology therapies market is the focus on combination therapies. Researchers and pharmaceutical companies are increasingly exploring the potential of combining different immunotherapy agents or pairing immunotherapies with traditional cancer treatments to enhance efficacy and overcome resistance mechanisms.
The role of hydroxyethylcellulose in aiding immune oncology therapies represents an emerging area of interest within this market. As a versatile polymer, hydroxyethylcellulose has potential applications in drug delivery systems and formulations for immunotherapy agents. Its ability to improve drug solubility, stability, and controlled release properties could enhance the effectiveness of immune oncology treatments.
While the market for immune oncology therapies continues to expand, challenges remain. These include high treatment costs, potential side effects, and the need for predictive biomarkers to identify patients most likely to respond to specific therapies. Addressing these challenges will be crucial for sustaining market growth and improving patient outcomes in the long term.
The global immune oncology therapies market is characterized by a diverse range of products, including checkpoint inhibitors, CAR-T cell therapies, cancer vaccines, and cytokine-based therapies. Among these, checkpoint inhibitors have emerged as the dominant category, accounting for a substantial portion of market revenue. The success of drugs like pembrolizumab and nivolumab has fueled further research and development in this area.
Geographically, North America leads the market, followed by Europe and Asia-Pacific. The United States, in particular, holds the largest market share due to its advanced healthcare infrastructure, high healthcare expenditure, and favorable reimbursement policies. However, emerging economies in Asia-Pacific, such as China and India, are expected to witness rapid growth in the coming years, driven by increasing healthcare investments and rising cancer incidence rates.
The market is highly competitive, with key players including Merck & Co., Bristol-Myers Squibb, Roche, AstraZeneca, and Pfizer. These companies are continuously investing in research and development to expand their product portfolios and maintain their market positions. Additionally, numerous smaller biotech firms and startups are entering the market with innovative approaches, contributing to the overall dynamism of the sector.
One of the key trends shaping the immune oncology therapies market is the focus on combination therapies. Researchers and pharmaceutical companies are increasingly exploring the potential of combining different immunotherapy agents or pairing immunotherapies with traditional cancer treatments to enhance efficacy and overcome resistance mechanisms.
The role of hydroxyethylcellulose in aiding immune oncology therapies represents an emerging area of interest within this market. As a versatile polymer, hydroxyethylcellulose has potential applications in drug delivery systems and formulations for immunotherapy agents. Its ability to improve drug solubility, stability, and controlled release properties could enhance the effectiveness of immune oncology treatments.
While the market for immune oncology therapies continues to expand, challenges remain. These include high treatment costs, potential side effects, and the need for predictive biomarkers to identify patients most likely to respond to specific therapies. Addressing these challenges will be crucial for sustaining market growth and improving patient outcomes in the long term.
HEC: Current Applications and Challenges
Hydroxyethylcellulose (HEC) is a versatile polymer widely used in various industries, including pharmaceuticals, cosmetics, and construction. In the context of immune oncology therapies, HEC has emerged as a promising excipient with potential to enhance drug delivery and efficacy. Currently, HEC is primarily utilized as a thickening agent, stabilizer, and binder in pharmaceutical formulations.
In immune oncology, HEC's applications are centered around its ability to improve drug solubility, control release rates, and enhance the stability of therapeutic agents. Its non-ionic nature and biocompatibility make it an attractive choice for formulating complex biopharmaceuticals, including monoclonal antibodies and immune checkpoint inhibitors. HEC's ability to form hydrogels has been exploited to create sustained-release platforms for immunomodulatory drugs, potentially improving their pharmacokinetic profiles and reducing dosing frequency.
One of the key applications of HEC in immune oncology is its use in nanoparticle-based drug delivery systems. HEC can be employed as a coating material or matrix component in nanocarriers, helping to encapsulate and protect sensitive immunotherapeutic agents. This approach has shown promise in enhancing the targeted delivery of drugs to tumor sites while minimizing systemic toxicity.
Despite its potential, several challenges persist in the widespread adoption of HEC in immune oncology therapies. One significant hurdle is the need for precise control over HEC's molecular weight and degree of substitution to achieve optimal performance in different formulations. Variations in these parameters can significantly affect drug release kinetics and overall therapeutic efficacy.
Another challenge lies in ensuring the consistency and reproducibility of HEC-based formulations across different batches and manufacturing scales. This is particularly critical in the production of complex biopharmaceuticals, where slight variations in excipient properties can impact drug stability and efficacy.
