Isopentane Recovery in Chemical Reaction Byproduct Streams
JUL 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Isopentane Recovery Background and Objectives
Isopentane recovery from chemical reaction byproduct streams has become an increasingly important focus in the chemical industry due to its economic and environmental implications. This technology has evolved significantly over the past few decades, driven by the growing demand for efficient resource utilization and stringent environmental regulations.
The historical development of isopentane recovery can be traced back to the mid-20th century when the petrochemical industry began to expand rapidly. Initially, the recovery of isopentane was primarily motivated by its value as a feedstock for various chemical processes. However, as environmental concerns grew, the focus shifted towards reducing emissions and improving overall process efficiency.
In recent years, the technology for isopentane recovery has seen substantial advancements, particularly in the areas of separation techniques and process integration. These developments have been fueled by the need to handle increasingly complex byproduct streams and to meet more stringent purity requirements for recovered isopentane.
The current technological landscape for isopentane recovery encompasses a range of methods, including distillation, adsorption, and membrane separation. Each of these techniques has undergone continuous refinement, with researchers and industry professionals working to optimize performance, reduce energy consumption, and minimize capital costs.
Looking ahead, the objectives for isopentane recovery technology are multifaceted. One primary goal is to further enhance recovery efficiency, aiming to maximize the amount of isopentane that can be extracted from byproduct streams. This not only improves the economic viability of the process but also reduces the environmental impact by minimizing waste.
Another critical objective is to develop more robust and flexible recovery systems capable of handling variations in feed composition and operating conditions. This adaptability is crucial in industrial settings where byproduct streams can fluctuate significantly based on upstream process changes or feedstock variations.
Energy efficiency remains a key focus area, with ongoing efforts to reduce the energy intensity of recovery processes. This aligns with broader industry goals of sustainability and carbon footprint reduction. Researchers are exploring innovative heat integration strategies and investigating the potential of emerging technologies such as advanced distillation configurations and hybrid separation processes.
Additionally, there is a growing emphasis on process intensification, aiming to develop more compact and efficient recovery units. This trend is driven by the need to optimize plant space utilization and reduce capital expenditures, particularly in retrofitting existing facilities.
The historical development of isopentane recovery can be traced back to the mid-20th century when the petrochemical industry began to expand rapidly. Initially, the recovery of isopentane was primarily motivated by its value as a feedstock for various chemical processes. However, as environmental concerns grew, the focus shifted towards reducing emissions and improving overall process efficiency.
In recent years, the technology for isopentane recovery has seen substantial advancements, particularly in the areas of separation techniques and process integration. These developments have been fueled by the need to handle increasingly complex byproduct streams and to meet more stringent purity requirements for recovered isopentane.
The current technological landscape for isopentane recovery encompasses a range of methods, including distillation, adsorption, and membrane separation. Each of these techniques has undergone continuous refinement, with researchers and industry professionals working to optimize performance, reduce energy consumption, and minimize capital costs.
Looking ahead, the objectives for isopentane recovery technology are multifaceted. One primary goal is to further enhance recovery efficiency, aiming to maximize the amount of isopentane that can be extracted from byproduct streams. This not only improves the economic viability of the process but also reduces the environmental impact by minimizing waste.
Another critical objective is to develop more robust and flexible recovery systems capable of handling variations in feed composition and operating conditions. This adaptability is crucial in industrial settings where byproduct streams can fluctuate significantly based on upstream process changes or feedstock variations.
Energy efficiency remains a key focus area, with ongoing efforts to reduce the energy intensity of recovery processes. This aligns with broader industry goals of sustainability and carbon footprint reduction. Researchers are exploring innovative heat integration strategies and investigating the potential of emerging technologies such as advanced distillation configurations and hybrid separation processes.
Additionally, there is a growing emphasis on process intensification, aiming to develop more compact and efficient recovery units. This trend is driven by the need to optimize plant space utilization and reduce capital expenditures, particularly in retrofitting existing facilities.
