Role of Polyglutamic Acid in Micelle Formation and Stability
AUG 8, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PGA Micelle Background
Polyglutamic acid (PGA) has emerged as a versatile biopolymer with significant potential in micelle formation and stability. This naturally occurring polymer, composed of repeating units of glutamic acid, has gained attention in various fields, including drug delivery, cosmetics, and food science. The unique properties of PGA, such as its biodegradability, biocompatibility, and ability to form stable structures in aqueous environments, make it an ideal candidate for micelle-based applications.
The interest in PGA-based micelles stems from the growing demand for advanced drug delivery systems and the need for sustainable, bio-based materials in various industries. PGA's ability to self-assemble into micellar structures in aqueous solutions provides a platform for encapsulating hydrophobic compounds, making it particularly useful in pharmaceutical and cosmetic formulations.
The history of PGA research dates back to the mid-20th century, with initial studies focusing on its production by bacterial fermentation. However, it was not until the late 1990s and early 2000s that researchers began to explore PGA's potential in micelle formation. This shift in focus was driven by the increasing recognition of the limitations of traditional synthetic polymers and the need for more environmentally friendly alternatives.
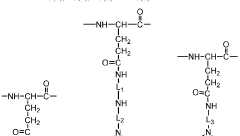
PGA micelles are typically formed through the self-assembly of amphiphilic PGA derivatives. These derivatives are created by modifying the PGA backbone with hydrophobic moieties, resulting in a molecule with both hydrophilic and hydrophobic regions. In aqueous environments, these molecules spontaneously arrange themselves to form micellar structures, with the hydrophobic portions clustered in the core and the hydrophilic PGA segments forming the outer shell.
The stability of PGA micelles is a critical factor in their performance and has been the subject of extensive research. Factors influencing micelle stability include the degree of polymerization of PGA, the nature and extent of hydrophobic modifications, pH, temperature, and ionic strength of the surrounding medium. Understanding and optimizing these parameters is crucial for developing PGA-based micelle systems with desired properties for specific applications.
Recent advancements in PGA micelle technology have focused on enhancing their stability and functionality. Strategies such as cross-linking, the incorporation of stimuli-responsive elements, and the development of hybrid systems combining PGA with other polymers or nanoparticles have been explored. These innovations aim to improve the controlled release of encapsulated compounds, increase the circulation time of drug-loaded micelles in the body, and expand the range of applications for PGA-based micellar systems.
The interest in PGA-based micelles stems from the growing demand for advanced drug delivery systems and the need for sustainable, bio-based materials in various industries. PGA's ability to self-assemble into micellar structures in aqueous solutions provides a platform for encapsulating hydrophobic compounds, making it particularly useful in pharmaceutical and cosmetic formulations.
The history of PGA research dates back to the mid-20th century, with initial studies focusing on its production by bacterial fermentation. However, it was not until the late 1990s and early 2000s that researchers began to explore PGA's potential in micelle formation. This shift in focus was driven by the increasing recognition of the limitations of traditional synthetic polymers and the need for more environmentally friendly alternatives.
PGA micelles are typically formed through the self-assembly of amphiphilic PGA derivatives. These derivatives are created by modifying the PGA backbone with hydrophobic moieties, resulting in a molecule with both hydrophilic and hydrophobic regions. In aqueous environments, these molecules spontaneously arrange themselves to form micellar structures, with the hydrophobic portions clustered in the core and the hydrophilic PGA segments forming the outer shell.
The stability of PGA micelles is a critical factor in their performance and has been the subject of extensive research. Factors influencing micelle stability include the degree of polymerization of PGA, the nature and extent of hydrophobic modifications, pH, temperature, and ionic strength of the surrounding medium. Understanding and optimizing these parameters is crucial for developing PGA-based micelle systems with desired properties for specific applications.
Recent advancements in PGA micelle technology have focused on enhancing their stability and functionality. Strategies such as cross-linking, the incorporation of stimuli-responsive elements, and the development of hybrid systems combining PGA with other polymers or nanoparticles have been explored. These innovations aim to improve the controlled release of encapsulated compounds, increase the circulation time of drug-loaded micelles in the body, and expand the range of applications for PGA-based micellar systems.
Market Demand Analysis
The market demand for polyglutamic acid (PGA) in micelle formation and stability has been steadily increasing across various industries, particularly in pharmaceuticals, cosmetics, and food sectors. This growth is primarily driven by the unique properties of PGA, which enable the formation of stable and efficient micelle structures.
