Sodium silicate as a precursor for aerogel synthesis
AUG 21, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Aerogel Synthesis Background and Objectives
Aerogels represent a remarkable class of advanced materials characterized by their ultra-low density, high porosity, and exceptional thermal insulation properties. The synthesis of aerogels has been a subject of intense research and development since their discovery in the 1930s. Sodium silicate, also known as water glass, has emerged as a promising precursor for aerogel synthesis due to its availability, cost-effectiveness, and versatility.
The primary objective of this research is to explore and optimize the use of sodium silicate as a precursor for aerogel synthesis. This investigation aims to address several key aspects of the synthesis process, including the development of efficient and scalable production methods, enhancement of material properties, and expansion of potential applications.
Historically, aerogel synthesis has primarily relied on alkoxide precursors, which are often expensive and require careful handling due to their sensitivity to moisture. The shift towards sodium silicate as a precursor represents a significant advancement in making aerogel production more economically viable and environmentally friendly. This transition aligns with the growing demand for sustainable and cost-effective materials in various industries.
The evolution of aerogel synthesis techniques has been driven by the need to overcome challenges such as long processing times, high production costs, and limitations in scalability. Sodium silicate-based synthesis methods offer potential solutions to these issues, paving the way for broader industrial adoption of aerogels.
Current research efforts are focused on optimizing the sol-gel process using sodium silicate, with particular emphasis on controlling the gelation kinetics, pore structure, and surface chemistry of the resulting aerogels. Additionally, there is significant interest in developing hybrid aerogels by incorporating organic components or functional nanoparticles into the silica network, further expanding the range of achievable properties and applications.
The potential applications of aerogels synthesized from sodium silicate span a wide range of fields, including thermal insulation for buildings and aerospace, environmental remediation, catalysis, and energy storage. As such, this research has far-reaching implications for addressing global challenges in energy efficiency, environmental protection, and sustainable development.
By advancing the understanding and capabilities of sodium silicate-based aerogel synthesis, this research aims to contribute to the broader goal of making aerogels more accessible and applicable in everyday life. The successful development of efficient and scalable production methods could lead to a new generation of high-performance, environmentally friendly materials with the potential to revolutionize multiple industries.
The primary objective of this research is to explore and optimize the use of sodium silicate as a precursor for aerogel synthesis. This investigation aims to address several key aspects of the synthesis process, including the development of efficient and scalable production methods, enhancement of material properties, and expansion of potential applications.
Historically, aerogel synthesis has primarily relied on alkoxide precursors, which are often expensive and require careful handling due to their sensitivity to moisture. The shift towards sodium silicate as a precursor represents a significant advancement in making aerogel production more economically viable and environmentally friendly. This transition aligns with the growing demand for sustainable and cost-effective materials in various industries.
The evolution of aerogel synthesis techniques has been driven by the need to overcome challenges such as long processing times, high production costs, and limitations in scalability. Sodium silicate-based synthesis methods offer potential solutions to these issues, paving the way for broader industrial adoption of aerogels.
Current research efforts are focused on optimizing the sol-gel process using sodium silicate, with particular emphasis on controlling the gelation kinetics, pore structure, and surface chemistry of the resulting aerogels. Additionally, there is significant interest in developing hybrid aerogels by incorporating organic components or functional nanoparticles into the silica network, further expanding the range of achievable properties and applications.
The potential applications of aerogels synthesized from sodium silicate span a wide range of fields, including thermal insulation for buildings and aerospace, environmental remediation, catalysis, and energy storage. As such, this research has far-reaching implications for addressing global challenges in energy efficiency, environmental protection, and sustainable development.
By advancing the understanding and capabilities of sodium silicate-based aerogel synthesis, this research aims to contribute to the broader goal of making aerogels more accessible and applicable in everyday life. The successful development of efficient and scalable production methods could lead to a new generation of high-performance, environmentally friendly materials with the potential to revolutionize multiple industries.