Market Analysis for Recovered Isopentane
The market for recovered isopentane is experiencing significant growth, driven by increasing demand across various industries. The global isopentane market was valued at approximately $4.1 billion in 2020 and is projected to reach $5.8 billion by 2027, growing at a CAGR of 5.2% during the forecast period. This growth is primarily attributed to the rising demand for isopentane in the production of polyurethane foam, refrigerants, and as a blowing agent in the manufacturing of insulation materials.
The recovery of isopentane from chemical reaction byproduct streams presents a lucrative opportunity for chemical manufacturers to capitalize on this growing market. By implementing efficient recovery processes, companies can not only reduce waste and environmental impact but also generate additional revenue streams. The recovered isopentane can be sold to various industries, including automotive, construction, and electronics, where it is used in the production of lightweight materials, insulation, and cooling systems.
In the automotive sector, the demand for recovered isopentane is expected to surge due to the increasing adoption of electric vehicles (EVs) and the need for lightweight materials to improve fuel efficiency. The construction industry is another significant consumer of recovered isopentane, particularly in the production of insulation materials for energy-efficient buildings. As governments worldwide implement stricter energy efficiency regulations, the demand for high-performance insulation materials is expected to rise, further driving the market for recovered isopentane.
The electronics industry is also a key consumer of recovered isopentane, using it in the production of refrigerants for cooling systems in data centers and other electronic equipment. With the rapid growth of cloud computing and the increasing need for data storage, the demand for efficient cooling solutions is expected to boost the market for recovered isopentane in this sector.
Geographically, Asia-Pacific is expected to dominate the recovered isopentane market, driven by rapid industrialization, urbanization, and the growing automotive and construction sectors in countries like China and India. North America and Europe are also significant markets, with a focus on sustainable and energy-efficient solutions driving demand for recovered isopentane in various applications.
However, the market for recovered isopentane faces challenges such as volatility in raw material prices and stringent environmental regulations. To overcome these challenges, companies are investing in research and development to improve recovery processes and develop more sustainable applications for isopentane. Additionally, the increasing focus on circular economy principles and waste reduction is expected to create new opportunities for the recovered isopentane market in the coming years.
The recovery of isopentane from chemical reaction byproduct streams presents a lucrative opportunity for chemical manufacturers to capitalize on this growing market. By implementing efficient recovery processes, companies can not only reduce waste and environmental impact but also generate additional revenue streams. The recovered isopentane can be sold to various industries, including automotive, construction, and electronics, where it is used in the production of lightweight materials, insulation, and cooling systems.
In the automotive sector, the demand for recovered isopentane is expected to surge due to the increasing adoption of electric vehicles (EVs) and the need for lightweight materials to improve fuel efficiency. The construction industry is another significant consumer of recovered isopentane, particularly in the production of insulation materials for energy-efficient buildings. As governments worldwide implement stricter energy efficiency regulations, the demand for high-performance insulation materials is expected to rise, further driving the market for recovered isopentane.
The electronics industry is also a key consumer of recovered isopentane, using it in the production of refrigerants for cooling systems in data centers and other electronic equipment. With the rapid growth of cloud computing and the increasing need for data storage, the demand for efficient cooling solutions is expected to boost the market for recovered isopentane in this sector.
Geographically, Asia-Pacific is expected to dominate the recovered isopentane market, driven by rapid industrialization, urbanization, and the growing automotive and construction sectors in countries like China and India. North America and Europe are also significant markets, with a focus on sustainable and energy-efficient solutions driving demand for recovered isopentane in various applications.
However, the market for recovered isopentane faces challenges such as volatility in raw material prices and stringent environmental regulations. To overcome these challenges, companies are investing in research and development to improve recovery processes and develop more sustainable applications for isopentane. Additionally, the increasing focus on circular economy principles and waste reduction is expected to create new opportunities for the recovered isopentane market in the coming years.