The interaction between HEC and various immunotherapeutic agents is not fully understood, necessitating extensive compatibility studies. There are concerns about potential immunogenicity or altered pharmacokinetics of drugs when formulated with HEC, requiring thorough investigation and long-term safety assessments.
Furthermore, regulatory considerations pose a challenge in the adoption of novel HEC-based formulations. Demonstrating bioequivalence and obtaining approval for new drug delivery systems incorporating HEC can be a time-consuming and costly process, potentially slowing down the development of innovative immune oncology therapies.
In immune oncology, HEC's applications are centered around its ability to improve drug solubility, control release rates, and enhance the stability of therapeutic agents. Its non-ionic nature and biocompatibility make it an attractive choice for formulating complex biopharmaceuticals, including monoclonal antibodies and immune checkpoint inhibitors. HEC's ability to form hydrogels has been exploited to create sustained-release platforms for immunomodulatory drugs, potentially improving their pharmacokinetic profiles and reducing dosing frequency.
One of the key applications of HEC in immune oncology is its use in nanoparticle-based drug delivery systems. HEC can be employed as a coating material or matrix component in nanocarriers, helping to encapsulate and protect sensitive immunotherapeutic agents. This approach has shown promise in enhancing the targeted delivery of drugs to tumor sites while minimizing systemic toxicity.
Despite its potential, several challenges persist in the widespread adoption of HEC in immune oncology therapies. One significant hurdle is the need for precise control over HEC's molecular weight and degree of substitution to achieve optimal performance in different formulations. Variations in these parameters can significantly affect drug release kinetics and overall therapeutic efficacy.
Another challenge lies in ensuring the consistency and reproducibility of HEC-based formulations across different batches and manufacturing scales. This is particularly critical in the production of complex biopharmaceuticals, where slight variations in excipient properties can impact drug stability and efficacy.
The interaction between HEC and various immunotherapeutic agents is not fully understood, necessitating extensive compatibility studies. There are concerns about potential immunogenicity or altered pharmacokinetics of drugs when formulated with HEC, requiring thorough investigation and long-term safety assessments.
Furthermore, regulatory considerations pose a challenge in the adoption of novel HEC-based formulations. Demonstrating bioequivalence and obtaining approval for new drug delivery systems incorporating HEC can be a time-consuming and costly process, potentially slowing down the development of innovative immune oncology therapies.
HEC-based Solutions in Immune Oncology
01 Use as a thickening agent in various formulations
Hydroxyethylcellulose is widely used as a thickening agent in various industries, including cosmetics, pharmaceuticals, and personal care products. It helps to improve the viscosity and stability of formulations, enhancing their texture and consistency.- Use in drilling fluids and well treatment compositions: Hydroxyethylcellulose is used as a viscosifier and fluid loss control agent in drilling fluids and well treatment compositions. It helps to improve the rheological properties of the fluids, enhance suspension of solids, and reduce fluid loss to the formation during drilling and well operations.
- Application in personal care and cosmetic products: Hydroxyethylcellulose is utilized as a thickening agent and stabilizer in personal care and cosmetic formulations. It provides texture, improves product consistency, and enhances the stability of emulsions in various products such as shampoos, lotions, and creams.
- Use in pharmaceutical formulations: Hydroxyethylcellulose is employed in pharmaceutical formulations as a binder, thickener, and controlled-release agent. It helps to modify drug release profiles, improve tablet properties, and enhance the stability of various dosage forms.
- Application in construction and building materials: Hydroxyethylcellulose is used as an additive in construction and building materials to improve workability, water retention, and adhesion properties. It is particularly useful in cement-based products, tile adhesives, and joint compounds.
- Use in textile and paper industries: Hydroxyethylcellulose finds applications in textile and paper industries as a sizing agent, binder, and surface treatment additive. It improves the strength, printability, and surface properties of textiles and paper products.