Market Analysis for Sodium Silicate-based Aerogels
The market for sodium silicate-based aerogels is experiencing significant growth, driven by their unique properties and diverse applications across various industries. These lightweight, highly porous materials offer exceptional thermal insulation, acoustic dampening, and filtration capabilities, making them attractive for use in construction, aerospace, oil and gas, and environmental remediation sectors.
In the construction industry, sodium silicate-based aerogels are gaining traction as high-performance insulation materials. Their superior thermal insulation properties, combined with fire resistance and moisture management capabilities, position them as a premium alternative to traditional insulation materials. The growing emphasis on energy-efficient buildings and stringent building codes are expected to fuel demand in this sector.
The aerospace industry represents another key market for sodium silicate-based aerogels. Their ultra-low density and excellent thermal insulation properties make them ideal for use in aircraft and spacecraft components, where weight reduction and thermal management are critical. As the aerospace industry continues to focus on fuel efficiency and performance optimization, the demand for these advanced materials is projected to increase.
In the oil and gas sector, sodium silicate-based aerogels find applications in pipeline insulation, subsea equipment, and cryogenic insulation. Their ability to withstand extreme temperatures and pressures while providing effective thermal insulation makes them valuable in offshore and onshore operations. The ongoing exploration of deep-sea reserves and the need for energy-efficient transportation systems are driving market growth in this sector.
Environmental remediation is an emerging application area for sodium silicate-based aerogels. Their high surface area and adsorption capabilities make them effective in water purification, air filtration, and oil spill cleanup. As environmental regulations become more stringent and the need for sustainable solutions grows, the demand for these materials in environmental applications is expected to rise.
The market for sodium silicate-based aerogels is characterized by a mix of established players and innovative startups. Key market players are investing in research and development to enhance material properties, reduce production costs, and expand application areas. Collaborations between material manufacturers and end-users are becoming more common, driving innovation and market expansion.
While the market shows promising growth potential, challenges such as high production costs and limited awareness of aerogel benefits in certain industries persist. However, ongoing technological advancements and increasing focus on sustainability are expected to address these challenges and further drive market growth in the coming years.
In the construction industry, sodium silicate-based aerogels are gaining traction as high-performance insulation materials. Their superior thermal insulation properties, combined with fire resistance and moisture management capabilities, position them as a premium alternative to traditional insulation materials. The growing emphasis on energy-efficient buildings and stringent building codes are expected to fuel demand in this sector.
The aerospace industry represents another key market for sodium silicate-based aerogels. Their ultra-low density and excellent thermal insulation properties make them ideal for use in aircraft and spacecraft components, where weight reduction and thermal management are critical. As the aerospace industry continues to focus on fuel efficiency and performance optimization, the demand for these advanced materials is projected to increase.
In the oil and gas sector, sodium silicate-based aerogels find applications in pipeline insulation, subsea equipment, and cryogenic insulation. Their ability to withstand extreme temperatures and pressures while providing effective thermal insulation makes them valuable in offshore and onshore operations. The ongoing exploration of deep-sea reserves and the need for energy-efficient transportation systems are driving market growth in this sector.
Environmental remediation is an emerging application area for sodium silicate-based aerogels. Their high surface area and adsorption capabilities make them effective in water purification, air filtration, and oil spill cleanup. As environmental regulations become more stringent and the need for sustainable solutions grows, the demand for these materials in environmental applications is expected to rise.
The market for sodium silicate-based aerogels is characterized by a mix of established players and innovative startups. Key market players are investing in research and development to enhance material properties, reduce production costs, and expand application areas. Collaborations between material manufacturers and end-users are becoming more common, driving innovation and market expansion.
While the market shows promising growth potential, challenges such as high production costs and limited awareness of aerogel benefits in certain industries persist. However, ongoing technological advancements and increasing focus on sustainability are expected to address these challenges and further drive market growth in the coming years.
