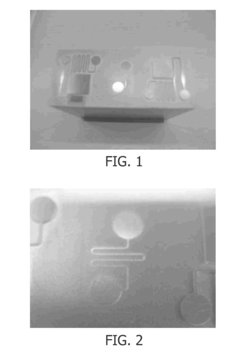
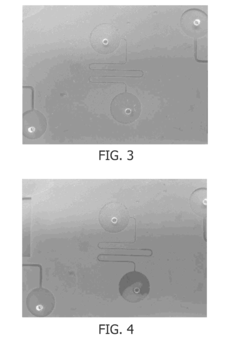
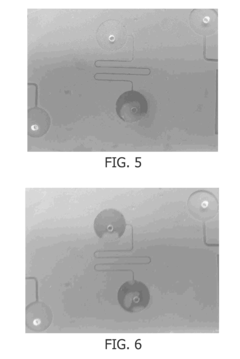
Silicone Rubber for Cutting-Edge Microfluidics Devices
JUL 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Silicone Rubber in Microfluidics: Evolution and Objectives
Silicone rubber has played a pivotal role in the evolution of microfluidics devices, revolutionizing the field since its inception in the 1990s. Initially developed for macro-scale applications, silicone rubber, particularly polydimethylsiloxane (PDMS), quickly became the material of choice for microfluidic chip fabrication due to its unique properties and versatility.
The journey of silicone rubber in microfluidics began with simple channel designs and has progressed to complex, multi-layered devices capable of performing intricate biological and chemical analyses. This evolution has been driven by the material's excellent optical transparency, gas permeability, and ease of fabrication, which align perfectly with the requirements of miniaturized fluidic systems.
As the field of microfluidics expanded, researchers and engineers continuously pushed the boundaries of silicone rubber applications. The material's flexibility allowed for the creation of integrated valves and pumps, enabling precise fluid control at the microscale. This capability opened up new possibilities for lab-on-a-chip devices and point-of-care diagnostics.
The objectives of silicone rubber research in cutting-edge microfluidics devices are multifaceted. One primary goal is to enhance the material's properties to meet the increasing demands of advanced applications. This includes improving chemical resistance, reducing non-specific adsorption of biomolecules, and enhancing long-term stability for prolonged use in biological environments.
Another crucial objective is to develop novel fabrication techniques that allow for even finer feature resolution and more complex 3D structures. Researchers are exploring methods such as 3D printing, multi-layer soft lithography, and hybrid material integration to create next-generation microfluidic devices with unprecedented functionality.
Furthermore, there is a growing focus on making silicone rubber-based microfluidic devices more accessible and scalable for industrial and clinical applications. This involves streamlining manufacturing processes, standardizing device designs, and developing automated assembly techniques to facilitate mass production and wider adoption of microfluidic technologies.
As we look to the future, the objectives of silicone rubber research in microfluidics are increasingly aligned with emerging fields such as organ-on-a-chip, personalized medicine, and environmental monitoring. The ultimate goal is to create highly integrated, multifunctional microfluidic platforms that can revolutionize healthcare, scientific research, and industrial processes, leveraging the unique properties of silicone rubber to their fullest potential.
The journey of silicone rubber in microfluidics began with simple channel designs and has progressed to complex, multi-layered devices capable of performing intricate biological and chemical analyses. This evolution has been driven by the material's excellent optical transparency, gas permeability, and ease of fabrication, which align perfectly with the requirements of miniaturized fluidic systems.
As the field of microfluidics expanded, researchers and engineers continuously pushed the boundaries of silicone rubber applications. The material's flexibility allowed for the creation of integrated valves and pumps, enabling precise fluid control at the microscale. This capability opened up new possibilities for lab-on-a-chip devices and point-of-care diagnostics.
The objectives of silicone rubber research in cutting-edge microfluidics devices are multifaceted. One primary goal is to enhance the material's properties to meet the increasing demands of advanced applications. This includes improving chemical resistance, reducing non-specific adsorption of biomolecules, and enhancing long-term stability for prolonged use in biological environments.
