Advanced catalysts in Photoelectrochemical Water Splitting: Role and development.
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEC Water Splitting Background and Objectives
Photoelectrochemical (PEC) water splitting has emerged as a promising technology for sustainable hydrogen production, leveraging solar energy to directly convert water into hydrogen and oxygen. This approach represents a significant advancement in renewable energy systems, offering a pathway to carbon-neutral fuel production without the intermediate electricity generation step required by conventional electrolysis.
The evolution of PEC water splitting technology dates back to the pioneering work of Fujishima and Honda in 1972, who demonstrated the photocatalytic decomposition of water using titanium dioxide electrodes. Since then, the field has witnessed remarkable progress, with research focusing on enhancing efficiency, stability, and cost-effectiveness of PEC systems. The technological trajectory has moved from simple semiconductor photoelectrodes to complex multi-junction devices incorporating various catalytic materials.
Current technological trends in PEC water splitting are primarily directed toward developing advanced catalysts that can overcome the fundamental limitations of water oxidation and reduction reactions. These catalysts play a crucial role in reducing activation energy barriers, improving charge separation, and enhancing overall system efficiency. The integration of nanomaterials, plasmonic structures, and heterojunction architectures represents the cutting edge of catalyst design in this domain.
The primary technical objectives in advanced PEC catalyst development include achieving solar-to-hydrogen conversion efficiencies exceeding 10%, developing catalysts with operational stability beyond 10,000 hours, and reducing system costs to below $2/kg H₂. Additionally, researchers aim to design earth-abundant catalysts that can replace precious metals, thereby addressing sustainability concerns and enabling large-scale deployment.
Another significant goal is the development of tandem photoelectrode systems that can effectively utilize a broader spectrum of solar radiation. This approach involves coupling semiconductors with complementary absorption properties to maximize photon harvesting and energy conversion efficiency. The ultimate vision is to create artificial photosynthetic systems that mimic natural processes but with significantly higher efficiency.
The technological roadmap for PEC water splitting catalysts also encompasses the integration of computational methods for rational catalyst design, in-situ characterization techniques for understanding reaction mechanisms, and scalable manufacturing processes for commercial viability. These advancements are expected to position PEC water splitting as a key technology in the future hydrogen economy, contributing to global efforts in decarbonization and sustainable energy production.
The evolution of PEC water splitting technology dates back to the pioneering work of Fujishima and Honda in 1972, who demonstrated the photocatalytic decomposition of water using titanium dioxide electrodes. Since then, the field has witnessed remarkable progress, with research focusing on enhancing efficiency, stability, and cost-effectiveness of PEC systems. The technological trajectory has moved from simple semiconductor photoelectrodes to complex multi-junction devices incorporating various catalytic materials.
Current technological trends in PEC water splitting are primarily directed toward developing advanced catalysts that can overcome the fundamental limitations of water oxidation and reduction reactions. These catalysts play a crucial role in reducing activation energy barriers, improving charge separation, and enhancing overall system efficiency. The integration of nanomaterials, plasmonic structures, and heterojunction architectures represents the cutting edge of catalyst design in this domain.
The primary technical objectives in advanced PEC catalyst development include achieving solar-to-hydrogen conversion efficiencies exceeding 10%, developing catalysts with operational stability beyond 10,000 hours, and reducing system costs to below $2/kg H₂. Additionally, researchers aim to design earth-abundant catalysts that can replace precious metals, thereby addressing sustainability concerns and enabling large-scale deployment.
Another significant goal is the development of tandem photoelectrode systems that can effectively utilize a broader spectrum of solar radiation. This approach involves coupling semiconductors with complementary absorption properties to maximize photon harvesting and energy conversion efficiency. The ultimate vision is to create artificial photosynthetic systems that mimic natural processes but with significantly higher efficiency.
The technological roadmap for PEC water splitting catalysts also encompasses the integration of computational methods for rational catalyst design, in-situ characterization techniques for understanding reaction mechanisms, and scalable manufacturing processes for commercial viability. These advancements are expected to position PEC water splitting as a key technology in the future hydrogen economy, contributing to global efforts in decarbonization and sustainable energy production.