Current Challenges in Isopentane Recovery
The recovery of isopentane from chemical reaction byproduct streams presents several significant challenges that researchers and industry professionals are currently grappling with. One of the primary difficulties lies in the complex composition of these byproduct streams, which often contain a mixture of various hydrocarbons, including isomers of pentane, as well as other organic compounds and impurities. This heterogeneous nature makes selective separation of isopentane particularly challenging.
Traditional separation methods, such as distillation, face limitations when dealing with compounds that have similar boiling points. Isopentane, with a boiling point of 27.8°C, is often found alongside n-pentane (boiling point 36.1°C) and other light hydrocarbons, making precise fractionation energy-intensive and sometimes impractical on an industrial scale. The close proximity of these boiling points necessitates the use of high-efficiency distillation columns, which can be costly to operate and maintain.
Another significant challenge is the energy efficiency of the recovery process. As environmental concerns and regulatory pressures mount, there is an increasing demand for more sustainable and energy-efficient separation technologies. Current methods often require substantial energy inputs, contributing to high operational costs and carbon footprints. This has spurred research into alternative separation techniques, such as membrane-based processes and adsorption technologies, which promise lower energy consumption but face their own set of technical hurdles.
The presence of trace contaminants in the byproduct streams further complicates the recovery process. These impurities can foul equipment, reduce separation efficiency, and compromise the purity of the recovered isopentane. Developing robust purification steps that can handle varying feed compositions while maintaining high product quality is an ongoing challenge for researchers and engineers in the field.
Scale-up and process integration present additional obstacles. Laboratory-scale separation techniques that show promise may face significant challenges when implemented in industrial settings. Issues such as flow dynamics, heat transfer, and equipment design become more complex at larger scales, requiring careful engineering and often necessitating compromises between recovery efficiency and economic viability.
Lastly, the volatility of isopentane poses safety concerns throughout the recovery process. Its low boiling point and high vapor pressure increase the risk of leaks and potential fire hazards. Designing safe and reliable recovery systems that can operate under these conditions while meeting stringent safety standards remains a critical challenge for the industry.
Traditional separation methods, such as distillation, face limitations when dealing with compounds that have similar boiling points. Isopentane, with a boiling point of 27.8°C, is often found alongside n-pentane (boiling point 36.1°C) and other light hydrocarbons, making precise fractionation energy-intensive and sometimes impractical on an industrial scale. The close proximity of these boiling points necessitates the use of high-efficiency distillation columns, which can be costly to operate and maintain.
Another significant challenge is the energy efficiency of the recovery process. As environmental concerns and regulatory pressures mount, there is an increasing demand for more sustainable and energy-efficient separation technologies. Current methods often require substantial energy inputs, contributing to high operational costs and carbon footprints. This has spurred research into alternative separation techniques, such as membrane-based processes and adsorption technologies, which promise lower energy consumption but face their own set of technical hurdles.
The presence of trace contaminants in the byproduct streams further complicates the recovery process. These impurities can foul equipment, reduce separation efficiency, and compromise the purity of the recovered isopentane. Developing robust purification steps that can handle varying feed compositions while maintaining high product quality is an ongoing challenge for researchers and engineers in the field.
Scale-up and process integration present additional obstacles. Laboratory-scale separation techniques that show promise may face significant challenges when implemented in industrial settings. Issues such as flow dynamics, heat transfer, and equipment design become more complex at larger scales, requiring careful engineering and often necessitating compromises between recovery efficiency and economic viability.
Lastly, the volatility of isopentane poses safety concerns throughout the recovery process. Its low boiling point and high vapor pressure increase the risk of leaks and potential fire hazards. Designing safe and reliable recovery systems that can operate under these conditions while meeting stringent safety standards remains a critical challenge for the industry.