Another crucial objective is to develop novel fabrication techniques that allow for even finer feature resolution and more complex 3D structures. Researchers are exploring methods such as 3D printing, multi-layer soft lithography, and hybrid material integration to create next-generation microfluidic devices with unprecedented functionality.
Furthermore, there is a growing focus on making silicone rubber-based microfluidic devices more accessible and scalable for industrial and clinical applications. This involves streamlining manufacturing processes, standardizing device designs, and developing automated assembly techniques to facilitate mass production and wider adoption of microfluidic technologies.
As we look to the future, the objectives of silicone rubber research in microfluidics are increasingly aligned with emerging fields such as organ-on-a-chip, personalized medicine, and environmental monitoring. The ultimate goal is to create highly integrated, multifunctional microfluidic platforms that can revolutionize healthcare, scientific research, and industrial processes, leveraging the unique properties of silicone rubber to their fullest potential.
Market Analysis for Advanced Microfluidic Devices
The market for advanced microfluidic devices is experiencing rapid growth, driven by increasing demand in various sectors such as healthcare, pharmaceuticals, and life sciences. The global microfluidics market is projected to reach significant value in the coming years, with a compound annual growth rate (CAGR) exceeding industry averages. This growth is primarily attributed to the rising adoption of point-of-care testing, personalized medicine, and drug discovery applications.
In the healthcare sector, microfluidic devices are revolutionizing diagnostic procedures by enabling faster, more accurate, and cost-effective testing. The COVID-19 pandemic has further accelerated the adoption of microfluidic-based diagnostic tools, highlighting their importance in rapid and efficient disease detection. This trend is expected to continue, with microfluidic devices playing a crucial role in addressing future healthcare challenges.
The pharmaceutical industry is another key driver of market growth, with microfluidic technologies being increasingly utilized in drug discovery and development processes. These devices offer advantages such as reduced sample volumes, faster reaction times, and improved control over experimental conditions, leading to more efficient and cost-effective drug screening and testing procedures.
Academic and research institutions are also contributing to market expansion, as they continue to explore new applications and push the boundaries of microfluidic technology. This ongoing research is expected to open up new market opportunities and drive innovation in the field.
Geographically, North America currently holds the largest market share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is anticipated to witness the highest growth rate in the coming years, driven by increasing healthcare expenditure, growing research activities, and rising adoption of advanced technologies in countries like China, Japan, and India.
Key market players are focusing on product innovation and strategic collaborations to maintain their competitive edge. The development of integrated microfluidic systems, combining multiple functionalities on a single chip, is a notable trend that is expected to shape the market landscape in the near future.
Despite the positive outlook, challenges such as high initial costs, technical complexities, and the need for specialized expertise may hinder market growth to some extent. However, ongoing advancements in materials science, including research on silicone rubber for cutting-edge microfluidic devices, are expected to address these challenges and further propel market expansion.
In the healthcare sector, microfluidic devices are revolutionizing diagnostic procedures by enabling faster, more accurate, and cost-effective testing. The COVID-19 pandemic has further accelerated the adoption of microfluidic-based diagnostic tools, highlighting their importance in rapid and efficient disease detection. This trend is expected to continue, with microfluidic devices playing a crucial role in addressing future healthcare challenges.
The pharmaceutical industry is another key driver of market growth, with microfluidic technologies being increasingly utilized in drug discovery and development processes. These devices offer advantages such as reduced sample volumes, faster reaction times, and improved control over experimental conditions, leading to more efficient and cost-effective drug screening and testing procedures.
Academic and research institutions are also contributing to market expansion, as they continue to explore new applications and push the boundaries of microfluidic technology. This ongoing research is expected to open up new market opportunities and drive innovation in the field.
Geographically, North America currently holds the largest market share, followed by Europe and Asia-Pacific. However, the Asia-Pacific region is anticipated to witness the highest growth rate in the coming years, driven by increasing healthcare expenditure, growing research activities, and rising adoption of advanced technologies in countries like China, Japan, and India.