Market Analysis for Hydrogen Production Technologies
The global hydrogen production market is experiencing significant growth, driven by increasing demand for clean energy solutions and decarbonization efforts across industries. Currently valued at approximately $130 billion, the market is projected to reach $220 billion by 2030, with a compound annual growth rate of 9.2% during the forecast period. This growth trajectory is particularly relevant for photoelectrochemical (PEC) water splitting technologies, which represent an emerging segment within the broader hydrogen production landscape.
Traditional hydrogen production methods, dominated by steam methane reforming (SMR), account for over 76% of global hydrogen production but face sustainability challenges due to associated carbon emissions. In contrast, water electrolysis currently represents only about 4% of production but is experiencing the fastest growth rate among all hydrogen production technologies, with a 14.5% annual increase projected through 2030.
Photoelectrochemical water splitting, while still in early commercialization stages, is positioned as a high-potential technology due to its ability to directly convert solar energy to hydrogen without intermediate electricity generation steps. Market analysis indicates that PEC technologies could capture up to 8% of the green hydrogen market by 2035, representing a potential market value of $15 billion.
Regional analysis shows Asia-Pacific leading hydrogen production market growth, with China, Japan, and South Korea making substantial investments in advanced catalyst research for PEC applications. Europe follows closely, with Germany, France, and the Netherlands establishing dedicated research clusters focused on sustainable hydrogen production technologies. North America, particularly the United States and Canada, maintains strong positions through university-industry partnerships advancing catalyst innovations.
The investment landscape for advanced catalysts in PEC water splitting has seen remarkable growth, with venture capital funding increasing from $340 million in 2018 to over $1.2 billion in 2022. Corporate R&D expenditure in this specific segment has similarly expanded, with major energy companies allocating an average of 12% of their research budgets to advanced catalyst development.
Market adoption barriers include high production costs compared to conventional methods, with current PEC hydrogen production costs ranging from $8-12/kg compared to $1-3/kg for SMR-produced hydrogen. However, technological advancements in catalyst efficiency and durability are expected to reduce production costs to $4-5/kg by 2028, significantly improving market competitiveness and accelerating adoption across industrial applications.
Traditional hydrogen production methods, dominated by steam methane reforming (SMR), account for over 76% of global hydrogen production but face sustainability challenges due to associated carbon emissions. In contrast, water electrolysis currently represents only about 4% of production but is experiencing the fastest growth rate among all hydrogen production technologies, with a 14.5% annual increase projected through 2030.
Photoelectrochemical water splitting, while still in early commercialization stages, is positioned as a high-potential technology due to its ability to directly convert solar energy to hydrogen without intermediate electricity generation steps. Market analysis indicates that PEC technologies could capture up to 8% of the green hydrogen market by 2035, representing a potential market value of $15 billion.
Regional analysis shows Asia-Pacific leading hydrogen production market growth, with China, Japan, and South Korea making substantial investments in advanced catalyst research for PEC applications. Europe follows closely, with Germany, France, and the Netherlands establishing dedicated research clusters focused on sustainable hydrogen production technologies. North America, particularly the United States and Canada, maintains strong positions through university-industry partnerships advancing catalyst innovations.
The investment landscape for advanced catalysts in PEC water splitting has seen remarkable growth, with venture capital funding increasing from $340 million in 2018 to over $1.2 billion in 2022. Corporate R&D expenditure in this specific segment has similarly expanded, with major energy companies allocating an average of 12% of their research budgets to advanced catalyst development.
Market adoption barriers include high production costs compared to conventional methods, with current PEC hydrogen production costs ranging from $8-12/kg compared to $1-3/kg for SMR-produced hydrogen. However, technological advancements in catalyst efficiency and durability are expected to reduce production costs to $4-5/kg by 2028, significantly improving market competitiveness and accelerating adoption across industrial applications.
