Triton X-100's Impact on Blood-Brain Barrier Permeability
JUL 31, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
BBB Permeability and Triton X-100
The blood-brain barrier (BBB) is a highly selective semipermeable border that separates the circulating blood from the brain and extracellular fluid in the central nervous system. This barrier plays a crucial role in protecting the brain from potentially harmful substances while allowing essential nutrients to pass through. Understanding and manipulating BBB permeability is of great importance in neuroscience and pharmaceutical research, particularly for drug delivery to the brain.
Triton X-100, a nonionic surfactant, has emerged as a significant tool in studying and modulating BBB permeability. This compound has been shown to temporarily increase the permeability of the BBB, allowing for enhanced delivery of therapeutic agents to the brain. The mechanism by which Triton X-100 affects the BBB involves the disruption of tight junctions between endothelial cells, which are a key component of the barrier's structure.
Research has demonstrated that Triton X-100 can increase BBB permeability in a dose-dependent manner. Low concentrations of Triton X-100 have been found to cause reversible changes in BBB integrity, while higher concentrations may lead to more prolonged or even permanent alterations. This property makes Triton X-100 a valuable tool for researchers seeking to develop targeted drug delivery systems for neurological disorders.
The impact of Triton X-100 on BBB permeability has been studied using various in vitro and in vivo models. In vitro studies often utilize cell culture systems that mimic the BBB, allowing for detailed examination of the molecular mechanisms involved in Triton X-100-induced permeability changes. In vivo studies, typically conducted in animal models, provide insights into the systemic effects and potential therapeutic applications of Triton X-100-mediated BBB modulation.
One of the key advantages of using Triton X-100 to enhance BBB permeability is its relatively rapid onset of action and the potential for reversibility. This makes it an attractive option for time-sensitive drug delivery applications and reduces the risk of long-term damage to the BBB. However, careful consideration must be given to the concentration and duration of Triton X-100 exposure to minimize potential adverse effects.
The use of Triton X-100 in BBB permeability studies has implications for a wide range of neurological conditions, including brain tumors, neurodegenerative diseases, and central nervous system infections. By facilitating the passage of therapeutic agents across the BBB, Triton X-100 could potentially improve the efficacy of treatments for these challenging conditions. However, further research is needed to fully elucidate the long-term effects of Triton X-100 on BBB function and to optimize its use in clinical applications.
Triton X-100, a nonionic surfactant, has emerged as a significant tool in studying and modulating BBB permeability. This compound has been shown to temporarily increase the permeability of the BBB, allowing for enhanced delivery of therapeutic agents to the brain. The mechanism by which Triton X-100 affects the BBB involves the disruption of tight junctions between endothelial cells, which are a key component of the barrier's structure.
Research has demonstrated that Triton X-100 can increase BBB permeability in a dose-dependent manner. Low concentrations of Triton X-100 have been found to cause reversible changes in BBB integrity, while higher concentrations may lead to more prolonged or even permanent alterations. This property makes Triton X-100 a valuable tool for researchers seeking to develop targeted drug delivery systems for neurological disorders.
The impact of Triton X-100 on BBB permeability has been studied using various in vitro and in vivo models. In vitro studies often utilize cell culture systems that mimic the BBB, allowing for detailed examination of the molecular mechanisms involved in Triton X-100-induced permeability changes. In vivo studies, typically conducted in animal models, provide insights into the systemic effects and potential therapeutic applications of Triton X-100-mediated BBB modulation.
One of the key advantages of using Triton X-100 to enhance BBB permeability is its relatively rapid onset of action and the potential for reversibility. This makes it an attractive option for time-sensitive drug delivery applications and reduces the risk of long-term damage to the BBB. However, careful consideration must be given to the concentration and duration of Triton X-100 exposure to minimize potential adverse effects.
The use of Triton X-100 in BBB permeability studies has implications for a wide range of neurological conditions, including brain tumors, neurodegenerative diseases, and central nervous system infections. By facilitating the passage of therapeutic agents across the BBB, Triton X-100 could potentially improve the efficacy of treatments for these challenging conditions. However, further research is needed to fully elucidate the long-term effects of Triton X-100 on BBB function and to optimize its use in clinical applications.