Key market players are focusing on product innovation and strategic collaborations to maintain their competitive edge. The development of integrated microfluidic systems, combining multiple functionalities on a single chip, is a notable trend that is expected to shape the market landscape in the near future.
Despite the positive outlook, challenges such as high initial costs, technical complexities, and the need for specialized expertise may hinder market growth to some extent. However, ongoing advancements in materials science, including research on silicone rubber for cutting-edge microfluidic devices, are expected to address these challenges and further propel market expansion.
Current Challenges in Silicone Rubber Microfluidics
Silicone rubber has emerged as a key material in the development of cutting-edge microfluidic devices. However, its widespread adoption and optimal performance face several significant challenges. One of the primary obstacles is the material's inherent hydrophobicity, which can lead to undesirable interactions with aqueous solutions commonly used in microfluidic applications. This property often results in non-specific adsorption of biomolecules and air bubble formation, compromising the efficiency and reliability of microfluidic operations.
Another critical challenge lies in the precision of feature replication during the fabrication process. While silicone rubber offers excellent flexibility and ease of molding, achieving consistent and high-resolution microstructures, especially at the sub-micron scale, remains problematic. This limitation can affect the functionality of complex microfluidic designs that require intricate channel geometries or precise flow control mechanisms.
The long-term stability of silicone rubber microfluidic devices presents an additional hurdle. Exposure to certain solvents and chemicals can cause swelling or degradation of the material, potentially altering the device's dimensions and compromising its performance over time. This susceptibility to chemical attack restricts the range of reagents that can be used in silicone-based microfluidic systems, limiting their applicability in certain chemical and biological assays.
Furthermore, the gas permeability of silicone rubber, while advantageous in some applications, poses challenges in maintaining stable environmental conditions within microfluidic channels. This property can lead to evaporation of small liquid volumes and changes in solution concentrations, affecting the reproducibility and reliability of experimental results.
The integration of additional functionalities, such as electrodes or sensors, into silicone rubber microfluidic devices also presents significant technical difficulties. The material's elastomeric nature and chemical composition make it challenging to achieve robust bonding with other materials or to incorporate conductive elements without compromising the device's integrity or performance.
Lastly, scaling up the production of silicone rubber microfluidic devices for commercial applications faces obstacles related to manufacturing consistency and cost-effectiveness. The current fabrication processes, often relying on manual steps, are not easily adaptable to high-volume production without sacrificing quality or increasing costs substantially.
Addressing these challenges requires interdisciplinary research efforts, combining materials science, surface chemistry, and advanced manufacturing techniques. Innovations in surface modification strategies, novel composite materials, and improved fabrication methods are crucial for overcoming the current limitations and unlocking the full potential of silicone rubber in next-generation microfluidic devices.
Another critical challenge lies in the precision of feature replication during the fabrication process. While silicone rubber offers excellent flexibility and ease of molding, achieving consistent and high-resolution microstructures, especially at the sub-micron scale, remains problematic. This limitation can affect the functionality of complex microfluidic designs that require intricate channel geometries or precise flow control mechanisms.
The long-term stability of silicone rubber microfluidic devices presents an additional hurdle. Exposure to certain solvents and chemicals can cause swelling or degradation of the material, potentially altering the device's dimensions and compromising its performance over time. This susceptibility to chemical attack restricts the range of reagents that can be used in silicone-based microfluidic systems, limiting their applicability in certain chemical and biological assays.
Furthermore, the gas permeability of silicone rubber, while advantageous in some applications, poses challenges in maintaining stable environmental conditions within microfluidic channels. This property can lead to evaporation of small liquid volumes and changes in solution concentrations, affecting the reproducibility and reliability of experimental results.
The integration of additional functionalities, such as electrodes or sensors, into silicone rubber microfluidic devices also presents significant technical difficulties. The material's elastomeric nature and chemical composition make it challenging to achieve robust bonding with other materials or to incorporate conductive elements without compromising the device's integrity or performance.