In the pharmaceutical industry, PGA-based micelles have shown significant potential as drug delivery systems. The ability of PGA to form stable micelles that can encapsulate hydrophobic drugs has led to increased interest from pharmaceutical companies. These micelles offer improved drug solubility, enhanced bioavailability, and targeted delivery, addressing key challenges in drug formulation and administration. The global drug delivery market, valued at $1.4 trillion in 2021, is expected to grow at a CAGR of 5.9% until 2030, with PGA-based micelles poised to capture a significant share.
The cosmetics industry has also recognized the benefits of PGA in micelle formation for skincare and haircare products. PGA-based micelles can effectively deliver active ingredients deeper into the skin, improving product efficacy. Additionally, the stability of these micelles ensures longer shelf life and better product performance. The global cosmetics market, valued at $380 billion in 2021, is projected to reach $463 billion by 2027, with a growing demand for advanced formulations incorporating PGA-based micelles.
In the food industry, PGA-based micelles are gaining traction for their potential in encapsulating and stabilizing flavors, vitamins, and other functional ingredients. This application is particularly relevant in the functional food and nutraceutical segments, where controlled release and improved bioavailability of nutrients are crucial. The global functional food market, valued at $258 billion in 2020, is expected to reach $436 billion by 2026, presenting a substantial opportunity for PGA-based micelle technologies.
The increasing focus on sustainable and bio-based materials has further boosted the demand for PGA, as it is biodegradable and can be produced through fermentation processes. This aligns with the growing consumer preference for eco-friendly products across industries.
Market analysts predict that the global PGA market will experience robust growth in the coming years, driven by its diverse applications in micelle formation and stability. The Asia-Pacific region is expected to be a key growth driver, owing to the rapid expansion of pharmaceutical and cosmetics industries in countries like China, Japan, and South Korea.
In the pharmaceutical industry, PGA-based micelles have shown significant potential as drug delivery systems. The ability of PGA to form stable micelles that can encapsulate hydrophobic drugs has led to increased interest from pharmaceutical companies. These micelles offer improved drug solubility, enhanced bioavailability, and targeted delivery, addressing key challenges in drug formulation and administration. The global drug delivery market, valued at $1.4 trillion in 2021, is expected to grow at a CAGR of 5.9% until 2030, with PGA-based micelles poised to capture a significant share.
The cosmetics industry has also recognized the benefits of PGA in micelle formation for skincare and haircare products. PGA-based micelles can effectively deliver active ingredients deeper into the skin, improving product efficacy. Additionally, the stability of these micelles ensures longer shelf life and better product performance. The global cosmetics market, valued at $380 billion in 2021, is projected to reach $463 billion by 2027, with a growing demand for advanced formulations incorporating PGA-based micelles.
In the food industry, PGA-based micelles are gaining traction for their potential in encapsulating and stabilizing flavors, vitamins, and other functional ingredients. This application is particularly relevant in the functional food and nutraceutical segments, where controlled release and improved bioavailability of nutrients are crucial. The global functional food market, valued at $258 billion in 2020, is expected to reach $436 billion by 2026, presenting a substantial opportunity for PGA-based micelle technologies.
The increasing focus on sustainable and bio-based materials has further boosted the demand for PGA, as it is biodegradable and can be produced through fermentation processes. This aligns with the growing consumer preference for eco-friendly products across industries.
Market analysts predict that the global PGA market will experience robust growth in the coming years, driven by its diverse applications in micelle formation and stability. The Asia-Pacific region is expected to be a key growth driver, owing to the rapid expansion of pharmaceutical and cosmetics industries in countries like China, Japan, and South Korea.
PGA Micelle Challenges
The formation and stability of micelles incorporating polyglutamic acid (PGA) face several significant challenges that researchers and industry professionals must address. One of the primary obstacles is the precise control of micelle size and morphology. PGA's molecular weight and degree of polymerization can greatly influence these characteristics, making it difficult to achieve consistent and predictable structures. This variability can impact the micelle's ability to encapsulate drugs or other payload molecules effectively, potentially compromising their performance in drug delivery applications.