Current Catalyst Challenges in PEC Water Splitting
Despite significant advancements in photoelectrochemical (PEC) water splitting technology, current catalysts face substantial challenges that impede commercial viability. The primary obstacle remains the trade-off between efficiency, stability, and cost. High-performance catalysts typically utilize noble metals like platinum and iridium, which deliver excellent catalytic activity but at prohibitive costs for large-scale implementation. This economic barrier significantly restricts widespread adoption of PEC systems.
Stability presents another critical challenge, particularly in the harsh operating environments of PEC cells. Most catalysts experience rapid degradation under prolonged exposure to electrolytes, especially in extreme pH conditions necessary for optimal water splitting. This degradation manifests as decreased catalytic activity, structural deterioration, and eventual catalyst failure, substantially reducing system lifespan and economic viability.
The oxygen evolution reaction (OER) at the photoanode remains particularly problematic, requiring complex four-electron transfer processes that create significant kinetic barriers. Current catalysts struggle to efficiently facilitate this reaction without substantial overpotential, resulting in energy losses that compromise overall system efficiency. Even state-of-the-art catalysts typically require overpotentials exceeding 300mV, far from the theoretical minimum.
Interfacial challenges between catalysts and semiconductor photoelectrodes further complicate development. Poor electrical contact, unfavorable band alignment, and charge recombination at these interfaces significantly reduce quantum efficiency. Additionally, many promising catalyst materials block incident light from reaching the photoactive substrate, creating a counterproductive effect that diminishes photocurrent generation.
Scalability issues persist across catalyst development. Laboratory-scale synthesis methods often involve complex procedures that resist industrial scaling. Techniques like atomic layer deposition produce excellent catalysts but remain prohibitively expensive and time-consuming for mass production. This disconnect between laboratory performance and industrial feasibility creates a significant barrier to commercialization.
Bifunctional catalysts capable of facilitating both hydrogen and oxygen evolution reactions have shown promise but currently lack the performance of specialized catalysts. The development of effective co-catalysts that can operate synergistically without mutual interference represents an ongoing challenge. Furthermore, the integration of catalysts with emerging photoelectrode materials, particularly those based on earth-abundant elements, requires significant optimization to achieve compatible interfaces and matched electronic properties.
Stability presents another critical challenge, particularly in the harsh operating environments of PEC cells. Most catalysts experience rapid degradation under prolonged exposure to electrolytes, especially in extreme pH conditions necessary for optimal water splitting. This degradation manifests as decreased catalytic activity, structural deterioration, and eventual catalyst failure, substantially reducing system lifespan and economic viability.
The oxygen evolution reaction (OER) at the photoanode remains particularly problematic, requiring complex four-electron transfer processes that create significant kinetic barriers. Current catalysts struggle to efficiently facilitate this reaction without substantial overpotential, resulting in energy losses that compromise overall system efficiency. Even state-of-the-art catalysts typically require overpotentials exceeding 300mV, far from the theoretical minimum.
Interfacial challenges between catalysts and semiconductor photoelectrodes further complicate development. Poor electrical contact, unfavorable band alignment, and charge recombination at these interfaces significantly reduce quantum efficiency. Additionally, many promising catalyst materials block incident light from reaching the photoactive substrate, creating a counterproductive effect that diminishes photocurrent generation.
Scalability issues persist across catalyst development. Laboratory-scale synthesis methods often involve complex procedures that resist industrial scaling. Techniques like atomic layer deposition produce excellent catalysts but remain prohibitively expensive and time-consuming for mass production. This disconnect between laboratory performance and industrial feasibility creates a significant barrier to commercialization.
Bifunctional catalysts capable of facilitating both hydrogen and oxygen evolution reactions have shown promise but currently lack the performance of specialized catalysts. The development of effective co-catalysts that can operate synergistically without mutual interference represents an ongoing challenge. Furthermore, the integration of catalysts with emerging photoelectrode materials, particularly those based on earth-abundant elements, requires significant optimization to achieve compatible interfaces and matched electronic properties.
