Triton X-100 as a Facilitator in Hematological Sample Preparation
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Triton X-100 in Hematology: Background and Objectives
Triton X-100, a nonionic surfactant, has emerged as a crucial component in hematological sample preparation over the past few decades. Its unique chemical properties, including its ability to solubilize proteins and lyse cell membranes, have made it an indispensable tool in various hematological applications. The evolution of Triton X-100 usage in hematology can be traced back to the 1960s when researchers first recognized its potential in blood sample processing.
The primary objective of utilizing Triton X-100 in hematological sample preparation is to enhance the accuracy and efficiency of blood analysis. By facilitating the lysis of red blood cells and stabilizing white blood cells, Triton X-100 enables more precise cell counting and differentiation. This is particularly crucial in diagnostic procedures, where the accurate quantification of blood cell populations is essential for identifying various hematological disorders.
As hematology techniques have advanced, the role of Triton X-100 has expanded beyond basic cell lysis. It now plays a significant part in flow cytometry, where it aids in permeabilizing cell membranes to allow intracellular staining. This application has revolutionized the field of immunophenotyping, enabling researchers and clinicians to gain deeper insights into cellular characteristics and functions.
The ongoing research on Triton X-100 in hematology aims to optimize its use in sample preparation protocols. Scientists are exploring the ideal concentrations and incubation times for different types of blood samples, as well as investigating potential synergistic effects when combined with other reagents. These efforts are driven by the need for more standardized and reproducible methods in clinical laboratories worldwide.
Another key objective in the research of Triton X-100 is to address potential limitations and side effects. While generally considered safe for laboratory use, there are concerns about its impact on certain cellular components and its potential to interfere with specific analytical techniques. Researchers are working to develop modified versions or alternative surfactants that maintain the beneficial properties of Triton X-100 while minimizing any adverse effects.
The environmental impact of Triton X-100 has also become a focus of recent research. As a non-biodegradable compound, its widespread use in laboratories raises concerns about long-term environmental accumulation. This has spurred investigations into more eco-friendly alternatives and the development of disposal methods that minimize environmental contamination.
The primary objective of utilizing Triton X-100 in hematological sample preparation is to enhance the accuracy and efficiency of blood analysis. By facilitating the lysis of red blood cells and stabilizing white blood cells, Triton X-100 enables more precise cell counting and differentiation. This is particularly crucial in diagnostic procedures, where the accurate quantification of blood cell populations is essential for identifying various hematological disorders.
As hematology techniques have advanced, the role of Triton X-100 has expanded beyond basic cell lysis. It now plays a significant part in flow cytometry, where it aids in permeabilizing cell membranes to allow intracellular staining. This application has revolutionized the field of immunophenotyping, enabling researchers and clinicians to gain deeper insights into cellular characteristics and functions.
The ongoing research on Triton X-100 in hematology aims to optimize its use in sample preparation protocols. Scientists are exploring the ideal concentrations and incubation times for different types of blood samples, as well as investigating potential synergistic effects when combined with other reagents. These efforts are driven by the need for more standardized and reproducible methods in clinical laboratories worldwide.
Another key objective in the research of Triton X-100 is to address potential limitations and side effects. While generally considered safe for laboratory use, there are concerns about its impact on certain cellular components and its potential to interfere with specific analytical techniques. Researchers are working to develop modified versions or alternative surfactants that maintain the beneficial properties of Triton X-100 while minimizing any adverse effects.
The environmental impact of Triton X-100 has also become a focus of recent research. As a non-biodegradable compound, its widespread use in laboratories raises concerns about long-term environmental accumulation. This has spurred investigations into more eco-friendly alternatives and the development of disposal methods that minimize environmental contamination.
Market Analysis for Hematological Sample Preparation
The hematological sample preparation market has been experiencing significant growth due to the increasing prevalence of blood disorders and the rising demand for advanced diagnostic techniques. The global market for hematology analyzers and reagents is projected to reach substantial figures in the coming years, driven by factors such as the growing aging population and the rising incidence of blood-related diseases.