Another major challenge lies in maintaining micelle stability under various physiological conditions. PGA-based micelles must withstand changes in pH, temperature, and ionic strength encountered in biological environments. The ionizable nature of PGA's carboxylic acid groups makes the micelles particularly sensitive to pH fluctuations, which can lead to premature disassembly or aggregation. This instability can result in the uncontrolled release of encapsulated compounds or reduced circulation time in the bloodstream, limiting the micelles' therapeutic efficacy.
The biodegradability of PGA, while advantageous for biocompatibility, presents challenges in terms of long-term storage and shelf life of PGA-based micellar formulations. Hydrolytic degradation of PGA can occur over time, altering the micelle structure and potentially compromising its functionality. This necessitates the development of robust stabilization techniques or advanced storage conditions to maintain the integrity of PGA micelles throughout their intended shelf life.
Scalability and reproducibility in the production of PGA micelles pose significant hurdles for industrial applications. The synthesis of PGA with consistent molecular weight distributions and the subsequent formation of uniform micelles on a large scale remain challenging. Variations in production processes can lead to batch-to-batch inconsistencies, affecting the quality and performance of the final product. This issue is particularly critical for pharmaceutical applications, where stringent regulatory requirements demand high levels of consistency and reproducibility.
Furthermore, the interaction between PGA and other components in complex formulations can present additional challenges. When incorporating PGA micelles into multi-component systems, such as those containing other polymers, surfactants, or active ingredients, unexpected interactions may occur. These interactions can alter the micelle structure, stability, or functionality, necessitating careful formulation design and extensive compatibility studies.
Lastly, the cost-effectiveness of PGA micelle production remains a significant challenge, particularly for large-scale applications. The synthesis of high-quality PGA and the subsequent micelle formation process can be resource-intensive, potentially limiting the commercial viability of PGA-based micellar systems. Developing more efficient and economical production methods is crucial for the widespread adoption of this technology across various industries.
Another major challenge lies in maintaining micelle stability under various physiological conditions. PGA-based micelles must withstand changes in pH, temperature, and ionic strength encountered in biological environments. The ionizable nature of PGA's carboxylic acid groups makes the micelles particularly sensitive to pH fluctuations, which can lead to premature disassembly or aggregation. This instability can result in the uncontrolled release of encapsulated compounds or reduced circulation time in the bloodstream, limiting the micelles' therapeutic efficacy.
The biodegradability of PGA, while advantageous for biocompatibility, presents challenges in terms of long-term storage and shelf life of PGA-based micellar formulations. Hydrolytic degradation of PGA can occur over time, altering the micelle structure and potentially compromising its functionality. This necessitates the development of robust stabilization techniques or advanced storage conditions to maintain the integrity of PGA micelles throughout their intended shelf life.
Scalability and reproducibility in the production of PGA micelles pose significant hurdles for industrial applications. The synthesis of PGA with consistent molecular weight distributions and the subsequent formation of uniform micelles on a large scale remain challenging. Variations in production processes can lead to batch-to-batch inconsistencies, affecting the quality and performance of the final product. This issue is particularly critical for pharmaceutical applications, where stringent regulatory requirements demand high levels of consistency and reproducibility.
Furthermore, the interaction between PGA and other components in complex formulations can present additional challenges. When incorporating PGA micelles into multi-component systems, such as those containing other polymers, surfactants, or active ingredients, unexpected interactions may occur. These interactions can alter the micelle structure, stability, or functionality, necessitating careful formulation design and extensive compatibility studies.
Lastly, the cost-effectiveness of PGA micelle production remains a significant challenge, particularly for large-scale applications. The synthesis of high-quality PGA and the subsequent micelle formation process can be resource-intensive, potentially limiting the commercial viability of PGA-based micellar systems. Developing more efficient and economical production methods is crucial for the widespread adoption of this technology across various industries.