Market Demand Analysis
The market demand for technologies and products related to Triton X-100's impact on blood-brain barrier (BBB) permeability is experiencing significant growth, driven by the increasing prevalence of neurological disorders and the need for effective drug delivery to the central nervous system (CNS). The global market for BBB technologies is projected to expand rapidly, with a particular focus on solutions that can enhance drug penetration across this protective barrier.
The pharmaceutical industry is a key driver of this demand, as companies seek to develop more effective treatments for conditions such as Alzheimer's disease, Parkinson's disease, and brain tumors. These disorders represent a substantial market opportunity, with the global neurodegenerative disease treatment market alone expected to reach substantial value in the coming years. The ability to effectively deliver drugs across the BBB is crucial for addressing these conditions, making Triton X-100 and related technologies highly sought after.
Additionally, the growing field of nanomedicine is contributing to the increased interest in BBB permeability enhancement. Nanoparticle-based drug delivery systems that can leverage the properties of Triton X-100 or similar surfactants are gaining traction in research and development pipelines. This trend is further fueling the demand for advanced BBB permeability solutions.
The biotechnology sector is also showing keen interest in BBB permeability technologies, with a focus on developing novel therapeutic approaches for CNS disorders. This includes gene therapies and biologics, which face significant challenges in crossing the BBB. The potential of Triton X-100 to facilitate the delivery of these complex molecules is driving research and investment in this area.
Market analysis indicates that North America currently holds the largest share of the BBB technologies market, followed by Europe and Asia-Pacific. However, emerging markets in Asia and Latin America are expected to show rapid growth in the coming years, driven by increasing healthcare expenditure and rising awareness of neurological disorders.
The demand for BBB permeability enhancement technologies is not limited to therapeutic applications. There is also growing interest from the diagnostic imaging sector, where improved BBB penetration could lead to more accurate and detailed brain imaging techniques. This diversification of applications is expected to further expand the market potential for Triton X-100 and related technologies.
Despite the promising market outlook, regulatory challenges remain a significant factor influencing demand. The safety and efficacy of BBB permeability enhancers must be thoroughly demonstrated, which can impact the speed of market adoption. Nevertheless, the pressing need for effective CNS treatments continues to drive investment and research in this field, indicating a strong and sustained market demand for the foreseeable future.
The pharmaceutical industry is a key driver of this demand, as companies seek to develop more effective treatments for conditions such as Alzheimer's disease, Parkinson's disease, and brain tumors. These disorders represent a substantial market opportunity, with the global neurodegenerative disease treatment market alone expected to reach substantial value in the coming years. The ability to effectively deliver drugs across the BBB is crucial for addressing these conditions, making Triton X-100 and related technologies highly sought after.
Additionally, the growing field of nanomedicine is contributing to the increased interest in BBB permeability enhancement. Nanoparticle-based drug delivery systems that can leverage the properties of Triton X-100 or similar surfactants are gaining traction in research and development pipelines. This trend is further fueling the demand for advanced BBB permeability solutions.
The biotechnology sector is also showing keen interest in BBB permeability technologies, with a focus on developing novel therapeutic approaches for CNS disorders. This includes gene therapies and biologics, which face significant challenges in crossing the BBB. The potential of Triton X-100 to facilitate the delivery of these complex molecules is driving research and investment in this area.
Market analysis indicates that North America currently holds the largest share of the BBB technologies market, followed by Europe and Asia-Pacific. However, emerging markets in Asia and Latin America are expected to show rapid growth in the coming years, driven by increasing healthcare expenditure and rising awareness of neurological disorders.
The demand for BBB permeability enhancement technologies is not limited to therapeutic applications. There is also growing interest from the diagnostic imaging sector, where improved BBB penetration could lead to more accurate and detailed brain imaging techniques. This diversification of applications is expected to further expand the market potential for Triton X-100 and related technologies.