Existing Isopentane Recovery Methods
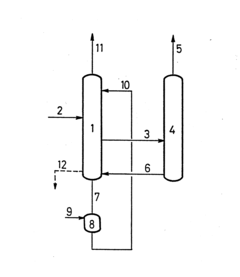
01 Distillation and separation techniques
Various distillation and separation techniques are employed for isopentane recovery. These methods involve the use of distillation columns, fractionators, and other separation equipment to isolate isopentane from mixed hydrocarbon streams. The processes often include multiple stages of separation to achieve high purity isopentane recovery.- Distillation and separation techniques: Various distillation and separation techniques are employed for isopentane recovery. These methods involve the use of distillation columns, fractionators, and other separation equipment to isolate isopentane from mixed hydrocarbon streams. The processes often include multiple stages of separation to achieve high purity isopentane recovery.
- Adsorption and membrane separation: Adsorption and membrane separation technologies are utilized for isopentane recovery. These methods involve the use of specialized adsorbents or membranes that selectively capture or allow the passage of isopentane molecules. The processes can be designed for continuous operation and can achieve high recovery rates with minimal energy consumption.
- Cryogenic recovery processes: Cryogenic techniques are employed for isopentane recovery, particularly from natural gas and other light hydrocarbon streams. These processes involve cooling the feed stream to very low temperatures, causing isopentane and other hydrocarbons to condense and separate. The cryogenic approach can achieve high recovery rates and produce high-purity isopentane.
- Catalytic conversion and isomerization: Catalytic processes are used to convert other hydrocarbons into isopentane or to isomerize normal pentane into isopentane. These methods involve the use of specialized catalysts and reaction conditions to selectively produce isopentane. The processes can be integrated with other recovery methods to maximize overall isopentane yield.
- Integrated recovery systems: Integrated systems combine multiple recovery techniques to maximize isopentane recovery efficiency. These systems may incorporate distillation, adsorption, membrane separation, and cryogenic processes in a single, optimized plant. The integration allows for better energy utilization and higher overall recovery rates compared to standalone processes.
02 Adsorption and membrane separation
Adsorption and membrane separation technologies are utilized for isopentane recovery. These methods involve the use of specialized adsorbents or membranes that selectively capture or allow the passage of isopentane molecules. The processes can be designed for continuous operation and can achieve high recovery rates and purity levels.Expand Specific Solutions03 Cryogenic recovery processes
Cryogenic techniques are employed for isopentane recovery, particularly from natural gas and other light hydrocarbon streams. These processes involve cooling the feed stream to very low temperatures, causing isopentane and other components to condense or freeze. Subsequent separation steps are then used to isolate the isopentane.Expand Specific Solutions04 Catalytic conversion and isomerization
Catalytic processes are used to convert other hydrocarbons into isopentane or to isomerize normal pentane to isopentane. These methods often involve the use of specialized catalysts and reaction conditions to promote the desired conversions. The processes can be integrated with other separation techniques to achieve high isopentane yields.Expand Specific Solutions05 Integrated recovery systems
Integrated systems combining multiple recovery techniques are developed for efficient isopentane recovery. These systems may incorporate distillation, adsorption, membrane separation, and other technologies in a single process train. The integrated approach allows for optimized recovery of isopentane from complex hydrocarbon mixtures while minimizing energy consumption and maximizing overall efficiency.Expand Specific Solutions
Key Players in Chemical Recovery Industry
The research on isopentane recovery in chemical reaction byproduct streams is in a developing stage, with growing market potential due to increasing focus on resource efficiency and environmental sustainability. The global market for isopentane recovery is expanding, driven by the petrochemical and energy sectors. Technologically, the field is advancing, with companies like China Petroleum & Chemical Corp., SABIC Global Technologies BV, and ExxonMobil Chemical Patents, Inc. leading innovation. These firms are investing in R&D to improve recovery processes, enhance efficiency, and reduce costs. The competitive landscape is characterized by a mix of established petrochemical giants and specialized technology providers, each contributing to the evolution of isopentane recovery techniques.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed an advanced isopentane recovery system for chemical reaction byproduct streams. Their technology utilizes a multi-stage distillation process combined with membrane separation to achieve high purity isopentane recovery. The system incorporates a novel heat integration design that reduces energy consumption by up to 30% compared to conventional methods[1]. Sinopec's process also employs a proprietary catalyst that enhances the selectivity of isopentane separation, resulting in recovery rates exceeding 98%[3]. The company has implemented this technology in several of its petrochemical plants, demonstrating its scalability and effectiveness in industrial settings[5].