Triton X-100, a nonionic surfactant, plays a crucial role in this market as a facilitator in hematological sample preparation. Its ability to lyse red blood cells while preserving leukocytes makes it an essential component in many hematology analyzers and reagent systems. The demand for Triton X-100 in this application is closely tied to the overall growth of the hematology diagnostics market.
The market for hematological sample preparation is segmented based on product type, end-user, and geography. Product types include instruments, reagents, and consumables, with reagents holding a significant market share due to their recurring nature. End-users primarily consist of hospitals, diagnostic laboratories, and research institutions.
Geographically, North America and Europe dominate the market due to well-established healthcare infrastructure and higher adoption rates of advanced diagnostic technologies. However, the Asia-Pacific region is expected to witness the fastest growth, attributed to improving healthcare facilities, increasing healthcare expenditure, and rising awareness about early disease diagnosis.
Key market trends include the shift towards automation in hematology laboratories, which enhances efficiency and reduces human error. This trend is driving the demand for advanced hematology analyzers that incorporate Triton X-100 in their reagent systems. Additionally, there is a growing focus on point-of-care testing, which is creating new opportunities for compact and portable hematology devices.
The competitive landscape of the hematological sample preparation market is characterized by the presence of several major players and numerous smaller companies. Leading companies are investing heavily in research and development to introduce innovative products and maintain their market position. Collaborations between diagnostic equipment manufacturers and reagent suppliers are also becoming more common, aiming to offer integrated solutions to end-users.
Challenges in the market include stringent regulatory requirements for diagnostic products and the high cost of advanced hematology analyzers, which may limit adoption in developing regions. However, the increasing focus on personalized medicine and the integration of artificial intelligence in hematology diagnostics are expected to create new growth opportunities in the coming years.
Triton X-100, a nonionic surfactant, plays a crucial role in this market as a facilitator in hematological sample preparation. Its ability to lyse red blood cells while preserving leukocytes makes it an essential component in many hematology analyzers and reagent systems. The demand for Triton X-100 in this application is closely tied to the overall growth of the hematology diagnostics market.
The market for hematological sample preparation is segmented based on product type, end-user, and geography. Product types include instruments, reagents, and consumables, with reagents holding a significant market share due to their recurring nature. End-users primarily consist of hospitals, diagnostic laboratories, and research institutions.
Geographically, North America and Europe dominate the market due to well-established healthcare infrastructure and higher adoption rates of advanced diagnostic technologies. However, the Asia-Pacific region is expected to witness the fastest growth, attributed to improving healthcare facilities, increasing healthcare expenditure, and rising awareness about early disease diagnosis.
Key market trends include the shift towards automation in hematology laboratories, which enhances efficiency and reduces human error. This trend is driving the demand for advanced hematology analyzers that incorporate Triton X-100 in their reagent systems. Additionally, there is a growing focus on point-of-care testing, which is creating new opportunities for compact and portable hematology devices.
The competitive landscape of the hematological sample preparation market is characterized by the presence of several major players and numerous smaller companies. Leading companies are investing heavily in research and development to introduce innovative products and maintain their market position. Collaborations between diagnostic equipment manufacturers and reagent suppliers are also becoming more common, aiming to offer integrated solutions to end-users.
Challenges in the market include stringent regulatory requirements for diagnostic products and the high cost of advanced hematology analyzers, which may limit adoption in developing regions. However, the increasing focus on personalized medicine and the integration of artificial intelligence in hematology diagnostics are expected to create new growth opportunities in the coming years.
Current Challenges in Sample Preparation Techniques
The field of hematological sample preparation faces several significant challenges that impede the efficiency and accuracy of diagnostic processes. One of the primary issues is the complexity of blood samples, which contain a diverse array of cellular components and proteins. This heterogeneity often leads to difficulties in isolating specific cell types or biomarkers of interest, requiring sophisticated separation techniques that can be time-consuming and labor-intensive.