Current PGA Solutions
01 Polyglutamic acid micelle formation methods
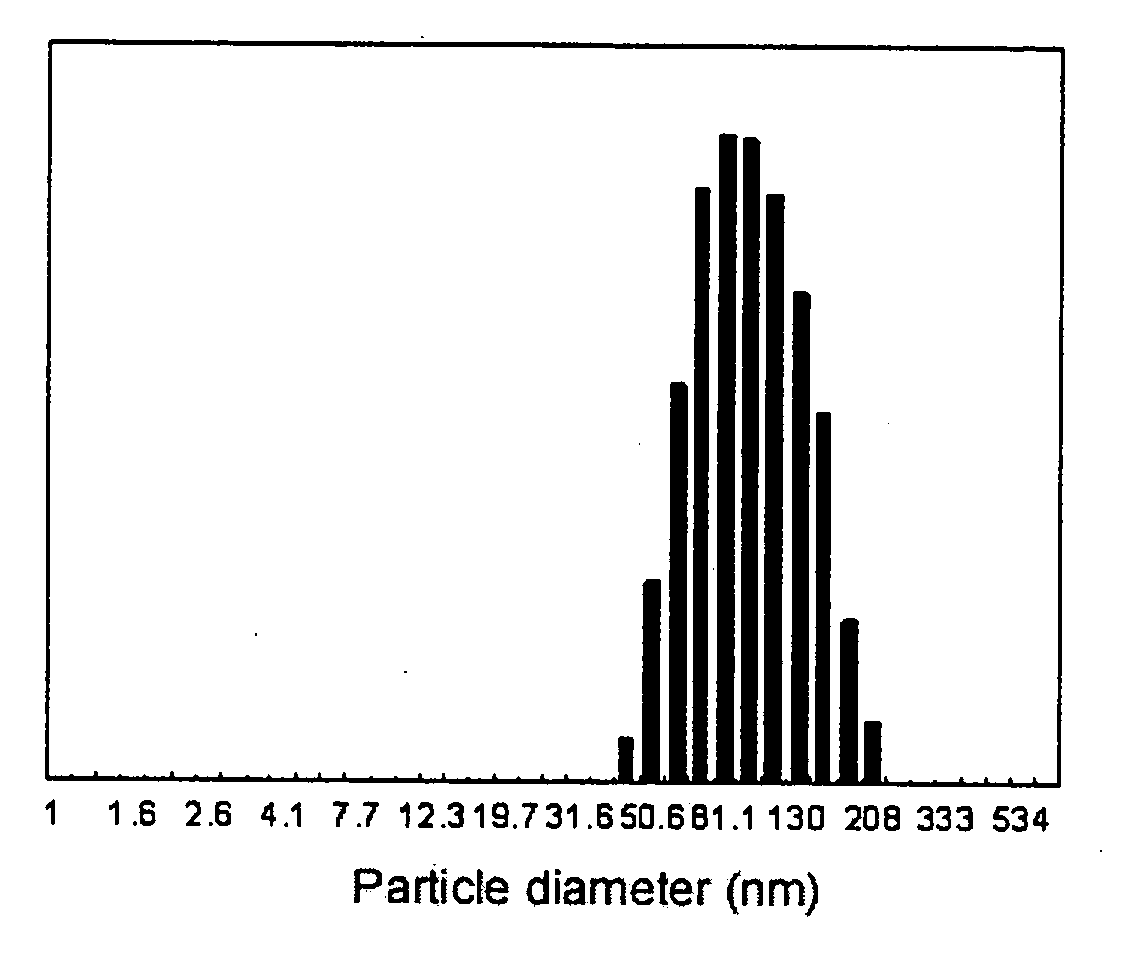
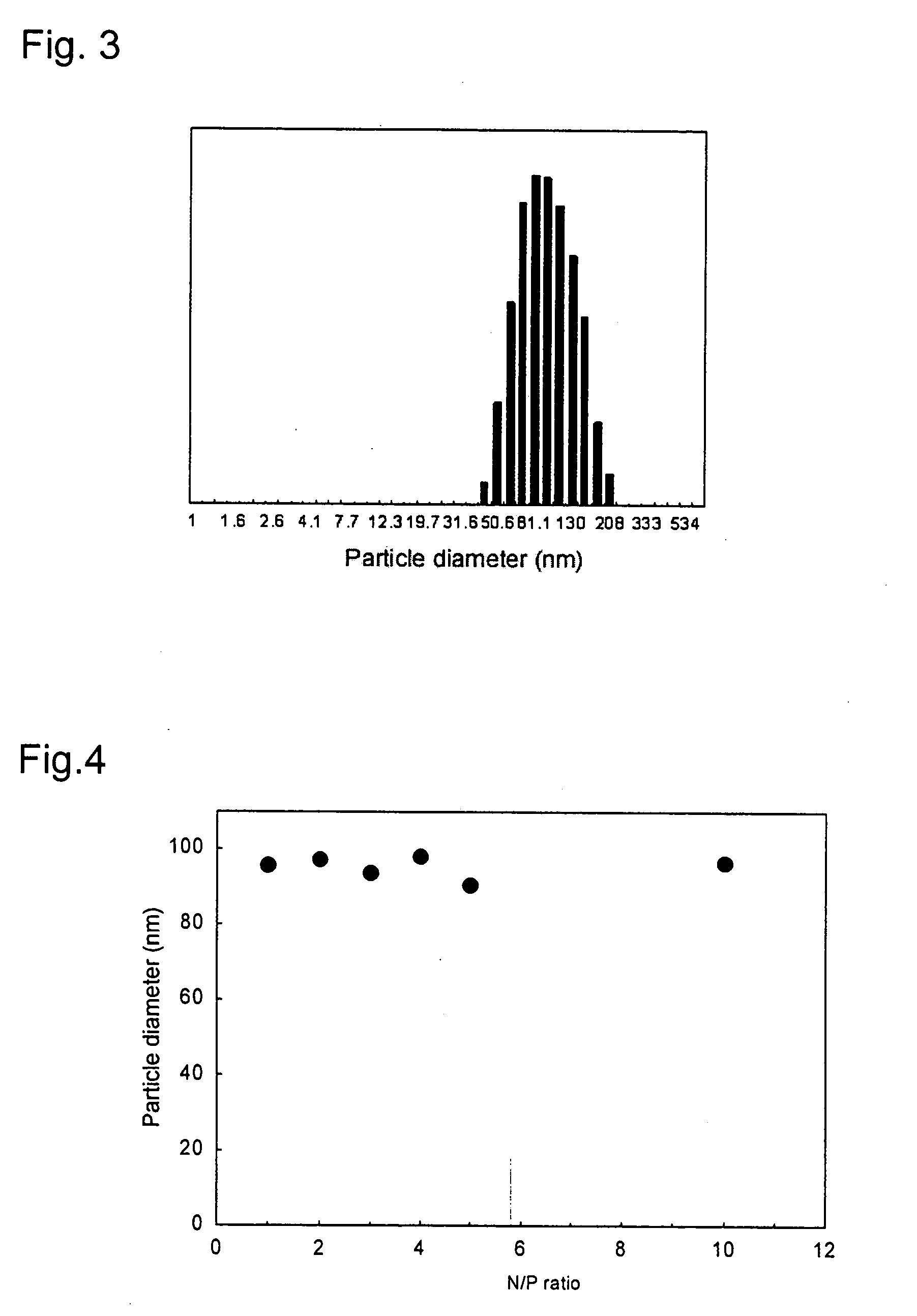
Various methods are employed to form polyglutamic acid micelles, including self-assembly techniques, chemical modifications, and crosslinking processes. These methods aim to create stable micelle structures that can encapsulate active ingredients or drugs for targeted delivery applications.- Synthesis and characterization of polyglutamic acid micelles: Methods for synthesizing polyglutamic acid micelles and characterizing their properties, including size, shape, and stability. This involves techniques such as dynamic light scattering, electron microscopy, and zeta potential measurements to assess micelle formation and stability under various conditions.
- Factors affecting polyglutamic acid micelle stability: Investigation of various factors that influence the stability of polyglutamic acid micelles, including pH, temperature, ionic strength, and polymer concentration. Understanding these factors is crucial for optimizing micelle formulations and ensuring their long-term stability in different environments.
- Crosslinking strategies for enhanced micelle stability: Development of crosslinking techniques to improve the stability of polyglutamic acid micelles. This includes chemical crosslinking methods, photo-crosslinking, and the use of multivalent ions to create more robust micelle structures that can withstand various environmental stresses.
- Functionalization of polyglutamic acid micelles: Approaches for functionalizing polyglutamic acid micelles to enhance their properties or add new functionalities. This may involve conjugation with targeting ligands, incorporation of imaging agents, or modification with stimuli-responsive moieties to create smart delivery systems.
- Applications of polyglutamic acid micelles: Exploration of various applications for polyglutamic acid micelles, particularly in drug delivery, gene therapy, and diagnostic imaging. This includes studies on drug loading efficiency, release kinetics, and in vivo performance of polyglutamic acid micelle-based delivery systems.