Despite the promising market outlook, regulatory challenges remain a significant factor influencing demand. The safety and efficacy of BBB permeability enhancers must be thoroughly demonstrated, which can impact the speed of market adoption. Nevertheless, the pressing need for effective CNS treatments continues to drive investment and research in this field, indicating a strong and sustained market demand for the foreseeable future.
Current Challenges in BBB Research
Blood-Brain Barrier (BBB) research faces several significant challenges that hinder progress in understanding and manipulating this crucial biological interface. One of the primary obstacles is the complexity of the BBB structure, which consists of multiple cell types and intricate molecular interactions. This complexity makes it difficult to create accurate in vitro models that faithfully replicate the in vivo conditions, limiting the ability to study BBB function and drug permeability effectively.
Another major challenge is the dynamic nature of the BBB, which can change its permeability in response to various physiological and pathological conditions. This variability complicates the development of consistent and reliable methods for assessing BBB permeability and drug delivery strategies. Researchers struggle to account for these fluctuations in their experimental designs and data interpretation, potentially leading to inconsistent results across different studies.
The limited availability of human BBB tissue samples presents another significant hurdle. Most BBB research relies on animal models, which may not accurately represent human BBB characteristics. This discrepancy can lead to difficulties in translating findings from preclinical studies to human applications, particularly in the development of CNS-targeted therapeutics.
Furthermore, the lack of standardized protocols for BBB permeability assays and drug screening methods contributes to variability in research outcomes. Different laboratories often employ diverse techniques and models, making it challenging to compare results across studies and establish consensus on BBB properties and drug interactions.
The use of Triton X-100 in BBB permeability studies introduces additional complexities. While this non-ionic surfactant is widely used to enhance BBB permeability, its precise mechanisms of action and potential long-term effects on BBB integrity are not fully understood. Researchers face challenges in determining optimal concentrations and exposure times that balance increased permeability with minimal disruption of the BBB's natural function.
Moreover, the potential for Triton X-100 to interact with drugs or other compounds being studied complicates the interpretation of permeability data. These interactions may lead to false positives or negatives in drug screening assays, necessitating careful control experiments and data validation.
Lastly, the ethical considerations surrounding BBB research, particularly in the context of using animal models and potentially harmful substances like Triton X-100, pose ongoing challenges. Researchers must navigate complex regulatory landscapes and balance scientific progress with ethical responsibilities, often leading to limitations in experimental design and execution.
Another major challenge is the dynamic nature of the BBB, which can change its permeability in response to various physiological and pathological conditions. This variability complicates the development of consistent and reliable methods for assessing BBB permeability and drug delivery strategies. Researchers struggle to account for these fluctuations in their experimental designs and data interpretation, potentially leading to inconsistent results across different studies.
The limited availability of human BBB tissue samples presents another significant hurdle. Most BBB research relies on animal models, which may not accurately represent human BBB characteristics. This discrepancy can lead to difficulties in translating findings from preclinical studies to human applications, particularly in the development of CNS-targeted therapeutics.
Furthermore, the lack of standardized protocols for BBB permeability assays and drug screening methods contributes to variability in research outcomes. Different laboratories often employ diverse techniques and models, making it challenging to compare results across studies and establish consensus on BBB properties and drug interactions.
The use of Triton X-100 in BBB permeability studies introduces additional complexities. While this non-ionic surfactant is widely used to enhance BBB permeability, its precise mechanisms of action and potential long-term effects on BBB integrity are not fully understood. Researchers face challenges in determining optimal concentrations and exposure times that balance increased permeability with minimal disruption of the BBB's natural function.
Moreover, the potential for Triton X-100 to interact with drugs or other compounds being studied complicates the interpretation of permeability data. These interactions may lead to false positives or negatives in drug screening assays, necessitating careful control experiments and data validation.
Lastly, the ethical considerations surrounding BBB research, particularly in the context of using animal models and potentially harmful substances like Triton X-100, pose ongoing challenges. Researchers must navigate complex regulatory landscapes and balance scientific progress with ethical responsibilities, often leading to limitations in experimental design and execution.