Lastly, scaling up the production of silicone rubber microfluidic devices for commercial applications faces obstacles related to manufacturing consistency and cost-effectiveness. The current fabrication processes, often relying on manual steps, are not easily adaptable to high-volume production without sacrificing quality or increasing costs substantially.
Addressing these challenges requires interdisciplinary research efforts, combining materials science, surface chemistry, and advanced manufacturing techniques. Innovations in surface modification strategies, novel composite materials, and improved fabrication methods are crucial for overcoming the current limitations and unlocking the full potential of silicone rubber in next-generation microfluidic devices.
Existing Silicone Rubber Solutions for Microfluidics
01 Composition and formulation of silicone rubber
Silicone rubber compositions typically include silicone polymers, fillers, and curing agents. The formulation can be adjusted to achieve specific properties such as hardness, elasticity, and heat resistance. Various additives may be incorporated to enhance performance characteristics or processing behavior.- Composition and preparation of silicone rubber: Silicone rubber is typically composed of silicone polymers, fillers, and curing agents. The preparation process often involves mixing these components, shaping the mixture, and then curing it to form the final rubber product. Various additives can be incorporated to enhance specific properties such as strength, flexibility, or heat resistance.
- Modification of silicone rubber properties: The properties of silicone rubber can be modified through the addition of specific compounds or by altering the polymer structure. This can include improving thermal stability, increasing electrical conductivity, enhancing mechanical strength, or adjusting the rubber's hardness. Such modifications allow for the customization of silicone rubber for various applications.
- Silicone rubber in medical and healthcare applications: Silicone rubber is widely used in medical and healthcare products due to its biocompatibility, flexibility, and durability. Applications include medical implants, prosthetics, drug delivery systems, and various medical devices. The material can be formulated to meet specific requirements for sterilization, long-term implantation, or controlled drug release.
- Silicone rubber composites and blends: Silicone rubber can be combined with other materials to create composites or blends with enhanced properties. This includes mixing with other polymers, incorporating nanoparticles, or adding reinforcing fibers. These composites can offer improved mechanical properties, thermal conductivity, or specific functional characteristics not achievable with pure silicone rubber.
- Manufacturing processes for silicone rubber products: Various manufacturing processes are employed to produce silicone rubber products, including injection molding, extrusion, compression molding, and 3D printing. Each process has specific advantages and is suited for different product types and production volumes. Advancements in manufacturing techniques focus on improving efficiency, reducing waste, and enabling the production of complex shapes and structures.
02 Manufacturing processes for silicone rubber products
Manufacturing methods for silicone rubber products include molding, extrusion, and casting techniques. The process may involve mixing components, shaping the material, and applying heat or other curing methods to achieve the final product form. Specialized equipment and controlled environments are often required for optimal production.Expand Specific Solutions03 Modifications and improvements to silicone rubber properties
Researchers continually work on modifying silicone rubber to enhance its properties. This may include improving heat resistance, increasing tensile strength, or enhancing chemical resistance. Techniques such as blending with other polymers, incorporating nanoparticles, or modifying the molecular structure are explored to achieve desired improvements.Expand Specific Solutions04 Applications of silicone rubber in various industries
Silicone rubber finds applications in diverse industries due to its unique properties. It is used in medical devices, automotive parts, electrical insulation, consumer products, and aerospace components. The material's biocompatibility, durability, and resistance to extreme temperatures make it suitable for a wide range of applications.Expand Specific Solutions05 Environmental and safety considerations in silicone rubber production
The production and use of silicone rubber involve considerations for environmental impact and safety. This includes developing eco-friendly manufacturing processes, ensuring the safety of workers handling the material, and addressing end-of-life disposal or recycling of silicone rubber products. Regulatory compliance and sustainable practices are increasingly important in this field.Expand Specific Solutions
Key Industry Players in Microfluidic Materials
The research on silicone rubber for cutting-edge microfluidics devices is in a rapidly evolving phase, with significant market potential due to increasing applications in healthcare, biotechnology, and analytical sciences. The global microfluidics market is experiencing robust growth, driven by technological advancements and rising demand for point-of-care diagnostics. Companies like Shin-Etsu Chemical, Wacker Chemie, and Dow Silicones are at the forefront, leveraging their expertise in silicone materials to develop innovative solutions. Academic institutions such as Northwestern University and École Normale Supérieure de Lyon are contributing to fundamental research, while collaborations between industry and academia are accelerating the technology's maturation and commercialization.