Current Challenges in Sodium Silicate Aerogel Production
The production of sodium silicate aerogels faces several significant challenges that hinder their widespread adoption and commercial viability. One of the primary obstacles is the high cost associated with the synthesis process. The precursor materials, particularly high-purity sodium silicate, can be expensive, and the multi-step production process requires specialized equipment and controlled environments, further driving up costs.
Another major challenge is the time-consuming nature of the synthesis process. Traditional methods often involve lengthy sol-gel transitions, aging periods, and drying stages, which can take days or even weeks to complete. This extended production time not only impacts manufacturing efficiency but also increases energy consumption and overall production costs.
The fragility and poor mechanical properties of sodium silicate aerogels present additional hurdles. These materials are known for their brittleness and low tensile strength, which limits their applications in scenarios where durability is crucial. Improving the mechanical robustness of aerogels without compromising their unique properties, such as low density and high porosity, remains a significant challenge for researchers and engineers.
Scalability is another critical issue facing sodium silicate aerogel production. While laboratory-scale synthesis can produce high-quality aerogels, translating these processes to industrial-scale manufacturing presents numerous difficulties. Maintaining consistent quality, controlling reaction parameters, and ensuring uniform drying across large volumes are complex tasks that require sophisticated process control and specialized equipment.
Environmental concerns also pose challenges in aerogel production. The use of certain solvents and chemicals in the synthesis process can have negative environmental impacts. Additionally, the high energy consumption associated with supercritical drying methods contributes to the carbon footprint of aerogel production. Developing more environmentally friendly synthesis routes and reducing energy requirements are important goals for sustainable aerogel manufacturing.
The shrinkage and cracking of aerogels during the drying process represent another significant challenge. As the liquid within the gel structure evaporates, capillary forces can cause the delicate network to collapse or develop cracks, compromising the integrity and properties of the final aerogel. Mitigating these effects while maintaining the desired porosity and surface area is a complex balancing act that researchers continue to address.
Lastly, the limited understanding of structure-property relationships in sodium silicate aerogels hinders targeted design and optimization. The complex interplay between processing parameters, nanostructure, and macroscopic properties is not fully elucidated, making it challenging to tailor aerogels for specific applications or to predict their performance under various conditions.
Another major challenge is the time-consuming nature of the synthesis process. Traditional methods often involve lengthy sol-gel transitions, aging periods, and drying stages, which can take days or even weeks to complete. This extended production time not only impacts manufacturing efficiency but also increases energy consumption and overall production costs.
The fragility and poor mechanical properties of sodium silicate aerogels present additional hurdles. These materials are known for their brittleness and low tensile strength, which limits their applications in scenarios where durability is crucial. Improving the mechanical robustness of aerogels without compromising their unique properties, such as low density and high porosity, remains a significant challenge for researchers and engineers.
Scalability is another critical issue facing sodium silicate aerogel production. While laboratory-scale synthesis can produce high-quality aerogels, translating these processes to industrial-scale manufacturing presents numerous difficulties. Maintaining consistent quality, controlling reaction parameters, and ensuring uniform drying across large volumes are complex tasks that require sophisticated process control and specialized equipment.
Environmental concerns also pose challenges in aerogel production. The use of certain solvents and chemicals in the synthesis process can have negative environmental impacts. Additionally, the high energy consumption associated with supercritical drying methods contributes to the carbon footprint of aerogel production. Developing more environmentally friendly synthesis routes and reducing energy requirements are important goals for sustainable aerogel manufacturing.
The shrinkage and cracking of aerogels during the drying process represent another significant challenge. As the liquid within the gel structure evaporates, capillary forces can cause the delicate network to collapse or develop cracks, compromising the integrity and properties of the final aerogel. Mitigating these effects while maintaining the desired porosity and surface area is a complex balancing act that researchers continue to address.
Lastly, the limited understanding of structure-property relationships in sodium silicate aerogels hinders targeted design and optimization. The complex interplay between processing parameters, nanostructure, and macroscopic properties is not fully elucidated, making it challenging to tailor aerogels for specific applications or to predict their performance under various conditions.