State-of-the-Art Catalyst Solutions
01 Metal oxide-based catalysts for enhanced efficiency
Metal oxide-based catalysts, particularly those incorporating transition metals like titanium, iron, and cobalt, have shown significant improvements in photoelectrochemical water splitting efficiency. These materials offer advantages including broad light absorption spectra, suitable band gaps, and excellent charge separation properties. Modified metal oxides with specific nanostructures can further enhance catalytic activity and stability under operating conditions, leading to higher hydrogen production rates.- Metal oxide-based catalysts for enhanced efficiency: Metal oxide-based catalysts, particularly those incorporating transition metals like titanium, iron, and cobalt, have shown significant improvements in photoelectrochemical water splitting efficiency. These materials offer advantageous band gap properties, high surface area, and excellent light absorption capabilities. Modified metal oxides with specific nanostructures can enhance charge separation and transfer, leading to improved hydrogen evolution rates and overall system efficiency.
- Nanostructured composite materials for stability enhancement: Nanostructured composite materials combining multiple functional components have demonstrated superior stability in photoelectrochemical water splitting systems. These composites often incorporate protective layers or stabilizing agents that prevent photocorrosion and degradation during operation. By engineering interfaces between different materials at the nanoscale, these catalysts maintain performance over extended periods while operating in harsh electrolyte environments, addressing one of the key challenges in practical water splitting applications.
- Noble metal-free catalysts for cost-effective water splitting: Development of noble metal-free catalysts represents a significant advancement in making photoelectrochemical water splitting economically viable. These catalysts utilize earth-abundant elements such as nickel, iron, and molybdenum compounds as alternatives to platinum and other precious metals. Through careful engineering of electronic structure and morphology, these materials achieve competitive catalytic activity while substantially reducing system costs, making large-scale hydrogen production more feasible.
- Doped semiconductor photocatalysts with enhanced light absorption: Doped semiconductor materials have emerged as effective photocatalysts with expanded light absorption ranges, particularly in the visible spectrum. By introducing specific dopants into semiconductor lattices, the electronic band structure can be modified to harvest a broader portion of the solar spectrum. These materials demonstrate improved charge carrier generation and separation, leading to higher quantum efficiencies and better utilization of solar energy for water splitting applications.
- Heterojunction systems for improved charge separation: Heterojunction catalyst systems combine multiple semiconductors with strategically aligned band structures to facilitate efficient charge carrier separation and transfer. These systems minimize recombination losses by creating directional pathways for electrons and holes to move toward their respective reaction sites. The engineered interfaces between materials create electric fields that enhance charge separation, resulting in significantly improved quantum efficiency and hydrogen production rates in photoelectrochemical water splitting applications.
02 Nanostructured composite materials for improved stability
Nanostructured composite materials combining multiple functional components have demonstrated superior stability in photoelectrochemical water splitting systems. These composites typically feature protective layers that prevent photocorrosion while maintaining efficient charge transfer. Core-shell structures, heterojunctions, and surface passivation techniques are employed to extend catalyst lifetime under harsh operating conditions, addressing one of the major challenges in practical water splitting applications.Expand Specific Solutions03 Noble metal-free catalysts for cost-effective water splitting