Shin-Etsu Chemical Co., Ltd.
Technical Solution: Shin-Etsu Chemical has developed advanced silicone rubber formulations specifically tailored for microfluidic devices. Their research focuses on improving the elastomeric properties and surface characteristics of silicone rubber to enhance its performance in microfluidic applications. The company has introduced a range of silicone elastomers with optimized viscosity, curing behavior, and mechanical properties[1]. These materials exhibit excellent chemical resistance and thermal stability, crucial for various microfluidic operations. Shin-Etsu has also developed specialized surface modification techniques to control the hydrophobicity/hydrophilicity of silicone rubber surfaces, enabling precise fluid control in microchannels[2]. Their latest innovations include silicone rubbers with reduced gas permeability and improved optical clarity, addressing key challenges in microfluidic device fabrication[3].
Strengths: Extensive experience in silicone chemistry, wide range of tailored formulations, and strong R&D capabilities. Weaknesses: Potential higher costs compared to generic alternatives and possible limitations in extreme miniaturization scenarios.
Corning, Inc.
Technical Solution: Corning has developed a proprietary silicone-based material platform for microfluidic devices, focusing on enhancing the optical and mechanical properties of silicone rubber. Their research has led to the creation of optically clear silicone elastomers with improved refractive index matching, crucial for high-resolution imaging in microfluidic applications[4]. Corning's technology also addresses the issue of silicone swelling in the presence of organic solvents, a common problem in microfluidic devices. They have engineered silicone rubbers with enhanced chemical resistance and reduced swelling, expanding the range of compatible solvents for microfluidic operations[5]. Additionally, Corning has developed novel surface functionalization methods for silicone rubber, enabling better control over protein adsorption and cell adhesion in bio-microfluidic devices[6].
Strengths: Strong expertise in materials science, particularly in optical materials and surface chemistry. Weaknesses: May have less specific focus on silicone rubber compared to dedicated silicone manufacturers.
Innovative Silicone Rubber Formulations for Microfluidics
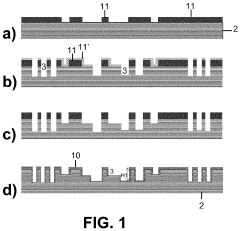
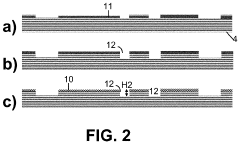
Method for Fabricating a Microfluidic Device
PatentPendingUS20210300752A1
Innovation
- A method involving direct bonding of silicon substrates with hydrophilic silicon oxide surfaces, followed by exposure to oxidative species and heat to form silicon oxide, which fills gaps and defects, enhancing fluid transport and channel sharpness.
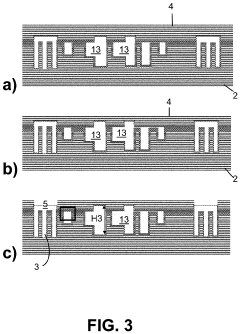
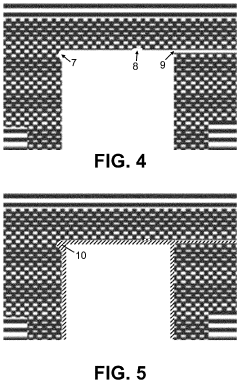
Microfluidic systems comprising a rubber material substrate
PatentInactiveUS20110294677A1
Innovation
- A microfluidic system using a rubber material with polar side groups linked via a linker comprising at least 6 atoms, which allows for capillary-driven fluid transport and self-sealing properties, reducing the need for valves, pressure chambers, and complex bonding methods, and enabling easier manufacturing and handling.