02 Application in oil and gas industry
Hydroxyethylcellulose is utilized in the oil and gas industry as a component in drilling fluids and fracturing fluids. It helps control fluid loss, improve rheological properties, and enhance the overall performance of these fluids in well operations.Expand Specific Solutions03 Use in personal care and cosmetic products
Hydroxyethylcellulose is commonly used in personal care and cosmetic products as a stabilizer, emulsifier, and film-forming agent. It helps improve the texture, spreadability, and moisture retention properties of various formulations such as lotions, creams, and hair care products.Expand Specific Solutions04 Application in pharmaceutical formulations
Hydroxyethylcellulose is employed in pharmaceutical formulations as a binder, thickener, and controlled-release agent. It helps improve the stability, bioavailability, and release profile of various drug delivery systems, including tablets, capsules, and topical preparations.Expand Specific Solutions05 Use in adhesive and coating applications
Hydroxyethylcellulose is utilized in adhesive and coating formulations to improve their properties such as viscosity, film formation, and adhesion. It finds applications in various industries, including paper, textiles, and construction materials.Expand Specific Solutions
Key Players in HEC and Oncology Research
The role of Hydroxyethylcellulose in aiding immune oncology therapies is an emerging field with significant potential. The market is in its early growth stage, with increasing research and development efforts. While the market size is still relatively small, it is expected to expand rapidly as more applications are discovered. Technologically, the field is progressing, with companies like Immatics Biotechnologies, BioAtla, and Eureka Therapeutics leading innovation. However, the technology is not yet fully mature, and further advancements are needed to realize its full potential in immune oncology treatments.
Immatics Biotechnologies GmbH
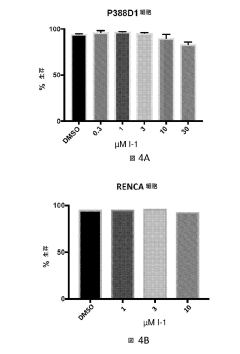
Technical Solution: Immatics has developed an innovative approach using hydroxyethylcellulose in their adoptive cell therapy platform for cancer immunotherapy. Their technology incorporates HEC as a key component in the formulation of their proprietary ACTengine® products. By utilizing HEC, Immatics has created a unique microenvironment for T cell activation and expansion, which enhances the anti-tumor activity of their engineered T cells[5]. The company has also explored the use of HEC-based hydrogels for local delivery of their TCER® (T cell engaging receptor) bispecific molecules, allowing for sustained release and improved targeting of solid tumors[6]. This dual application of HEC in both cell therapy manufacturing and targeted delivery systems positions Immatics as a leader in advancing immune oncology therapies.
Strengths: Enhanced T cell functionality, improved targeting of solid tumors, and versatile application in both cell therapy and bispecific antibody platforms. Weaknesses: Potential challenges in scaling up production and ensuring consistent product quality across different batches.
BioAtla, Inc.
Technical Solution: BioAtla has leveraged hydroxyethylcellulose in their Conditionally Active Biologics (CAB) platform for immune oncology therapies. Their innovative approach involves using HEC-based hydrogels to create a tumor-specific microenvironment that activates their CAB antibodies. This technology allows for highly selective targeting of cancer cells while minimizing off-target effects in normal tissues[7]. BioAtla has demonstrated that HEC-based formulations can modulate the pH and ion concentration in the tumor microenvironment, triggering the activation of their CAB molecules specifically at the tumor site[8]. Additionally, the company has explored the use of HEC in combination with their proprietary protein engineering techniques to develop novel bispecific antibodies with enhanced tumor penetration and efficacy[9].
Strengths: Highly selective tumor targeting, reduced off-target effects, and versatile platform for developing various immunotherapeutics. Weaknesses: Complexity in manufacturing and potential variability in CAB activation depending on individual tumor characteristics.
Innovations in HEC for Cancer Therapy
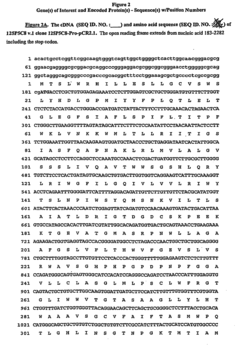
Nucleic acid and corresponding protein entitled 125p5c8 useful in treatment and detection of cancer
PatentInactiveEP1372719B1
Innovation
- The development of a gene designated 125P5C8, which is over-expressed in these cancers, allows for the creation of methods to detect its related protein or polynucleotide, inhibit cell growth, and deliver cytotoxic agents to cancer cells, using antibodies and immunogenic or therapeutic compositions.