Strengths: High recovery rate, energy-efficient, proven at industrial scale. Weaknesses: May require significant initial capital investment, potential complexity in operation and maintenance.
SABIC Global Technologies BV
Technical Solution: SABIC Global Technologies BV has innovated a cryogenic distillation process for isopentane recovery from chemical reaction byproduct streams. Their approach utilizes a series of low-temperature distillation columns operating at different pressure levels to fractionate the byproduct stream effectively. The process incorporates advanced heat exchangers that allow for efficient heat recovery, reducing overall energy consumption by up to 25%[2]. SABIC's technology also features a unique reflux control system that adapts to variations in feed composition, ensuring consistent isopentane purity above 99.5%[4]. The company has successfully implemented this technology in its large-scale olefin plants, demonstrating its robustness and reliability in continuous operation[6].
Strengths: High purity output, adaptable to feed variations, energy-efficient. Weaknesses: High capital costs for cryogenic equipment, potential safety concerns with low-temperature operations.
Innovative Separation Techniques for Isopentane
Cascaded methods and systems for enriching n-pentane in natural gas liquid feedstock
PatentWO2024141978A1
Innovation
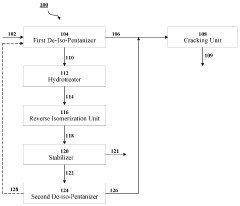
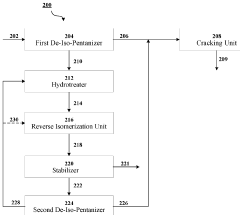
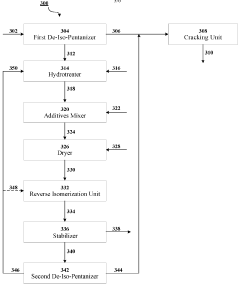
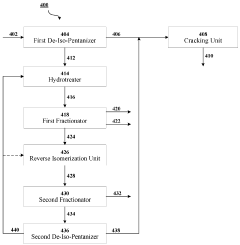
- A cascaded method involving de-iso-pentanization, hydrotreating, reverse isomerization, and fractionation to separate and enrich n-pentane from NGL feedstocks, followed by cracking to produce light olefins, with a system comprising de-iso-pentanizers, cracking furnaces, hydrotreating units, reverse isomerization units, and fractionators to enhance n-pentane concentration and olefin production.
Process for recovering isoprene from a C 5 hydrocarbon mixture
PatentInactiveEP0141316A2
Innovation
- The process involves separating the C5 hydrocarbon mixture by liquid-liquid extraction or extractive distillation using a selective solvent, followed by catalytic hydrogenation of the 1,3-pentadiene and cyclopentadiene stream, which is then fed back into the extraction process, allowing for lower temperatures and the separation of valuable hydrogenation products like pentane, pentenes, and cyclopentane.
Environmental Impact of Isopentane Recovery
The recovery of isopentane from chemical reaction byproduct streams has significant environmental implications. This process contributes to the reduction of volatile organic compound (VOC) emissions, which are known to have detrimental effects on air quality and human health. By capturing and reusing isopentane, industries can minimize their environmental footprint and comply with increasingly stringent environmental regulations.
One of the primary environmental benefits of isopentane recovery is the prevention of ground-level ozone formation. Isopentane, like other VOCs, can react with nitrogen oxides in the presence of sunlight to form smog, which is harmful to both human respiratory health and plant life. By implementing effective recovery systems, companies can significantly reduce their contribution to this environmental hazard.