Existing Sodium Silicate Aerogel Synthesis Techniques
01 Sol-gel synthesis method
The sol-gel method is a common technique for synthesizing sodium silicate aerogels. This process involves the formation of a colloidal solution (sol) that gradually evolves towards the formation of a gel-like network. The method typically includes steps such as hydrolysis, condensation, aging, and drying. This technique allows for precise control over the pore structure and surface properties of the resulting aerogel.- Sol-gel synthesis method: The sol-gel method is a common technique for synthesizing sodium silicate aerogels. This process involves the formation of a colloidal solution (sol) that gradually evolves towards the formation of a gel-like network. The method typically includes steps such as hydrolysis, condensation, aging, and drying. It allows for precise control over the pore structure and surface properties of the resulting aerogel.
- Supercritical drying technique: Supercritical drying is a crucial step in aerogel synthesis to preserve the porous structure. This technique involves replacing the liquid in the gel with a supercritical fluid, typically carbon dioxide, which is then vented off as a gas. This process minimizes surface tension and prevents the collapse of the delicate pore structure, resulting in a highly porous aerogel with low density.
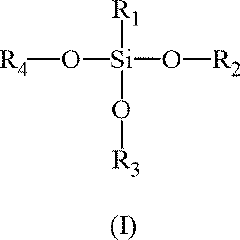
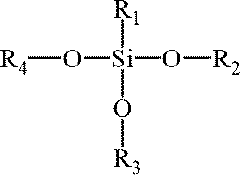
- Surface modification of aerogels: Surface modification techniques are employed to enhance the properties of sodium silicate aerogels. These methods can improve hydrophobicity, mechanical strength, or specific functionalities. Common approaches include silylation, polymer reinforcement, or the incorporation of functional groups. Such modifications can expand the range of applications for sodium silicate aerogels.
- Ambient pressure drying method: Ambient pressure drying is an alternative to supercritical drying for aerogel synthesis. This method involves modifying the gel's surface chemistry to reduce capillary pressure during drying, allowing the process to occur at atmospheric pressure. While it may result in some shrinkage, this technique is more cost-effective and easier to scale up compared to supercritical drying.
- Incorporation of additives and reinforcements: Various additives and reinforcements can be incorporated during the synthesis of sodium silicate aerogels to enhance their properties. These may include other metal oxides, polymers, or nanoparticles. Such additions can improve mechanical strength, thermal insulation properties, or introduce specific functionalities like catalytic activity or electromagnetic shielding.
02 Supercritical drying process
Supercritical drying is a crucial step in aerogel synthesis to preserve the porous structure. This process involves replacing the liquid in the gel with a supercritical fluid, typically carbon dioxide, which is then vented off as a gas. This method prevents the collapse of the delicate pore structure that would occur during conventional drying due to surface tension effects.Expand Specific Solutions03 Use of additives and modifiers
Various additives and modifiers can be incorporated during the synthesis process to enhance the properties of sodium silicate aerogels. These may include surfactants, catalysts, or other chemical agents that can influence the gel formation, pore structure, or surface characteristics of the final aerogel. The choice of additives can significantly impact the performance and application of the resulting material.Expand Specific Solutions04 Ambient pressure drying techniques
Alternative drying methods that operate at ambient pressure have been developed to simplify the aerogel synthesis process. These techniques often involve the use of surface modification agents or specific solvent exchange procedures to minimize capillary forces during drying. While potentially less energy-intensive than supercritical drying, these methods may result in some shrinkage or density changes in the final aerogel.Expand Specific Solutions05 Post-synthesis modifications
After the initial synthesis, sodium silicate aerogels can undergo various post-synthesis modifications to tailor their properties for specific applications. These modifications may include surface functionalization, thermal treatments, or the incorporation of additional materials into the aerogel structure. Such processes can enhance properties like hydrophobicity, mechanical strength, or thermal insulation capacity.Expand Specific Solutions
Key Players in Aerogel Research and Manufacturing
The research on sodium silicate as a precursor for aerogel synthesis is in a developing stage, with growing market potential due to aerogels' unique properties. The global aerogel market is expanding, driven by applications in various industries. Key players like Aspen Aerogels, LG Chem, and CNCEC Hualu New Materials are advancing the technology, while research institutions such as MIT, Newcastle University, and Zhejiang University contribute to innovation. The involvement of both established companies and academic institutions indicates a competitive landscape with opportunities for technological breakthroughs and market growth in this emerging field.