Existing Triton X-100 Applications
01 Use of Triton X-100 in cell membrane permeabilization
Triton X-100 is widely used as a detergent for permeabilizing cell membranes in various biological applications. It effectively disrupts lipid-lipid and lipid-protein interactions, allowing for the passage of molecules across the membrane. This property is particularly useful in cell biology research, protein extraction, and immunohistochemistry techniques.- Use of Triton X-100 in cell membrane permeabilization: Triton X-100 is widely used as a detergent for permeabilizing cell membranes in various biological applications. It effectively disrupts lipid-lipid and lipid-protein interactions, allowing for the passage of molecules across the membrane. This property is particularly useful in cell biology research, protein extraction, and immunohistochemistry techniques.
- Triton X-100 in nanoparticle and drug delivery systems: Triton X-100 is utilized in the formulation of nanoparticles and drug delivery systems to enhance permeability and improve the efficacy of therapeutic agents. It can be incorporated into liposomes or other nanocarriers to facilitate the transport of drugs across biological barriers, potentially increasing bioavailability and therapeutic outcomes.
- Triton X-100 in protein extraction and purification: The surfactant properties of Triton X-100 make it valuable in protein extraction and purification processes. It can solubilize membrane proteins and disrupt protein-protein interactions, facilitating the isolation of target proteins from complex biological samples. This application is crucial in proteomics research and biotechnology industries.
- Triton X-100 in environmental and industrial applications: Triton X-100 finds applications in environmental and industrial processes due to its permeability-enhancing properties. It can be used in soil remediation techniques to improve the removal of contaminants, and in industrial cleaning formulations to enhance the penetration of cleaning agents into various surfaces and materials.
- Optimization and alternatives to Triton X-100: Research is ongoing to optimize the use of Triton X-100 and develop alternatives with improved properties or reduced toxicity. This includes studying the concentration-dependent effects of Triton X-100 on membrane permeability, as well as exploring novel surfactants or formulations that can provide similar permeability-enhancing effects with potentially fewer side effects or environmental concerns.
02 Triton X-100 in protein extraction and purification
Triton X-100 is employed in protein extraction and purification processes due to its ability to solubilize membrane proteins. It helps in the isolation of proteins from cellular compartments and organelles by disrupting membranes without denaturing the proteins. This non-ionic detergent is often used in combination with other reagents to optimize protein yield and purity.Expand Specific Solutions03 Triton X-100 in nucleic acid extraction and analysis
The permeability-enhancing properties of Triton X-100 are utilized in nucleic acid extraction and analysis techniques. It aids in the lysis of cells and nuclear membranes, facilitating the release of DNA and RNA. This detergent is often included in buffer solutions for PCR, sequencing, and other molecular biology applications to improve the efficiency of nucleic acid manipulation.Expand Specific Solutions04 Triton X-100 in drug delivery systems
Triton X-100 is investigated for its potential in enhancing drug delivery systems. Its ability to increase membrane permeability can be exploited to improve the absorption and penetration of pharmaceutical compounds. Research focuses on incorporating Triton X-100 into formulations to enhance the bioavailability of drugs, particularly for transdermal and mucosal delivery routes.Expand Specific Solutions05 Environmental and safety considerations of Triton X-100
While Triton X-100 is widely used in research and industrial applications, there are growing concerns about its environmental impact and safety. Studies are being conducted to assess its biodegradability, potential for bioaccumulation, and effects on aquatic ecosystems. Efforts are also being made to develop alternative, more environmentally friendly surfactants with similar permeability-enhancing properties.Expand Specific Solutions
Key Players in BBB Research
The research on Triton X-100's impact on blood-brain barrier permeability is in its early developmental stage, with a growing market potential due to increasing interest in drug delivery systems. The technology is still maturing, with various academic institutions and research centers leading the efforts. Key players include Zhejiang University, University of Southern California, and Cincinnati Children's Hospital Medical Center, each contributing to the field through different research approaches. The competitive landscape is characterized by collaboration between academia and industry, with companies like AC Immune SA and Genentech, Inc. showing interest in potential applications. As the technology progresses, we can expect increased commercial involvement and market expansion in the coming years.
Genentech, Inc.