Another critical challenge is the preservation of sample integrity during the preparation process. Blood cells, particularly leukocytes, are highly sensitive to environmental changes and can rapidly degrade or alter their characteristics once drawn from the body. This necessitates swift processing and the use of specialized preservatives, which can complicate standardization efforts across different laboratories and healthcare settings.
The presence of interfering substances in blood samples poses yet another hurdle. Lipids, proteins, and other biomolecules can interfere with downstream analytical techniques, leading to inaccurate results or false positives. Developing effective methods to remove these interferents without compromising the target analytes remains an ongoing challenge in the field.
Automation of sample preparation processes is an area of significant interest, but it comes with its own set of challenges. While automated systems can improve throughput and reduce human error, they often struggle with the variability inherent in biological samples. Adapting these systems to handle diverse sample types and volumes while maintaining precision is a complex task that requires continuous refinement.
The need for miniaturization and point-of-care testing has also introduced new challenges in sample preparation. Developing techniques that can effectively process small sample volumes while maintaining sensitivity and specificity is crucial for advancing portable diagnostic devices. This often requires innovative approaches to sample concentration and purification within confined spaces.
Standardization across different laboratories and healthcare settings remains a persistent challenge. Variations in sample preparation protocols can lead to discrepancies in test results, complicating inter-laboratory comparisons and potentially affecting patient care. Establishing universally accepted standards for sample preparation, particularly for novel biomarkers or rare cell populations, is an ongoing effort in the field.
In the context of using Triton X-100 as a facilitator in hematological sample preparation, specific challenges arise. While Triton X-100 is effective in lysing cells and solubilizing membrane proteins, its use can sometimes lead to the denaturation of certain proteins of interest. Balancing the concentration of Triton X-100 to achieve optimal lysis without compromising the integrity of target molecules requires careful optimization. Additionally, the removal of Triton X-100 from samples post-preparation can be challenging, potentially interfering with downstream applications such as mass spectrometry or certain immunoassays.
Another critical challenge is the preservation of sample integrity during the preparation process. Blood cells, particularly leukocytes, are highly sensitive to environmental changes and can rapidly degrade or alter their characteristics once drawn from the body. This necessitates swift processing and the use of specialized preservatives, which can complicate standardization efforts across different laboratories and healthcare settings.
The presence of interfering substances in blood samples poses yet another hurdle. Lipids, proteins, and other biomolecules can interfere with downstream analytical techniques, leading to inaccurate results or false positives. Developing effective methods to remove these interferents without compromising the target analytes remains an ongoing challenge in the field.
Automation of sample preparation processes is an area of significant interest, but it comes with its own set of challenges. While automated systems can improve throughput and reduce human error, they often struggle with the variability inherent in biological samples. Adapting these systems to handle diverse sample types and volumes while maintaining precision is a complex task that requires continuous refinement.
The need for miniaturization and point-of-care testing has also introduced new challenges in sample preparation. Developing techniques that can effectively process small sample volumes while maintaining sensitivity and specificity is crucial for advancing portable diagnostic devices. This often requires innovative approaches to sample concentration and purification within confined spaces.
Standardization across different laboratories and healthcare settings remains a persistent challenge. Variations in sample preparation protocols can lead to discrepancies in test results, complicating inter-laboratory comparisons and potentially affecting patient care. Establishing universally accepted standards for sample preparation, particularly for novel biomarkers or rare cell populations, is an ongoing effort in the field.
In the context of using Triton X-100 as a facilitator in hematological sample preparation, specific challenges arise. While Triton X-100 is effective in lysing cells and solubilizing membrane proteins, its use can sometimes lead to the denaturation of certain proteins of interest. Balancing the concentration of Triton X-100 to achieve optimal lysis without compromising the integrity of target molecules requires careful optimization. Additionally, the removal of Triton X-100 from samples post-preparation can be challenging, potentially interfering with downstream applications such as mass spectrometry or certain immunoassays.