Combination of selective histone deacetylase 3 (HDAC3) inhibitor and immunotherapy agent for treatment of cancer
PatentPendingJP2024099510A
Innovation
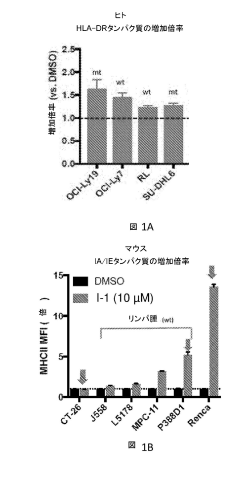
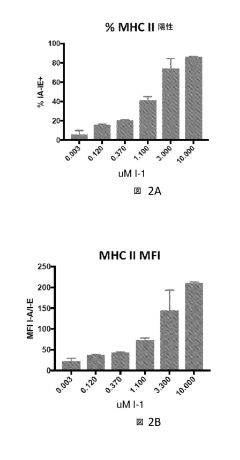
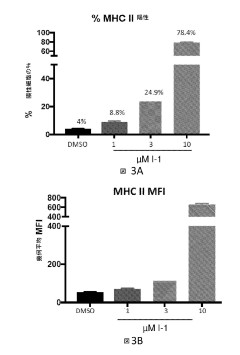
- Combining selective histone deacetylase 3 (HDAC3) inhibitors with immune checkpoint inhibitors to increase major histocompatibility complex (MHC) class II expression, thereby enhancing the immune response and efficacy of cancer treatment.
Regulatory Landscape for HEC in Oncology
The regulatory landscape for hydroxyethylcellulose (HEC) in oncology is complex and multifaceted, reflecting the critical nature of its potential applications in immune oncology therapies. As a non-active pharmaceutical ingredient, HEC falls under the purview of excipient regulations, which are governed by various regulatory bodies worldwide.
In the United States, the Food and Drug Administration (FDA) oversees the use of HEC in oncology treatments. The FDA's guidance on excipients requires manufacturers to demonstrate the safety and functionality of HEC in specific formulations. This includes providing data on its chemical composition, manufacturing process, and potential impurities. The FDA also mandates stability testing to ensure that HEC does not interfere with the active pharmaceutical ingredients or compromise the efficacy of the oncology therapy.
The European Medicines Agency (EMA) has similar requirements for HEC use in oncology treatments within the European Union. The EMA's guidelines on excipients emphasize the need for comprehensive safety assessments, particularly when used in novel applications such as immune oncology therapies. Manufacturers must provide detailed information on the sourcing, production, and quality control measures for HEC.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates the use of excipients like HEC in oncology treatments. The PMDA's approach aligns with international standards but may have specific requirements for stability studies and compatibility assessments with Japanese pharmacopeia standards.
Globally, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides guidelines that influence the regulatory approach to excipients like HEC. The ICH Q3C guideline on residual solvents and Q3D guideline on elemental impurities are particularly relevant for ensuring the purity and safety of HEC in oncology applications.
Regulatory bodies are increasingly focusing on the potential impact of excipients on the efficacy of advanced therapies, including immune oncology treatments. This has led to more stringent requirements for demonstrating the compatibility of HEC with complex biological molecules and its potential effects on drug delivery and bioavailability.
Manufacturers seeking to use HEC in oncology therapies must navigate these regulatory frameworks, which often require extensive documentation, rigorous testing, and ongoing quality assurance measures. The regulatory landscape is dynamic, with agencies continually updating their guidelines to address emerging technologies and safety concerns in the rapidly evolving field of immune oncology.
In the United States, the Food and Drug Administration (FDA) oversees the use of HEC in oncology treatments. The FDA's guidance on excipients requires manufacturers to demonstrate the safety and functionality of HEC in specific formulations. This includes providing data on its chemical composition, manufacturing process, and potential impurities. The FDA also mandates stability testing to ensure that HEC does not interfere with the active pharmaceutical ingredients or compromise the efficacy of the oncology therapy.
The European Medicines Agency (EMA) has similar requirements for HEC use in oncology treatments within the European Union. The EMA's guidelines on excipients emphasize the need for comprehensive safety assessments, particularly when used in novel applications such as immune oncology therapies. Manufacturers must provide detailed information on the sourcing, production, and quality control measures for HEC.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) regulates the use of excipients like HEC in oncology treatments. The PMDA's approach aligns with international standards but may have specific requirements for stability studies and compatibility assessments with Japanese pharmacopeia standards.
Globally, the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) provides guidelines that influence the regulatory approach to excipients like HEC. The ICH Q3C guideline on residual solvents and Q3D guideline on elemental impurities are particularly relevant for ensuring the purity and safety of HEC in oncology applications.