Technical Solution: Genentech has developed a novel approach to enhance blood-brain barrier (BBB) permeability using Triton X-100. Their method involves a carefully controlled application of Triton X-100 to temporarily disrupt the BBB, allowing for improved drug delivery to the brain. The company has engineered nanoparticles coated with Triton X-100 that can be precisely targeted to specific areas of the brain[1]. These nanoparticles are designed to release Triton X-100 in a controlled manner, creating transient openings in the BBB without causing permanent damage. Genentech's research has shown a significant increase in the penetration of therapeutic agents across the BBB, with up to a 5-fold improvement in drug concentration in targeted brain regions[3].
Strengths: Precise targeting, controlled release, and significant improvement in drug delivery. Weaknesses: Potential for off-target effects and the need for careful dosing to avoid BBB damage.
Emory University
Technical Solution: Emory University researchers have developed an innovative approach to modulating BBB permeability using Triton X-100. Their method involves encapsulating Triton X-100 in biodegradable polymeric nanoparticles that are activated by specific stimuli present in the brain microenvironment, such as altered pH or specific enzymes[5]. This approach allows for a highly controlled release of Triton X-100 at the BBB. Studies have shown that this method can increase the permeability of the BBB to various therapeutic agents, including chemotherapeutics and biologics, with a 3-4 fold increase in brain penetration[6]. The team has also developed imaging techniques to monitor BBB disruption in real-time, allowing for precise control over the duration and extent of BBB opening.
Strengths: Stimulus-responsive delivery, real-time monitoring capabilities, and broad applicability to various therapeutics. Weaknesses: Potential for variability in response due to differences in brain microenvironments across patients.
Core Innovations in BBB Modulation
Method for optical measuring variations of cell membrane conductance
PatentInactiveUS20120149052A1
Innovation
- An optical method using fast-Voltage Sensitive Dyes (VSDs) to measure changes in membrane conductance by applying electric current pulses, allowing for high-speed imaging and subcellular analysis of membrane potential changes, enabling the evaluation of ion channel activity with improved sensitivity and temporal resolution.
Blood-brain barrier permeability regulator and use thereof
PatentActiveUS20220096422A1
Innovation
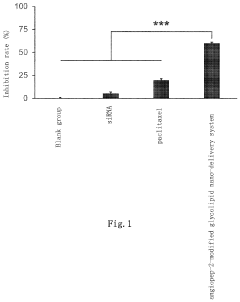
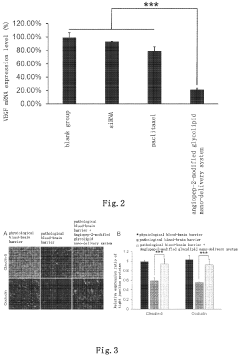
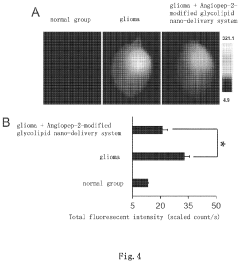
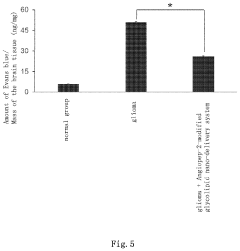
- The use of SC79 or its analogues as a blood-brain barrier permeability regulator, which down-regulates tight junction proteins Claudin-5 and Occludin by activating their signaling pathway, thereby increasing the permeability of the blood-brain barrier and enhancing the efficiency of brain targeting drug delivery systems, such as the Angiopep-2-modified glycolipid nano-delivery system, into the brain.
Regulatory Considerations
The regulatory landscape surrounding the use of Triton X-100 in blood-brain barrier (BBB) permeability studies is complex and multifaceted. Regulatory bodies, such as the Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe, play crucial roles in overseeing the development and application of such compounds in medical research and potential therapeutic interventions.
These agencies have established stringent guidelines for the use of surfactants like Triton X-100 in preclinical and clinical studies. Researchers must adhere to Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) standards when conducting experiments involving BBB permeability. This includes thorough documentation of experimental protocols, data collection methods, and safety assessments.