Furthermore, the recovery process helps conserve natural resources. Isopentane is derived from petroleum, and its recovery allows for the reuse of this valuable hydrocarbon, reducing the demand for fresh raw materials. This circular approach aligns with sustainability principles and helps mitigate the environmental impact associated with the extraction and processing of fossil fuels.
The energy efficiency of isopentane recovery systems also plays a crucial role in their environmental impact. Advanced recovery technologies can be designed to minimize energy consumption, thereby reducing the overall carbon footprint of the chemical production process. This aspect is particularly important as industries strive to meet carbon reduction targets and transition towards more sustainable operations.
Water conservation is another environmental benefit of isopentane recovery. Some recovery methods may involve water-based processes, and by implementing closed-loop systems, water usage can be optimized, reducing the strain on local water resources. This is especially critical in water-stressed regions where industrial water consumption is a significant concern.
However, it is important to consider potential negative environmental impacts associated with isopentane recovery. The process may require additional energy inputs, which could offset some of the environmental gains if not managed properly. Additionally, the disposal or treatment of any waste products generated during the recovery process must be carefully handled to prevent secondary environmental issues.
In conclusion, the environmental impact of isopentane recovery in chemical reaction byproduct streams is largely positive when implemented with best practices. It offers a pathway to reduce air pollution, conserve resources, and improve overall environmental performance in chemical manufacturing processes. As technology advances, the potential for even greater environmental benefits through more efficient and sustainable recovery methods continues to grow.
One of the primary environmental benefits of isopentane recovery is the prevention of ground-level ozone formation. Isopentane, like other VOCs, can react with nitrogen oxides in the presence of sunlight to form smog, which is harmful to both human respiratory health and plant life. By implementing effective recovery systems, companies can significantly reduce their contribution to this environmental hazard.
Furthermore, the recovery process helps conserve natural resources. Isopentane is derived from petroleum, and its recovery allows for the reuse of this valuable hydrocarbon, reducing the demand for fresh raw materials. This circular approach aligns with sustainability principles and helps mitigate the environmental impact associated with the extraction and processing of fossil fuels.
The energy efficiency of isopentane recovery systems also plays a crucial role in their environmental impact. Advanced recovery technologies can be designed to minimize energy consumption, thereby reducing the overall carbon footprint of the chemical production process. This aspect is particularly important as industries strive to meet carbon reduction targets and transition towards more sustainable operations.
Water conservation is another environmental benefit of isopentane recovery. Some recovery methods may involve water-based processes, and by implementing closed-loop systems, water usage can be optimized, reducing the strain on local water resources. This is especially critical in water-stressed regions where industrial water consumption is a significant concern.
However, it is important to consider potential negative environmental impacts associated with isopentane recovery. The process may require additional energy inputs, which could offset some of the environmental gains if not managed properly. Additionally, the disposal or treatment of any waste products generated during the recovery process must be carefully handled to prevent secondary environmental issues.
In conclusion, the environmental impact of isopentane recovery in chemical reaction byproduct streams is largely positive when implemented with best practices. It offers a pathway to reduce air pollution, conserve resources, and improve overall environmental performance in chemical manufacturing processes. As technology advances, the potential for even greater environmental benefits through more efficient and sustainable recovery methods continues to grow.
Economic Feasibility of Recovery Processes
The economic feasibility of isopentane recovery processes in chemical reaction byproduct streams is a critical factor in determining the viability of implementing such systems. The primary consideration is the balance between the costs associated with recovery and the potential revenue generated from the recovered isopentane.
Capital expenditure (CAPEX) for isopentane recovery systems typically includes the installation of distillation columns, heat exchangers, storage tanks, and associated piping and instrumentation. The scale of the operation and the desired recovery rate significantly influence these initial costs. For large-scale chemical plants, the CAPEX can range from several hundred thousand to millions of dollars, depending on the complexity of the system and the purity requirements of the recovered isopentane.