Regulatory bodies are increasingly focusing on the potential impact of excipients on the efficacy of advanced therapies, including immune oncology treatments. This has led to more stringent requirements for demonstrating the compatibility of HEC with complex biological molecules and its potential effects on drug delivery and bioavailability.
Manufacturers seeking to use HEC in oncology therapies must navigate these regulatory frameworks, which often require extensive documentation, rigorous testing, and ongoing quality assurance measures. The regulatory landscape is dynamic, with agencies continually updating their guidelines to address emerging technologies and safety concerns in the rapidly evolving field of immune oncology.
Safety and Biocompatibility of HEC
The safety and biocompatibility of Hydroxyethylcellulose (HEC) are crucial factors in its potential application for immune oncology therapies. HEC has been widely used in various pharmaceutical and cosmetic products, demonstrating a generally favorable safety profile. Extensive toxicological studies have shown that HEC exhibits low toxicity when administered orally, dermally, or through other routes.
In vitro studies have consistently demonstrated the biocompatibility of HEC with various cell types, including immune cells relevant to oncology therapies. These studies have shown minimal cytotoxicity and no significant interference with cellular functions. Furthermore, HEC does not appear to elicit strong immune responses or allergic reactions, making it a suitable candidate for use in immune-related applications.
Animal studies have further supported the safety of HEC for in vivo use. Long-term exposure studies in rodents and other animal models have shown no significant adverse effects on organ systems or overall health. Additionally, HEC has not demonstrated carcinogenic or mutagenic properties in standard toxicological assessments.
The biodegradability of HEC is another important aspect of its safety profile. The polymer can be broken down by various enzymes in the body, reducing the risk of long-term accumulation and associated complications. This property is particularly relevant for its potential use in oncology therapies, where repeated administrations may be necessary.
In the context of immune oncology therapies, the interaction between HEC and the immune system is of particular interest. Research has shown that HEC does not significantly suppress or overstimulate immune responses, maintaining a neutral impact on immune function. This characteristic is advantageous for its potential role as a carrier or adjuvant in immune-based cancer treatments.
Regulatory bodies, including the FDA and EMA, have recognized HEC as generally safe for use in various applications. It is listed as an inactive ingredient in numerous approved pharmaceutical products, further attesting to its safety profile. However, as with any material considered for novel therapeutic applications, additional specific safety assessments may be required to ensure its suitability for use in immune oncology therapies.
While the overall safety and biocompatibility of HEC are well-established, ongoing research continues to explore its behavior in specific physiological contexts relevant to cancer immunotherapy. This includes studies on its potential interactions with tumor microenvironments, its impact on drug delivery to target tissues, and any long-term effects on immune cell populations.
In vitro studies have consistently demonstrated the biocompatibility of HEC with various cell types, including immune cells relevant to oncology therapies. These studies have shown minimal cytotoxicity and no significant interference with cellular functions. Furthermore, HEC does not appear to elicit strong immune responses or allergic reactions, making it a suitable candidate for use in immune-related applications.
Animal studies have further supported the safety of HEC for in vivo use. Long-term exposure studies in rodents and other animal models have shown no significant adverse effects on organ systems or overall health. Additionally, HEC has not demonstrated carcinogenic or mutagenic properties in standard toxicological assessments.
The biodegradability of HEC is another important aspect of its safety profile. The polymer can be broken down by various enzymes in the body, reducing the risk of long-term accumulation and associated complications. This property is particularly relevant for its potential use in oncology therapies, where repeated administrations may be necessary.
In the context of immune oncology therapies, the interaction between HEC and the immune system is of particular interest. Research has shown that HEC does not significantly suppress or overstimulate immune responses, maintaining a neutral impact on immune function. This characteristic is advantageous for its potential role as a carrier or adjuvant in immune-based cancer treatments.
Regulatory bodies, including the FDA and EMA, have recognized HEC as generally safe for use in various applications. It is listed as an inactive ingredient in numerous approved pharmaceutical products, further attesting to its safety profile. However, as with any material considered for novel therapeutic applications, additional specific safety assessments may be required to ensure its suitability for use in immune oncology therapies.
While the overall safety and biocompatibility of HEC are well-established, ongoing research continues to explore its behavior in specific physiological contexts relevant to cancer immunotherapy. This includes studies on its potential interactions with tumor microenvironments, its impact on drug delivery to target tissues, and any long-term effects on immune cell populations.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!