Development of noble metal-free catalysts represents a significant advancement in making photoelectrochemical water splitting economically viable. These catalysts utilize earth-abundant elements such as nickel, molybdenum, and sulfides/phosphides of transition metals. Through careful engineering of electronic structure and morphology, these materials achieve catalytic performance comparable to precious metal catalysts while substantially reducing system costs and dependency on scarce resources.Expand Specific Solutions04 Doped semiconductor photocatalysts with enhanced light absorption
Doping semiconductor materials with specific elements has proven effective in extending light absorption into the visible spectrum, addressing a key limitation in photoelectrochemical efficiency. These doped photocatalysts feature modified band structures that enable harvesting of a broader portion of the solar spectrum. Various dopants including nitrogen, carbon, and transition metals are strategically incorporated to optimize charge carrier generation and separation, resulting in significantly improved solar-to-hydrogen conversion efficiencies.Expand Specific Solutions05 Z-scheme heterojunction systems for efficient charge separation
Z-scheme heterojunction systems represent an advanced catalyst design that mimics natural photosynthesis for efficient photoelectrochemical water splitting. These systems incorporate two different semiconductors with complementary band structures, connected by electron mediators or direct contact interfaces. This architecture facilitates spatial separation of reduction and oxidation reactions while maintaining strong redox potential for both hydrogen and oxygen evolution, effectively overcoming the charge recombination limitations of single-component catalysts.Expand Specific Solutions
Key Patents and Scientific Breakthroughs
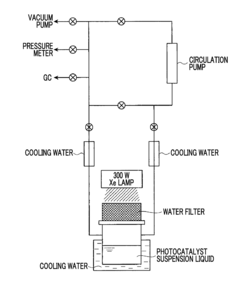
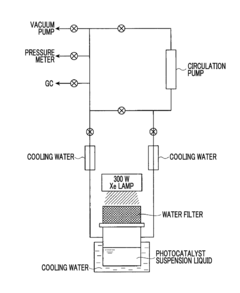
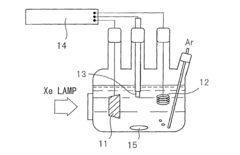
Photocatalyst for water splitting, production method for same, and photoelectrode for water splitting
PatentActiveUS10022713B2
Innovation
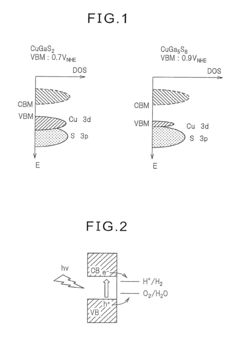
- A photocatalyst comprising barium niobium oxynitride with a supported promoter, specifically cobalt oxides or metallic cobalt, is developed, where the promoter is integrated in a specific range to enhance the absorption of long-wavelength light, thereby improving catalytic activity for water splitting.
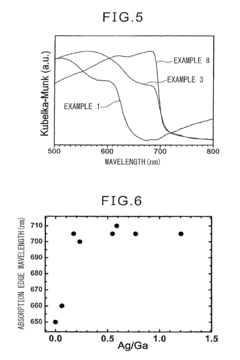
Photocatalyst for water splitting comprising gallium selenide and photoelectrode for water splitting comprising the same
PatentActiveUS9975115B2
Innovation
- A photocatalyst comprising Ga selenide, Ag-Ga selenide, or both, with a higher valence band maximum positioning, allowing for higher water splitting activity, and optionally incorporating Rh or Pt as promoters to enhance reaction rates.
Scalability and Manufacturing Considerations
The scalability and manufacturing of advanced catalysts for photoelectrochemical (PEC) water splitting presents significant challenges that must be addressed for commercial viability. Current laboratory-scale synthesis methods often involve complex procedures, expensive precursors, and specialized equipment that are difficult to translate to industrial production. Batch-to-batch variations in catalyst performance remain a persistent issue, highlighting the need for standardized manufacturing protocols and quality control measures.
Cost considerations represent a major barrier to widespread implementation. Noble metal-based catalysts such as platinum, iridium, and ruthenium demonstrate superior catalytic activity but their scarcity and high cost limit large-scale deployment. The development of earth-abundant alternatives based on transition metals (nickel, iron, cobalt) has shown promise, yet these materials often require complex nanostructuring or doping to achieve comparable efficiency, complicating mass production efforts.
Manufacturing techniques must evolve to accommodate industrial-scale production while maintaining nanoscale precision. Emerging approaches include continuous flow synthesis, which offers better control over reaction parameters and improved reproducibility compared to traditional batch methods. Spray pyrolysis and electrodeposition show potential for scaling catalyst production, while atomic layer deposition provides exceptional control over catalyst composition and structure, albeit at higher costs.