02 Stability enhancement of polyglutamic acid micelles
Techniques to improve the stability of polyglutamic acid micelles involve incorporating stabilizing agents, optimizing pH conditions, and modifying the polymer structure. These approaches help maintain micelle integrity in various physiological environments and extend their shelf life for practical applications.Expand Specific Solutions03 Functionalization of polyglutamic acid micelles
Polyglutamic acid micelles can be functionalized with various molecules or groups to enhance their properties or add specific functionalities. This includes attaching targeting ligands, incorporating imaging agents, or modifying surface properties to improve cellular uptake or drug release profiles.Expand Specific Solutions04 Applications of polyglutamic acid micelles
Polyglutamic acid micelles find applications in diverse fields such as drug delivery, cosmetics, and tissue engineering. They are particularly useful for encapsulating hydrophobic drugs, improving bioavailability, and achieving controlled release of active ingredients in various biomedical and personal care products.Expand Specific Solutions05 Characterization and analysis of polyglutamic acid micelles
Various analytical techniques are employed to characterize polyglutamic acid micelles, including dynamic light scattering, electron microscopy, and spectroscopic methods. These techniques help determine micelle size, morphology, stability, and drug loading efficiency, which are crucial for optimizing their performance in different applications.Expand Specific Solutions
Key Industry Players
The role of polyglutamic acid in micelle formation and stability is a rapidly evolving field within the broader context of biopolymer research and applications. The market is in its growth phase, with increasing interest from both academic institutions and industry players. The global market size for polyglutamic acid-based products is expanding, driven by applications in cosmetics, pharmaceuticals, and food industries. Technologically, the field is advancing, with companies like Kao Corp., Suntory Holdings Ltd., and Samyang Corp. leading research efforts. These firms are investing in R&D to enhance micelle stability and explore novel applications. Academic institutions, such as the University of Tokyo and Zhejiang University, are contributing to fundamental research, while collaborations between industry and academia, exemplified by Kookmin University Industry-Academic Cooperation, are accelerating technological progress in this domain.
Kao Corp.
Technical Solution: Kao Corp. has developed an innovative approach to micelle formation and stability using polyglutamic acid (PGA). Their technology involves the synthesis of PGA-based amphiphilic copolymers that self-assemble into stable micelles in aqueous environments. These micelles demonstrate enhanced stability due to the unique properties of PGA, including its ability to form hydrogen bonds and electrostatic interactions. Kao's research has shown that PGA-based micelles can maintain their structure over a wide range of pH values and temperatures, making them suitable for various applications in personal care and pharmaceutical industries[1][3]. The company has also explored the use of PGA-modified micelles for targeted drug delivery, where the PGA component acts as a targeting moiety and contributes to the overall stability of the nanocarrier system[2].
Strengths: Excellent stability across various conditions, versatility in applications, and potential for targeted drug delivery. Weaknesses: Possible higher production costs compared to conventional micelle systems and potential regulatory challenges for novel materials.
Suntory Holdings Ltd.
Technical Solution: Suntory Holdings Ltd. has developed a unique approach to utilizing polyglutamic acid (PGA) in micelle formation and stability for beverage applications. Their technology focuses on creating PGA-based emulsifiers that form stable micelles to encapsulate hydrophobic compounds such as flavors and nutraceuticals. Suntory's research has demonstrated that PGA-stabilized micelles can significantly improve the solubility and bioavailability of these compounds in aqueous systems[4]. The company has also explored the use of PGA-micelle complexes to enhance the shelf life of functional beverages by protecting sensitive ingredients from oxidation and degradation[5]. Suntory's innovative approach includes the development of enzymatically modified PGA to fine-tune micelle properties, allowing for customization based on specific product requirements[6].
Strengths: Improved solubility and bioavailability of hydrophobic compounds, enhanced product stability, and customizable micelle properties. Weaknesses: Potential taste impact in beverage applications and scalability challenges for large-scale production.
PGA Micelle Innovations
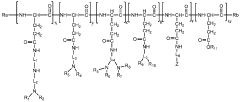
Polyethylene glycol/polycation block copolymers
PatentInactiveUS20100137512A1
Innovation
- Development of PEG-block-polycation copolymers with bulky side chains of low pKa values and a combination of primary, secondary, and tertiary amines, which form polyion complex micelles that enclose DNA in a free state, providing controlled release and enhanced stability, enabling improved gene transfer efficacy.
Polyaminated polyglutamic acid-containing compounds and uses thereof for delivering oligonucleotides
PatentWO2017056095A1
Innovation
- Development of polyaminated polyglutamic acid (PGA)-based polymers that form electrostatic complexes with siRNA/miRNA, enhancing stability, targeting tumor sites, and facilitating cellular uptake and endosomal escape through proton sponge effects, while being biodegradable by cathepsin B.