Aspen Aerogels, Inc.
Technical Solution: Aspen Aerogels has developed a proprietary process for synthesizing silica aerogels using sodium silicate as a precursor. Their method involves a sol-gel process where sodium silicate is first acidified to form silicic acid, which then undergoes polymerization and condensation to create a wet gel network. This gel is then subjected to supercritical drying to remove the liquid phase while preserving the nanoporous structure, resulting in a highly porous and lightweight aerogel[1]. The company has optimized this process to produce aerogels with superior thermal insulation properties, achieving thermal conductivities as low as 0.014 W/mK[2]. Aspen's aerogels are also engineered to be hydrophobic, enhancing their durability and performance in various applications[3].
Strengths: Scalable production process, excellent thermal insulation properties, and versatility in applications. Weaknesses: High production costs and energy-intensive manufacturing process.
Massachusetts Institute of Technology
Technical Solution: MIT researchers have pioneered advanced techniques for synthesizing aerogels from sodium silicate precursors. Their approach focuses on controlling the nanostructure of the aerogel during the sol-gel process by manipulating pH, temperature, and catalyst concentrations. This allows for precise tuning of pore size distribution and surface area, resulting in aerogels with tailored properties[4]. MIT has also developed a novel ambient pressure drying method that significantly reduces production costs compared to traditional supercritical drying[5]. Additionally, they have explored the incorporation of functional additives during synthesis to create multifunctional aerogels with enhanced mechanical strength and specific chemical properties[6].
Strengths: Cutting-edge research in nanostructure control and cost-effective production methods. Weaknesses: Some techniques may be challenging to scale up for industrial production.
Innovative Approaches in Sodium Silicate Precursor Utilization
Synthesis of silica airgel from sodium silicate solution at atmospheric pressure.
PatentActiveTH175734A
Innovation
- Synthesis of silica aerogel from sodium silicate solution without removing sodium ions first, allowing for a more streamlined process.
- Simultaneous surface functional group conversion and water replacement with n-hexane, leading to efficient hydrophobization.
- Atmospheric pressure synthesis resulting in high-quality aerogels with low density, high porosity, and large surface area without the need for supercritical drying.
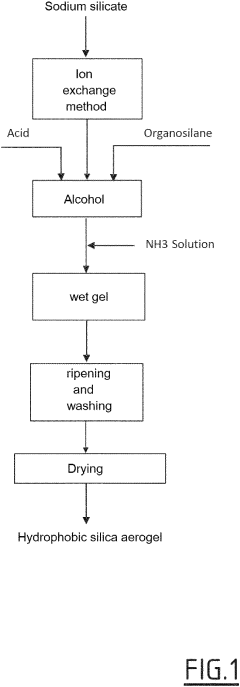
Process for synthesising a "one pot" hydrophobic silica aerogel from a silica precursor
PatentPendingUS20230227319A1
Innovation
- A 'one-pot' synthesis method using an aqueous silica precursor, ion exchanger resin-treated silicate solution, organosilane, and basic catalyst, followed by controlled drying to produce hydrophobic silica aerogels, reducing alkoxide precursor usage and synthesis time, suitable for industrial-scale preparation.