Triton X-100 Applications in Sample Preparation
01 Use of Triton X-100 in biochemical applications
Triton X-100 is widely used in various biochemical applications, particularly in cell lysis and protein extraction processes. It is an effective non-ionic detergent that can solubilize membrane proteins and disrupt cell membranes without denaturing proteins. This makes it valuable in research and diagnostic applications involving protein isolation and purification.- Use in cell lysis and protein extraction: Triton X-100 is widely used as a detergent for cell lysis and protein extraction in biochemical research. It effectively solubilizes cell membranes and helps release intracellular proteins while maintaining their native structure. This non-ionic surfactant is particularly useful in isolating membrane-bound proteins and preparing samples for various analytical techniques.
- Application in nucleic acid isolation: Triton X-100 plays a crucial role in nucleic acid isolation protocols. It aids in the disruption of cell membranes and nuclear envelopes, facilitating the release of DNA and RNA. The surfactant properties of Triton X-100 help to separate nucleic acids from other cellular components, improving the purity and yield of isolated genetic material.
- Use in enzyme assays and protein studies: Triton X-100 is employed in various enzyme assays and protein studies. It helps maintain enzyme activity by preventing protein aggregation and stabilizing protein structures. The surfactant can also be used to solubilize membrane-bound enzymes, allowing for their characterization and functional studies in solution.
- Application in membrane protein solubilization: Triton X-100 is effective in solubilizing membrane proteins while preserving their native structure and function. This property makes it valuable in studying integral membrane proteins, ion channels, and receptors. The surfactant helps extract these proteins from lipid bilayers, enabling their purification and subsequent analysis.
- Use in industrial and cleaning applications: Beyond its biochemical applications, Triton X-100 finds use in various industrial and cleaning processes. It serves as an effective emulsifier, wetting agent, and dispersant in formulations for paints, coatings, and cleaning products. The surfactant's ability to reduce surface tension makes it useful in enhancing the performance of these products.
02 Triton X-100 in analytical methods and assays
Triton X-100 is utilized in various analytical methods and assays, including enzyme-linked immunosorbent assays (ELISA) and polymerase chain reaction (PCR) techniques. It helps to reduce non-specific binding and improve the sensitivity and specificity of these tests. The surfactant properties of Triton X-100 also make it useful in sample preparation and as a component in buffer solutions for analytical procedures.Expand Specific Solutions03 Application of Triton X-100 in industrial processes
Triton X-100 finds applications in various industrial processes due to its excellent emulsifying and dispersing properties. It is used in the formulation of cleaning products, paints, and coatings. In the textile industry, it serves as a wetting agent and emulsifier. The surfactant is also employed in the production of nanoparticles and in certain polymerization processes.Expand Specific Solutions04 Triton X-100 in pharmaceutical and cosmetic formulations
Triton X-100 is used in pharmaceutical and cosmetic formulations as a solubilizing agent and emulsifier. It helps to improve the stability and bioavailability of certain drug formulations. In cosmetics, it can be found in some cleansers and personal care products. However, its use in these applications is subject to regulatory guidelines and concentration limits.Expand Specific Solutions05 Environmental and safety considerations of Triton X-100
While Triton X-100 is widely used, there are environmental and safety considerations associated with its use. It can be toxic to aquatic organisms and may persist in the environment. Efforts are being made to develop more environmentally friendly alternatives. In laboratory and industrial settings, proper handling and disposal procedures are necessary to minimize environmental impact and ensure worker safety.Expand Specific Solutions
Key Players in Hematology Reagent Industry
The research on Triton X-100 as a facilitator in hematological sample preparation is in a developing stage, with a growing market due to increasing demand for efficient blood sample processing in clinical diagnostics. The technology's maturity is moderate, with ongoing advancements from key players. Companies like Sunshine Lake Pharma, Origissay Diagnostics, and Sekisui Chemical are actively contributing to the field, while research institutions such as Korea Research Institute of Chemical Technology and Yissum Research Development are driving innovation. The competitive landscape is diverse, including both established pharmaceutical companies and specialized biotechnology firms, indicating a dynamic and evolving market with potential for further growth and technological improvements.