Operating expenses (OPEX) are another crucial component of the economic analysis. These include energy costs for heating and cooling in the distillation process, maintenance of equipment, labor costs for operation and supervision, and any additional chemicals or materials required for the recovery process. The energy intensity of the recovery process is particularly significant, as distillation can be energy-intensive, especially when high purity is required.
The revenue potential from recovered isopentane is directly linked to market prices and demand. Isopentane has various applications, including use as a blowing agent in foam production, a propellant in aerosols, and a component in gasoline blending. The market value of isopentane can fluctuate based on factors such as crude oil prices and regulatory changes affecting its applications. As of recent market data, industrial-grade isopentane typically sells for $1,000 to $1,500 per metric ton, though this can vary significantly based on purity and market conditions.
The recovery rate and purity of the isopentane are critical factors affecting both costs and potential revenue. Higher recovery rates and purity levels generally increase both the capital and operating costs but also enhance the value of the recovered product. Typical recovery rates in well-designed systems can range from 85% to 98%, depending on the specific process and equipment used.
Payback period and return on investment (ROI) calculations are essential tools in assessing the economic feasibility. For isopentane recovery systems, payback periods can vary widely, typically ranging from 1 to 5 years, depending on the scale of operation, efficiency of the recovery process, and market conditions. Factors such as regulatory compliance benefits and potential carbon credit opportunities can also influence the overall economic assessment.
In conclusion, the economic feasibility of isopentane recovery processes depends on a complex interplay of factors including capital costs, operating expenses, recovery efficiency, market demand, and regulatory environment. A thorough cost-benefit analysis, considering both short-term and long-term economic impacts, is crucial for making informed decisions about implementing such recovery systems in chemical reaction byproduct streams.
Capital expenditure (CAPEX) for isopentane recovery systems typically includes the installation of distillation columns, heat exchangers, storage tanks, and associated piping and instrumentation. The scale of the operation and the desired recovery rate significantly influence these initial costs. For large-scale chemical plants, the CAPEX can range from several hundred thousand to millions of dollars, depending on the complexity of the system and the purity requirements of the recovered isopentane.
Operating expenses (OPEX) are another crucial component of the economic analysis. These include energy costs for heating and cooling in the distillation process, maintenance of equipment, labor costs for operation and supervision, and any additional chemicals or materials required for the recovery process. The energy intensity of the recovery process is particularly significant, as distillation can be energy-intensive, especially when high purity is required.
The revenue potential from recovered isopentane is directly linked to market prices and demand. Isopentane has various applications, including use as a blowing agent in foam production, a propellant in aerosols, and a component in gasoline blending. The market value of isopentane can fluctuate based on factors such as crude oil prices and regulatory changes affecting its applications. As of recent market data, industrial-grade isopentane typically sells for $1,000 to $1,500 per metric ton, though this can vary significantly based on purity and market conditions.
The recovery rate and purity of the isopentane are critical factors affecting both costs and potential revenue. Higher recovery rates and purity levels generally increase both the capital and operating costs but also enhance the value of the recovered product. Typical recovery rates in well-designed systems can range from 85% to 98%, depending on the specific process and equipment used.
Payback period and return on investment (ROI) calculations are essential tools in assessing the economic feasibility. For isopentane recovery systems, payback periods can vary widely, typically ranging from 1 to 5 years, depending on the scale of operation, efficiency of the recovery process, and market conditions. Factors such as regulatory compliance benefits and potential carbon credit opportunities can also influence the overall economic assessment.
In conclusion, the economic feasibility of isopentane recovery processes depends on a complex interplay of factors including capital costs, operating expenses, recovery efficiency, market demand, and regulatory environment. A thorough cost-benefit analysis, considering both short-term and long-term economic impacts, is crucial for making informed decisions about implementing such recovery systems in chemical reaction byproduct streams.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!