Regulatory Considerations for Microfluidic Devices
The regulatory landscape for microfluidic devices is complex and evolving, reflecting the rapid advancements in this field. As these devices increasingly incorporate silicone rubber components, manufacturers must navigate a multifaceted regulatory environment. In the United States, the Food and Drug Administration (FDA) oversees the approval process for microfluidic devices used in medical applications. These devices often fall under the category of in vitro diagnostic (IVD) devices, subject to the regulatory framework outlined in the Code of Federal Regulations Title 21.
The FDA's approach to regulating microfluidic devices depends on their intended use and risk classification. Low-risk devices may be eligible for the 510(k) clearance pathway, requiring demonstration of substantial equivalence to a predicate device. Higher-risk devices or those with novel applications may require the more rigorous Premarket Approval (PMA) process, involving clinical trials to establish safety and efficacy.
In the European Union, microfluidic devices must comply with the In Vitro Diagnostic Medical Devices Regulation (IVDR), which came into full effect in May 2022. This regulation introduces stricter requirements for clinical evidence, post-market surveillance, and traceability. Manufacturers must obtain CE marking to indicate compliance with EU health, safety, and environmental protection standards.
Regulatory bodies are particularly concerned with the biocompatibility and chemical inertness of materials used in microfluidic devices, including silicone rubber. ISO 10993 standards for biocompatibility testing are often referenced in regulatory submissions. Manufacturers must demonstrate that their silicone rubber formulations do not leach harmful substances or interfere with the device's analytical performance.
Quality management systems are crucial for regulatory compliance. Both the FDA and EU regulators expect manufacturers to implement robust quality systems, such as those outlined in ISO 13485 for medical devices. These systems must address design controls, risk management, and post-market surveillance specific to microfluidic technologies.
As microfluidic devices often handle sensitive biological samples, data privacy and security regulations like HIPAA in the US and GDPR in the EU may also apply. Manufacturers must ensure their devices and associated software systems comply with these data protection requirements.
The global nature of the microfluidics market necessitates consideration of international regulatory harmonization efforts. Initiatives like the Medical Device Single Audit Program (MDSAP) aim to streamline regulatory processes across multiple jurisdictions, potentially easing the burden on manufacturers seeking multi-market approvals.
The FDA's approach to regulating microfluidic devices depends on their intended use and risk classification. Low-risk devices may be eligible for the 510(k) clearance pathway, requiring demonstration of substantial equivalence to a predicate device. Higher-risk devices or those with novel applications may require the more rigorous Premarket Approval (PMA) process, involving clinical trials to establish safety and efficacy.
In the European Union, microfluidic devices must comply with the In Vitro Diagnostic Medical Devices Regulation (IVDR), which came into full effect in May 2022. This regulation introduces stricter requirements for clinical evidence, post-market surveillance, and traceability. Manufacturers must obtain CE marking to indicate compliance with EU health, safety, and environmental protection standards.
Regulatory bodies are particularly concerned with the biocompatibility and chemical inertness of materials used in microfluidic devices, including silicone rubber. ISO 10993 standards for biocompatibility testing are often referenced in regulatory submissions. Manufacturers must demonstrate that their silicone rubber formulations do not leach harmful substances or interfere with the device's analytical performance.
Quality management systems are crucial for regulatory compliance. Both the FDA and EU regulators expect manufacturers to implement robust quality systems, such as those outlined in ISO 13485 for medical devices. These systems must address design controls, risk management, and post-market surveillance specific to microfluidic technologies.
As microfluidic devices often handle sensitive biological samples, data privacy and security regulations like HIPAA in the US and GDPR in the EU may also apply. Manufacturers must ensure their devices and associated software systems comply with these data protection requirements.
The global nature of the microfluidics market necessitates consideration of international regulatory harmonization efforts. Initiatives like the Medical Device Single Audit Program (MDSAP) aim to streamline regulatory processes across multiple jurisdictions, potentially easing the burden on manufacturers seeking multi-market approvals.
Environmental Impact of Silicone Rubber in Microfluidics
The environmental impact of silicone rubber in microfluidics is a critical consideration as the technology continues to advance. Silicone rubber, particularly polydimethylsiloxane (PDMS), has become a ubiquitous material in microfluidic device fabrication due to its favorable properties. However, its widespread use raises concerns about potential environmental consequences.