Sekisui Chemical Co., Ltd.
Technical Solution: Sekisui Chemical has developed advanced hematology reagents and systems that utilize Triton X-100 in various sample preparation steps. Their technology incorporates Triton X-100 in both lysing and permeabilizing solutions for blood cell analysis. Sekisui's approach optimizes the concentration and combination of Triton X-100 with other surfactants to achieve efficient red blood cell lysis while preserving white blood cell morphology[9]. This allows for accurate differentiation and counting of white blood cell subpopulations, including the challenging separation of basophils and other granulocytes. Additionally, Sekisui has developed specialized reagents using Triton X-100 for flow cytometry applications, enabling intracellular staining of various blood cell types[10]. Their technology has been implemented in automated hematology analyzers, offering high-throughput capabilities for clinical laboratories.
Strengths: Efficient red blood cell lysis, accurate white blood cell differentiation, and compatibility with both automated hematology analyzers and flow cytometry. Weaknesses: Potential interference with certain cellular markers and need for careful optimization of reagent formulations.
Thrombinoscope BV
Technical Solution: Thrombinoscope BV has developed a specialized technology for assessing blood coagulation dynamics using Triton X-100 as a facilitator. Their Calibrated Automated Thrombogram (CAT) system incorporates Triton X-100 in the preparation of platelet-rich plasma samples for thrombin generation assays[5]. The company's method utilizes a carefully optimized concentration of Triton X-100 to partially lyse platelets, exposing phosphatidylserine and enhancing the procoagulant activity of the sample. This approach allows for more sensitive and reproducible measurements of thrombin generation, providing valuable insights into hemostatic disorders and anticoagulant therapies[6]. Thrombinoscope's technology has been widely adopted in clinical research and is increasingly used in routine diagnostic settings.
Strengths: Enhanced sensitivity in thrombin generation assays, improved reproducibility in coagulation studies, and applicability to both research and clinical diagnostics. Weaknesses: Specialized application limited to coagulation studies and potential variability in results depending on sample handling.
Innovations in Triton X-100 Formulations
Substituted benzoindoles as SPLA2 inhibitors
PatentWO2002050028A8
Innovation
- Development of novel benz[f]indole compounds that selectively inhibit mammalian SPLA2, thereby preventing the release of fatty acids and their deleterious products, which are used in pharmaceutical compositions for treating inflammatory diseases.
Detergent and method for purifying a biotherapeutic
PatentPendingUS20240327454A1
Innovation
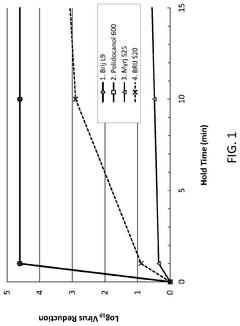
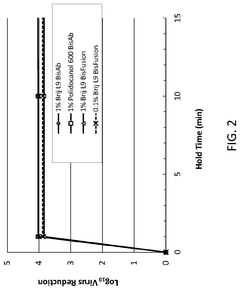
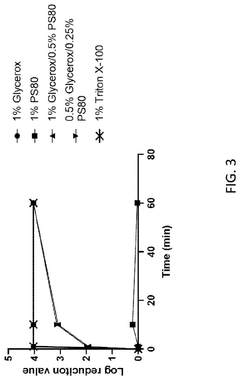
- The use of Laureth-9 as an environmentally compatible detergent for viral inactivation, cell lysis, and removal of impurities such as host cell proteins and endotoxins, which does not adversely impact product quality, is proposed. Laureth-9 is incorporated into the biotherapeutic manufacturing process for viral inactivation, cell lysis, and purification steps, demonstrating log reduction values comparable to or exceeding those of Triton X-100.
Safety and Environmental Considerations
The use of Triton X-100 in hematological sample preparation necessitates careful consideration of safety and environmental factors. As a non-ionic surfactant, Triton X-100 poses potential risks to both laboratory personnel and the environment, requiring stringent safety protocols and disposal procedures.