Environmental Impact of Sodium Silicate Aerogel Production
The production of sodium silicate aerogels has significant environmental implications that warrant careful consideration. The process involves the use of sodium silicate as a precursor, which is generally considered less harmful than some alternative materials. However, the production and disposal of sodium silicate aerogels still present environmental challenges that need to be addressed.
One of the primary environmental concerns is the energy consumption associated with the synthesis of sodium silicate aerogels. The process typically requires high temperatures and pressures, leading to substantial energy use and associated greenhouse gas emissions. This energy-intensive nature of production contributes to the carbon footprint of the final product, potentially offsetting some of its environmental benefits in applications such as thermal insulation.
Water usage is another critical environmental factor in the production of sodium silicate aerogels. The synthesis process often involves aqueous solutions, and subsequent washing and drying steps can consume significant amounts of water. This high water demand may strain local water resources, particularly in water-scarce regions, and necessitates proper wastewater treatment to prevent pollution.
The use of chemical additives and solvents in the production process also raises environmental concerns. While sodium silicate itself is relatively benign, other chemicals used in the synthesis, such as acid catalysts or organic solvents for surface modification, may pose risks if not properly managed. These substances can potentially contaminate soil and water if released into the environment, highlighting the importance of proper handling and disposal practices.
Waste generation is an additional environmental aspect to consider. The production of sodium silicate aerogels may result in by-products or unused materials that require appropriate disposal. Improper management of these wastes can lead to soil and water pollution, emphasizing the need for efficient recycling and waste reduction strategies in the manufacturing process.
On a positive note, the lightweight nature of sodium silicate aerogels can contribute to reduced transportation-related emissions when compared to heavier alternative materials. Additionally, their excellent insulation properties can lead to energy savings in various applications, potentially offsetting some of the environmental impacts associated with their production.
The end-of-life disposal of sodium silicate aerogels is another environmental consideration. While these materials are generally non-toxic, their disposal in landfills may not be the most environmentally friendly option. Research into recycling and reuse possibilities for spent aerogels could help mitigate this issue and promote a more circular economy approach to their lifecycle.
One of the primary environmental concerns is the energy consumption associated with the synthesis of sodium silicate aerogels. The process typically requires high temperatures and pressures, leading to substantial energy use and associated greenhouse gas emissions. This energy-intensive nature of production contributes to the carbon footprint of the final product, potentially offsetting some of its environmental benefits in applications such as thermal insulation.
Water usage is another critical environmental factor in the production of sodium silicate aerogels. The synthesis process often involves aqueous solutions, and subsequent washing and drying steps can consume significant amounts of water. This high water demand may strain local water resources, particularly in water-scarce regions, and necessitates proper wastewater treatment to prevent pollution.
The use of chemical additives and solvents in the production process also raises environmental concerns. While sodium silicate itself is relatively benign, other chemicals used in the synthesis, such as acid catalysts or organic solvents for surface modification, may pose risks if not properly managed. These substances can potentially contaminate soil and water if released into the environment, highlighting the importance of proper handling and disposal practices.
Waste generation is an additional environmental aspect to consider. The production of sodium silicate aerogels may result in by-products or unused materials that require appropriate disposal. Improper management of these wastes can lead to soil and water pollution, emphasizing the need for efficient recycling and waste reduction strategies in the manufacturing process.
On a positive note, the lightweight nature of sodium silicate aerogels can contribute to reduced transportation-related emissions when compared to heavier alternative materials. Additionally, their excellent insulation properties can lead to energy savings in various applications, potentially offsetting some of the environmental impacts associated with their production.
The end-of-life disposal of sodium silicate aerogels is another environmental consideration. While these materials are generally non-toxic, their disposal in landfills may not be the most environmentally friendly option. Research into recycling and reuse possibilities for spent aerogels could help mitigate this issue and promote a more circular economy approach to their lifecycle.
Scalability and Cost Analysis of Synthesis Methods
The scalability and cost analysis of sodium silicate-based aerogel synthesis methods is crucial for determining their industrial viability and potential for large-scale production. Traditional sol-gel processes using sodium silicate as a precursor have shown promising results in laboratory settings, but face challenges when scaled up to industrial levels.