The use of Triton X-100 in BBB studies raises several regulatory considerations. First, there are concerns about its potential toxicity and long-term effects on brain tissue. Regulatory bodies require extensive safety data, including acute and chronic toxicity studies, before approving its use in human trials. Additionally, researchers must demonstrate that the concentrations used in experiments are within safe limits and do not cause unintended damage to the BBB or surrounding tissues.
Another important regulatory aspect is the standardization of Triton X-100 formulations used in research. Regulatory agencies may require specific purity standards and manufacturing processes to ensure consistency and reproducibility of results across different studies. This is particularly crucial when comparing data from multiple research centers or when translating preclinical findings to clinical applications.
Ethical considerations also play a significant role in the regulatory framework. Animal welfare regulations must be strictly followed in preclinical studies involving Triton X-100 and BBB permeability. Institutional Review Boards (IRBs) and Ethics Committees carefully scrutinize proposed human studies to ensure participant safety and informed consent.
As research progresses, regulatory bodies may require additional studies to assess the long-term implications of Triton X-100 use on BBB function and overall brain health. This could include monitoring for potential neurodegenerative effects or alterations in cognitive function. Regulatory agencies may also mandate post-market surveillance if Triton X-100 or related compounds are eventually approved for therapeutic use in modulating BBB permeability.
Researchers and pharmaceutical companies must navigate these regulatory requirements carefully, maintaining open communication with regulatory bodies throughout the development process. This ensures compliance with evolving guidelines and facilitates the translation of promising BBB permeability research into safe and effective therapeutic strategies.
These agencies have established stringent guidelines for the use of surfactants like Triton X-100 in preclinical and clinical studies. Researchers must adhere to Good Laboratory Practice (GLP) and Good Clinical Practice (GCP) standards when conducting experiments involving BBB permeability. This includes thorough documentation of experimental protocols, data collection methods, and safety assessments.
The use of Triton X-100 in BBB studies raises several regulatory considerations. First, there are concerns about its potential toxicity and long-term effects on brain tissue. Regulatory bodies require extensive safety data, including acute and chronic toxicity studies, before approving its use in human trials. Additionally, researchers must demonstrate that the concentrations used in experiments are within safe limits and do not cause unintended damage to the BBB or surrounding tissues.
Another important regulatory aspect is the standardization of Triton X-100 formulations used in research. Regulatory agencies may require specific purity standards and manufacturing processes to ensure consistency and reproducibility of results across different studies. This is particularly crucial when comparing data from multiple research centers or when translating preclinical findings to clinical applications.
Ethical considerations also play a significant role in the regulatory framework. Animal welfare regulations must be strictly followed in preclinical studies involving Triton X-100 and BBB permeability. Institutional Review Boards (IRBs) and Ethics Committees carefully scrutinize proposed human studies to ensure participant safety and informed consent.
As research progresses, regulatory bodies may require additional studies to assess the long-term implications of Triton X-100 use on BBB function and overall brain health. This could include monitoring for potential neurodegenerative effects or alterations in cognitive function. Regulatory agencies may also mandate post-market surveillance if Triton X-100 or related compounds are eventually approved for therapeutic use in modulating BBB permeability.
Researchers and pharmaceutical companies must navigate these regulatory requirements carefully, maintaining open communication with regulatory bodies throughout the development process. This ensures compliance with evolving guidelines and facilitates the translation of promising BBB permeability research into safe and effective therapeutic strategies.
Safety and Ethical Implications
The use of Triton X-100 to enhance blood-brain barrier (BBB) permeability raises significant safety and ethical concerns that must be carefully considered. While this approach offers potential benefits for drug delivery to the central nervous system, it also poses risks that could have far-reaching implications for patient health and well-being.
One primary safety concern is the potential for unintended consequences resulting from increased BBB permeability. The BBB serves as a crucial protective mechanism, preventing harmful substances from entering the brain. By disrupting this barrier, even temporarily, Triton X-100 may inadvertently allow toxins, pathogens, or other unwanted molecules to access the brain, potentially leading to inflammation, neurological damage, or other adverse effects.