In laboratory settings, proper handling of Triton X-100 is crucial to minimize exposure risks. Personal protective equipment (PPE), including gloves, lab coats, and safety goggles, should be mandatory when working with this chemical. Adequate ventilation is essential to prevent inhalation of vapors, particularly during sample preparation processes that may generate aerosols. Spill containment and cleanup procedures must be established and communicated to all personnel to mitigate potential hazards.
The toxicological profile of Triton X-100 warrants attention. While it is generally considered to have low acute toxicity, prolonged or repeated exposure may cause skin irritation and sensitization. Ingestion can lead to gastrointestinal distress, and eye contact may result in severe irritation. Comprehensive safety data sheets (SDS) should be readily available, and regular safety training for laboratory staff is imperative to ensure proper handling and emergency response.
Environmental considerations are equally critical when using Triton X-100. The surfactant's persistence in aquatic environments and potential for bioaccumulation raise concerns about its ecological impact. Proper disposal methods are essential to prevent environmental contamination. Waste containing Triton X-100 should be collected separately and treated as hazardous waste, adhering to local and national regulations for chemical disposal.
Research institutions and clinical laboratories must implement robust waste management systems to handle Triton X-100 and other potentially harmful chemicals used in hematological sample preparation. This may include on-site treatment facilities or partnerships with specialized waste management companies to ensure environmentally responsible disposal.
The development of alternative, more environmentally friendly surfactants for hematological sample preparation is an active area of research. Efforts to find substitutes that maintain efficacy while reducing environmental impact are ongoing. These alternatives may include biodegradable surfactants or novel sample preparation techniques that minimize or eliminate the need for potentially harmful chemicals.
Regulatory compliance is a crucial aspect of using Triton X-100 in laboratory settings. Institutions must stay informed about evolving regulations regarding the use and disposal of such chemicals. This includes adherence to occupational health and safety standards, environmental protection laws, and specific guidelines for clinical and research laboratories.
In laboratory settings, proper handling of Triton X-100 is crucial to minimize exposure risks. Personal protective equipment (PPE), including gloves, lab coats, and safety goggles, should be mandatory when working with this chemical. Adequate ventilation is essential to prevent inhalation of vapors, particularly during sample preparation processes that may generate aerosols. Spill containment and cleanup procedures must be established and communicated to all personnel to mitigate potential hazards.
The toxicological profile of Triton X-100 warrants attention. While it is generally considered to have low acute toxicity, prolonged or repeated exposure may cause skin irritation and sensitization. Ingestion can lead to gastrointestinal distress, and eye contact may result in severe irritation. Comprehensive safety data sheets (SDS) should be readily available, and regular safety training for laboratory staff is imperative to ensure proper handling and emergency response.
Environmental considerations are equally critical when using Triton X-100. The surfactant's persistence in aquatic environments and potential for bioaccumulation raise concerns about its ecological impact. Proper disposal methods are essential to prevent environmental contamination. Waste containing Triton X-100 should be collected separately and treated as hazardous waste, adhering to local and national regulations for chemical disposal.
Research institutions and clinical laboratories must implement robust waste management systems to handle Triton X-100 and other potentially harmful chemicals used in hematological sample preparation. This may include on-site treatment facilities or partnerships with specialized waste management companies to ensure environmentally responsible disposal.
The development of alternative, more environmentally friendly surfactants for hematological sample preparation is an active area of research. Efforts to find substitutes that maintain efficacy while reducing environmental impact are ongoing. These alternatives may include biodegradable surfactants or novel sample preparation techniques that minimize or eliminate the need for potentially harmful chemicals.
Regulatory compliance is a crucial aspect of using Triton X-100 in laboratory settings. Institutions must stay informed about evolving regulations regarding the use and disposal of such chemicals. This includes adherence to occupational health and safety standards, environmental protection laws, and specific guidelines for clinical and research laboratories.