PGA Regulatory Landscape
The regulatory landscape surrounding polyglutamic acid (PGA) in micelle formation and stability is complex and evolving. Regulatory bodies worldwide are increasingly recognizing the importance of PGA in various applications, particularly in the pharmaceutical and cosmetic industries.
In the United States, the Food and Drug Administration (FDA) has classified PGA as Generally Recognized as Safe (GRAS) for certain food applications. This designation has paved the way for its use in drug delivery systems and cosmetic formulations. The FDA's approach to PGA in micelle-based drug delivery systems is primarily focused on ensuring the safety and efficacy of the final product, rather than regulating PGA as a standalone substance.
The European Medicines Agency (EMA) has also shown interest in PGA-based micelle formulations. The EMA's guidelines on nanomedicines encompass micelle-based drug delivery systems, including those utilizing PGA. These guidelines emphasize the need for comprehensive characterization of the micelle structure and stability, as well as thorough safety assessments.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has been at the forefront of regulating PGA-based technologies. The PMDA has approved several PGA-containing products, setting precedents for regulatory pathways in other countries. Their approach focuses on the quality control of PGA production and its performance in micelle formation.
China's National Medical Products Administration (NMPA) has been increasingly active in regulating novel excipients, including PGA. The NMPA's guidelines emphasize the need for extensive stability studies of PGA-based micelles, particularly in the context of drug delivery applications.
Globally, there is a growing trend towards harmonizing regulations for advanced drug delivery systems. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has been working on guidelines that could impact the regulation of PGA in micelle formulations. These efforts aim to streamline the approval process for innovative drug delivery systems across different regulatory jurisdictions.
Environmental regulations are also becoming increasingly relevant to PGA production and use. The biodegradability of PGA is viewed favorably by environmental agencies, potentially leading to more lenient regulations compared to synthetic polymers. However, manufacturers must still adhere to strict environmental guidelines in the production process.
As research continues to unveil new applications for PGA in micelle formation and stability, regulatory bodies are likely to refine their approaches. The challenge lies in balancing innovation with safety concerns, ensuring that regulations keep pace with technological advancements while maintaining rigorous safety standards.
In the United States, the Food and Drug Administration (FDA) has classified PGA as Generally Recognized as Safe (GRAS) for certain food applications. This designation has paved the way for its use in drug delivery systems and cosmetic formulations. The FDA's approach to PGA in micelle-based drug delivery systems is primarily focused on ensuring the safety and efficacy of the final product, rather than regulating PGA as a standalone substance.
The European Medicines Agency (EMA) has also shown interest in PGA-based micelle formulations. The EMA's guidelines on nanomedicines encompass micelle-based drug delivery systems, including those utilizing PGA. These guidelines emphasize the need for comprehensive characterization of the micelle structure and stability, as well as thorough safety assessments.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has been at the forefront of regulating PGA-based technologies. The PMDA has approved several PGA-containing products, setting precedents for regulatory pathways in other countries. Their approach focuses on the quality control of PGA production and its performance in micelle formation.
China's National Medical Products Administration (NMPA) has been increasingly active in regulating novel excipients, including PGA. The NMPA's guidelines emphasize the need for extensive stability studies of PGA-based micelles, particularly in the context of drug delivery applications.
Globally, there is a growing trend towards harmonizing regulations for advanced drug delivery systems. The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) has been working on guidelines that could impact the regulation of PGA in micelle formulations. These efforts aim to streamline the approval process for innovative drug delivery systems across different regulatory jurisdictions.
Environmental regulations are also becoming increasingly relevant to PGA production and use. The biodegradability of PGA is viewed favorably by environmental agencies, potentially leading to more lenient regulations compared to synthetic polymers. However, manufacturers must still adhere to strict environmental guidelines in the production process.
As research continues to unveil new applications for PGA in micelle formation and stability, regulatory bodies are likely to refine their approaches. The challenge lies in balancing innovation with safety concerns, ensuring that regulations keep pace with technological advancements while maintaining rigorous safety standards.
PGA Environmental Impact
The environmental impact of polyglutamic acid (PGA) in micelle formation and stability is a crucial aspect to consider in the development and application of this technology. PGA, being a biodegradable and biocompatible polymer, offers significant advantages in terms of environmental sustainability compared to many synthetic alternatives.