One of the primary environmental advantages of silicone rubber in microfluidics is its durability and reusability. Microfluidic devices made from PDMS can often be cleaned and reused multiple times, reducing the need for frequent replacements and minimizing waste generation. This characteristic aligns well with sustainable manufacturing practices and helps to conserve resources in the long term.
Despite its benefits, the production of silicone rubber involves energy-intensive processes and the use of potentially harmful chemicals. The synthesis of PDMS requires the use of chlorosilanes and other organosilicon compounds, which can have negative environmental impacts if not properly managed. Additionally, the curing process of silicone rubber often involves the release of volatile organic compounds (VOCs), contributing to air pollution if not adequately controlled.
The disposal of silicone rubber microfluidic devices at the end of their lifecycle presents another environmental challenge. While silicone rubber is generally considered inert and non-toxic, it is not biodegradable. This means that discarded devices can persist in the environment for extended periods, potentially contributing to plastic pollution in landfills or marine ecosystems if not properly disposed of or recycled.
Efforts are being made to address these environmental concerns. Researchers are exploring more sustainable production methods for silicone rubber, including the use of renewable resources and greener synthesis processes. Some studies have focused on developing bio-based alternatives to traditional silicone rubber, aiming to reduce the reliance on petrochemical-derived materials.
The miniaturization of microfluidic devices using silicone rubber can lead to reduced reagent consumption and waste generation in various applications, such as chemical analysis and biological testing. This aspect contributes positively to the overall environmental footprint of laboratory processes and industrial applications that utilize microfluidic technology.
As the field of microfluidics continues to evolve, there is a growing emphasis on lifecycle assessment and sustainable design practices. Researchers and manufacturers are increasingly considering the environmental impact of materials and processes used in microfluidic device fabrication, including silicone rubber. This holistic approach aims to balance the performance benefits of silicone rubber with its environmental implications, driving innovation towards more sustainable solutions in cutting-edge microfluidics devices.
One of the primary environmental advantages of silicone rubber in microfluidics is its durability and reusability. Microfluidic devices made from PDMS can often be cleaned and reused multiple times, reducing the need for frequent replacements and minimizing waste generation. This characteristic aligns well with sustainable manufacturing practices and helps to conserve resources in the long term.
Despite its benefits, the production of silicone rubber involves energy-intensive processes and the use of potentially harmful chemicals. The synthesis of PDMS requires the use of chlorosilanes and other organosilicon compounds, which can have negative environmental impacts if not properly managed. Additionally, the curing process of silicone rubber often involves the release of volatile organic compounds (VOCs), contributing to air pollution if not adequately controlled.
The disposal of silicone rubber microfluidic devices at the end of their lifecycle presents another environmental challenge. While silicone rubber is generally considered inert and non-toxic, it is not biodegradable. This means that discarded devices can persist in the environment for extended periods, potentially contributing to plastic pollution in landfills or marine ecosystems if not properly disposed of or recycled.
Efforts are being made to address these environmental concerns. Researchers are exploring more sustainable production methods for silicone rubber, including the use of renewable resources and greener synthesis processes. Some studies have focused on developing bio-based alternatives to traditional silicone rubber, aiming to reduce the reliance on petrochemical-derived materials.
The miniaturization of microfluidic devices using silicone rubber can lead to reduced reagent consumption and waste generation in various applications, such as chemical analysis and biological testing. This aspect contributes positively to the overall environmental footprint of laboratory processes and industrial applications that utilize microfluidic technology.
As the field of microfluidics continues to evolve, there is a growing emphasis on lifecycle assessment and sustainable design practices. Researchers and manufacturers are increasingly considering the environmental impact of materials and processes used in microfluidic device fabrication, including silicone rubber. This holistic approach aims to balance the performance benefits of silicone rubber with its environmental implications, driving innovation towards more sustainable solutions in cutting-edge microfluidics devices.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!