Regulatory Compliance in Hematological Testing
Regulatory compliance is a critical aspect of hematological testing, ensuring the safety, accuracy, and reliability of diagnostic procedures. In the context of using Triton X-100 as a facilitator in hematological sample preparation, adherence to regulatory standards is paramount. Various regulatory bodies, such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe, have established guidelines and regulations governing the use of chemicals in medical diagnostics.
The use of Triton X-100 in hematological sample preparation must comply with Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) standards. These standards ensure that the reagents used in diagnostic procedures are of consistent quality and that the testing processes are reproducible and reliable. Laboratories and manufacturers must maintain detailed documentation of their procedures, including the sourcing, handling, and storage of Triton X-100.
Quality control measures are essential to maintain regulatory compliance. Regular calibration of equipment, validation of testing methods, and proficiency testing are required to ensure the accuracy of results obtained using Triton X-100 in sample preparation. Laboratories must also implement robust quality management systems to track and document all aspects of the testing process.
Safety considerations are another crucial aspect of regulatory compliance. The use of Triton X-100 must adhere to occupational health and safety regulations, including proper handling, storage, and disposal procedures. Material Safety Data Sheets (MSDS) must be readily available, and staff must be trained in the safe handling of this chemical.
Environmental regulations also play a role in the use of Triton X-100 in hematological testing. Proper disposal methods must be employed to prevent environmental contamination, and laboratories may need to comply with local and national environmental protection laws.
Regulatory bodies often require validation studies to demonstrate the effectiveness and safety of using Triton X-100 in hematological sample preparation. These studies must show that the use of Triton X-100 does not interfere with the accuracy of test results or introduce any unintended effects on the samples.
Continuous monitoring and reporting of any adverse events or unexpected results related to the use of Triton X-100 in hematological testing are essential for maintaining regulatory compliance. Laboratories and manufacturers must have systems in place to promptly report such incidents to the appropriate regulatory authorities.
As regulations evolve, it is crucial for laboratories and manufacturers to stay informed about changes in regulatory requirements and update their processes accordingly. This may involve periodic training of staff, updating standard operating procedures, and participating in industry conferences and workshops focused on regulatory compliance in hematological testing.
The use of Triton X-100 in hematological sample preparation must comply with Good Laboratory Practice (GLP) and Good Manufacturing Practice (GMP) standards. These standards ensure that the reagents used in diagnostic procedures are of consistent quality and that the testing processes are reproducible and reliable. Laboratories and manufacturers must maintain detailed documentation of their procedures, including the sourcing, handling, and storage of Triton X-100.
Quality control measures are essential to maintain regulatory compliance. Regular calibration of equipment, validation of testing methods, and proficiency testing are required to ensure the accuracy of results obtained using Triton X-100 in sample preparation. Laboratories must also implement robust quality management systems to track and document all aspects of the testing process.
Safety considerations are another crucial aspect of regulatory compliance. The use of Triton X-100 must adhere to occupational health and safety regulations, including proper handling, storage, and disposal procedures. Material Safety Data Sheets (MSDS) must be readily available, and staff must be trained in the safe handling of this chemical.
Environmental regulations also play a role in the use of Triton X-100 in hematological testing. Proper disposal methods must be employed to prevent environmental contamination, and laboratories may need to comply with local and national environmental protection laws.
Regulatory bodies often require validation studies to demonstrate the effectiveness and safety of using Triton X-100 in hematological sample preparation. These studies must show that the use of Triton X-100 does not interfere with the accuracy of test results or introduce any unintended effects on the samples.
Continuous monitoring and reporting of any adverse events or unexpected results related to the use of Triton X-100 in hematological testing are essential for maintaining regulatory compliance. Laboratories and manufacturers must have systems in place to promptly report such incidents to the appropriate regulatory authorities.
As regulations evolve, it is crucial for laboratories and manufacturers to stay informed about changes in regulatory requirements and update their processes accordingly. This may involve periodic training of staff, updating standard operating procedures, and participating in industry conferences and workshops focused on regulatory compliance in hematological testing.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!