One of the primary advantages of using sodium silicate as a precursor is its relatively low cost compared to other silica sources, such as tetraethyl orthosilicate (TEOS). This cost-effectiveness is particularly important for large-scale production, as raw material expenses significantly impact the overall manufacturing costs. However, the scalability of sodium silicate-based processes is limited by several factors.
The gelation time and drying process are critical aspects that affect scalability. Sodium silicate gels typically require longer gelation times compared to TEOS-based gels, which can lead to increased production cycles and reduced throughput. Additionally, the drying process, often involving supercritical drying, is time-consuming and energy-intensive, further impacting production efficiency and costs.
To address these challenges, researchers have explored various modifications to the synthesis process. One approach involves the use of catalysts to accelerate gelation and reduce processing time. Another strategy focuses on optimizing the drying process through the development of ambient pressure drying techniques, which could significantly reduce energy consumption and production costs.
The scalability of sodium silicate-based aerogel synthesis is also influenced by the equipment and infrastructure required. While the initial setup costs may be lower compared to other precursors, the need for specialized equipment for supercritical drying can be a significant investment. However, recent advancements in continuous flow reactors and spray drying techniques show promise for improving process efficiency and reducing equipment costs.
From a cost perspective, the use of sodium silicate offers advantages in terms of raw material expenses, but the overall production costs must consider factors such as energy consumption, processing time, and equipment maintenance. A comprehensive cost analysis should also account for potential variations in sodium silicate quality and availability, as these factors can impact the consistency and scalability of the production process.
In conclusion, while sodium silicate presents an attractive option for cost-effective aerogel synthesis, significant challenges remain in scaling up production to industrial levels. Ongoing research and development efforts are focused on addressing these challenges through process optimization, innovative drying techniques, and the development of more efficient production equipment. The successful resolution of these scalability and cost issues will be crucial for the widespread adoption of sodium silicate-based aerogels in various industrial applications.
One of the primary advantages of using sodium silicate as a precursor is its relatively low cost compared to other silica sources, such as tetraethyl orthosilicate (TEOS). This cost-effectiveness is particularly important for large-scale production, as raw material expenses significantly impact the overall manufacturing costs. However, the scalability of sodium silicate-based processes is limited by several factors.
The gelation time and drying process are critical aspects that affect scalability. Sodium silicate gels typically require longer gelation times compared to TEOS-based gels, which can lead to increased production cycles and reduced throughput. Additionally, the drying process, often involving supercritical drying, is time-consuming and energy-intensive, further impacting production efficiency and costs.
To address these challenges, researchers have explored various modifications to the synthesis process. One approach involves the use of catalysts to accelerate gelation and reduce processing time. Another strategy focuses on optimizing the drying process through the development of ambient pressure drying techniques, which could significantly reduce energy consumption and production costs.
The scalability of sodium silicate-based aerogel synthesis is also influenced by the equipment and infrastructure required. While the initial setup costs may be lower compared to other precursors, the need for specialized equipment for supercritical drying can be a significant investment. However, recent advancements in continuous flow reactors and spray drying techniques show promise for improving process efficiency and reducing equipment costs.
From a cost perspective, the use of sodium silicate offers advantages in terms of raw material expenses, but the overall production costs must consider factors such as energy consumption, processing time, and equipment maintenance. A comprehensive cost analysis should also account for potential variations in sodium silicate quality and availability, as these factors can impact the consistency and scalability of the production process.
In conclusion, while sodium silicate presents an attractive option for cost-effective aerogel synthesis, significant challenges remain in scaling up production to industrial levels. Ongoing research and development efforts are focused on addressing these challenges through process optimization, innovative drying techniques, and the development of more efficient production equipment. The successful resolution of these scalability and cost issues will be crucial for the widespread adoption of sodium silicate-based aerogels in various industrial applications.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!