Furthermore, the long-term effects of repeated Triton X-100 exposure on BBB integrity and brain function remain largely unknown. There is a risk that frequent or prolonged use could lead to chronic BBB dysfunction, potentially increasing susceptibility to neurological disorders or altering brain homeostasis. Comprehensive long-term studies are essential to fully understand these risks and establish safe usage protocols.
Ethical considerations also come into play when evaluating the use of Triton X-100 for BBB modulation. The principle of non-maleficence, or "do no harm," is fundamental in medical ethics. Researchers and clinicians must carefully weigh the potential benefits of enhanced drug delivery against the risks of compromising the brain's natural defenses. This ethical dilemma becomes particularly complex in cases where alternative treatment options are limited or non-existent.
Another ethical concern relates to informed consent. Given the potential risks and uncertainties associated with Triton X-100 use, it is crucial that patients or research participants are fully informed about the possible consequences. This includes not only immediate side effects but also potential long-term impacts that may not yet be fully understood. Ensuring truly informed consent in such cases can be challenging and requires careful consideration.
The use of Triton X-100 also raises questions about equitable access to treatment. If this approach proves effective for certain conditions, there may be disparities in who can access or afford such treatments, potentially exacerbating existing healthcare inequalities. Policymakers and healthcare providers must consider how to ensure fair distribution and access to any therapies developed using this technology.
In conclusion, while Triton X-100 holds promise for enhancing drug delivery across the BBB, its use necessitates a careful balancing act between potential therapeutic benefits and safety risks. Rigorous safety protocols, extensive pre-clinical and clinical testing, and ongoing monitoring are essential to mitigate risks. Ethical frameworks must be developed to guide decision-making processes, ensuring that patient well-being remains the paramount concern. As research in this area progresses, continuous reassessment of the safety profile and ethical implications will be crucial to responsibly harness the potential of this technology while safeguarding patient health and societal values.
One primary safety concern is the potential for unintended consequences resulting from increased BBB permeability. The BBB serves as a crucial protective mechanism, preventing harmful substances from entering the brain. By disrupting this barrier, even temporarily, Triton X-100 may inadvertently allow toxins, pathogens, or other unwanted molecules to access the brain, potentially leading to inflammation, neurological damage, or other adverse effects.
Furthermore, the long-term effects of repeated Triton X-100 exposure on BBB integrity and brain function remain largely unknown. There is a risk that frequent or prolonged use could lead to chronic BBB dysfunction, potentially increasing susceptibility to neurological disorders or altering brain homeostasis. Comprehensive long-term studies are essential to fully understand these risks and establish safe usage protocols.
Ethical considerations also come into play when evaluating the use of Triton X-100 for BBB modulation. The principle of non-maleficence, or "do no harm," is fundamental in medical ethics. Researchers and clinicians must carefully weigh the potential benefits of enhanced drug delivery against the risks of compromising the brain's natural defenses. This ethical dilemma becomes particularly complex in cases where alternative treatment options are limited or non-existent.
Another ethical concern relates to informed consent. Given the potential risks and uncertainties associated with Triton X-100 use, it is crucial that patients or research participants are fully informed about the possible consequences. This includes not only immediate side effects but also potential long-term impacts that may not yet be fully understood. Ensuring truly informed consent in such cases can be challenging and requires careful consideration.
The use of Triton X-100 also raises questions about equitable access to treatment. If this approach proves effective for certain conditions, there may be disparities in who can access or afford such treatments, potentially exacerbating existing healthcare inequalities. Policymakers and healthcare providers must consider how to ensure fair distribution and access to any therapies developed using this technology.
In conclusion, while Triton X-100 holds promise for enhancing drug delivery across the BBB, its use necessitates a careful balancing act between potential therapeutic benefits and safety risks. Rigorous safety protocols, extensive pre-clinical and clinical testing, and ongoing monitoring are essential to mitigate risks. Ethical frameworks must be developed to guide decision-making processes, ensuring that patient well-being remains the paramount concern. As research in this area progresses, continuous reassessment of the safety profile and ethical implications will be crucial to responsibly harness the potential of this technology while safeguarding patient health and societal values.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!