One of the primary environmental benefits of PGA-based micelles is their biodegradability. When released into the environment, PGA can be broken down by naturally occurring microorganisms, reducing the long-term accumulation of polymer waste in ecosystems. This characteristic is particularly important in applications such as drug delivery systems or personal care products, where the micelles may eventually be released into water systems.
The production of PGA also presents a more environmentally friendly alternative to many synthetic polymers. PGA can be produced through bacterial fermentation, utilizing renewable resources as feedstock. This bio-based production method reduces reliance on petrochemical resources and potentially lowers the carbon footprint associated with polymer production.
In aquatic environments, PGA-based micelles demonstrate low toxicity to marine organisms. Studies have shown that PGA does not significantly accumulate in the food chain or cause adverse effects on aquatic life at typical concentrations. This is a crucial advantage over some synthetic surfactants that can persist in the environment and harm marine ecosystems.
The stability of PGA micelles in various environmental conditions contributes to their eco-friendly profile. These micelles can maintain their structure across a range of pH levels and temperatures found in natural environments, reducing the likelihood of premature release of encapsulated materials. This stability ensures that the micelles perform their intended function efficiently, minimizing the need for excess material use.
Furthermore, the use of PGA in micelle formation can lead to improved efficiency in various applications, potentially reducing the overall amount of materials needed. For instance, in drug delivery systems, PGA micelles can enhance the solubility and targeted delivery of drugs, potentially reducing the required dosage and minimizing the environmental release of pharmaceutical compounds.
However, it is important to note that the large-scale production and use of PGA-based micelles may have some environmental considerations. The fermentation process for PGA production requires energy and resources, and the disposal of byproducts needs careful management. Additionally, while PGA is biodegradable, the rate of degradation can vary depending on environmental conditions, and the long-term effects of widespread use are still being studied.
In conclusion, the environmental impact of PGA in micelle formation and stability is generally positive, offering a more sustainable alternative to many synthetic polymers. Its biodegradability, low toxicity, and potential for efficient resource use make it an attractive option for environmentally conscious applications. However, ongoing research and life cycle assessments are necessary to fully understand and optimize its environmental performance across different applications and scales of use.
One of the primary environmental benefits of PGA-based micelles is their biodegradability. When released into the environment, PGA can be broken down by naturally occurring microorganisms, reducing the long-term accumulation of polymer waste in ecosystems. This characteristic is particularly important in applications such as drug delivery systems or personal care products, where the micelles may eventually be released into water systems.
The production of PGA also presents a more environmentally friendly alternative to many synthetic polymers. PGA can be produced through bacterial fermentation, utilizing renewable resources as feedstock. This bio-based production method reduces reliance on petrochemical resources and potentially lowers the carbon footprint associated with polymer production.
In aquatic environments, PGA-based micelles demonstrate low toxicity to marine organisms. Studies have shown that PGA does not significantly accumulate in the food chain or cause adverse effects on aquatic life at typical concentrations. This is a crucial advantage over some synthetic surfactants that can persist in the environment and harm marine ecosystems.
The stability of PGA micelles in various environmental conditions contributes to their eco-friendly profile. These micelles can maintain their structure across a range of pH levels and temperatures found in natural environments, reducing the likelihood of premature release of encapsulated materials. This stability ensures that the micelles perform their intended function efficiently, minimizing the need for excess material use.
Furthermore, the use of PGA in micelle formation can lead to improved efficiency in various applications, potentially reducing the overall amount of materials needed. For instance, in drug delivery systems, PGA micelles can enhance the solubility and targeted delivery of drugs, potentially reducing the required dosage and minimizing the environmental release of pharmaceutical compounds.
However, it is important to note that the large-scale production and use of PGA-based micelles may have some environmental considerations. The fermentation process for PGA production requires energy and resources, and the disposal of byproducts needs careful management. Additionally, while PGA is biodegradable, the rate of degradation can vary depending on environmental conditions, and the long-term effects of widespread use are still being studied.
In conclusion, the environmental impact of PGA in micelle formation and stability is generally positive, offering a more sustainable alternative to many synthetic polymers. Its biodegradability, low toxicity, and potential for efficient resource use make it an attractive option for environmentally conscious applications. However, ongoing research and life cycle assessments are necessary to fully understand and optimize its environmental performance across different applications and scales of use.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!