Substrate integration presents another manufacturing challenge, as catalyst deposition must ensure strong adhesion, uniform coverage, and electrical connectivity while withstanding harsh electrochemical conditions. Roll-to-roll processing technologies are being adapted for flexible PEC systems, potentially enabling high-throughput production of integrated devices.
Environmental considerations and sustainability metrics are increasingly important in manufacturing assessment. Life cycle analyses reveal that energy-intensive synthesis methods can undermine the environmental benefits of hydrogen production. Developing green synthesis routes that minimize toxic reagents, reduce energy consumption, and enable catalyst recycling is becoming essential for truly sustainable PEC systems.
Standardization efforts are gradually emerging to address the fragmented approaches in catalyst manufacturing. Industry consortia and research institutions are working to establish benchmarking protocols and performance metrics that facilitate comparison between different catalyst systems and manufacturing methods. These standards will be crucial for quality assurance in commercial production and for regulatory compliance as the technology matures toward market readiness.
Cost considerations represent a major barrier to widespread implementation. Noble metal-based catalysts such as platinum, iridium, and ruthenium demonstrate superior catalytic activity but their scarcity and high cost limit large-scale deployment. The development of earth-abundant alternatives based on transition metals (nickel, iron, cobalt) has shown promise, yet these materials often require complex nanostructuring or doping to achieve comparable efficiency, complicating mass production efforts.
Manufacturing techniques must evolve to accommodate industrial-scale production while maintaining nanoscale precision. Emerging approaches include continuous flow synthesis, which offers better control over reaction parameters and improved reproducibility compared to traditional batch methods. Spray pyrolysis and electrodeposition show potential for scaling catalyst production, while atomic layer deposition provides exceptional control over catalyst composition and structure, albeit at higher costs.
Substrate integration presents another manufacturing challenge, as catalyst deposition must ensure strong adhesion, uniform coverage, and electrical connectivity while withstanding harsh electrochemical conditions. Roll-to-roll processing technologies are being adapted for flexible PEC systems, potentially enabling high-throughput production of integrated devices.
Environmental considerations and sustainability metrics are increasingly important in manufacturing assessment. Life cycle analyses reveal that energy-intensive synthesis methods can undermine the environmental benefits of hydrogen production. Developing green synthesis routes that minimize toxic reagents, reduce energy consumption, and enable catalyst recycling is becoming essential for truly sustainable PEC systems.
Standardization efforts are gradually emerging to address the fragmented approaches in catalyst manufacturing. Industry consortia and research institutions are working to establish benchmarking protocols and performance metrics that facilitate comparison between different catalyst systems and manufacturing methods. These standards will be crucial for quality assurance in commercial production and for regulatory compliance as the technology matures toward market readiness.
Environmental Impact and Sustainability Assessment
Photoelectrochemical (PEC) water splitting using advanced catalysts represents a promising pathway toward sustainable hydrogen production, yet its environmental implications require thorough assessment. The life cycle analysis of catalyst materials reveals significant variations in environmental footprints. Noble metal-based catalysts (platinum, ruthenium, iridium) demonstrate superior catalytic performance but present substantial environmental concerns due to resource scarcity and energy-intensive mining processes. In contrast, earth-abundant catalysts based on transition metals (nickel, iron, cobalt) offer reduced environmental impact despite lower efficiency.
The manufacturing processes for advanced catalysts involve multiple chemical treatments and high-temperature syntheses that contribute to their carbon footprint. Nanostructured catalysts, while enhancing performance through increased surface area, often require specialized fabrication techniques with additional environmental costs. Recent advancements in green synthesis methods, including biomass-derived precursors and ambient-temperature processes, have shown potential to reduce these impacts by up to 40% compared to conventional methods.
Water consumption represents another critical environmental consideration. While PEC systems directly utilize water as a feedstock, the water footprint extends to catalyst production and system manufacturing. Encouragingly, the operational water requirements for PEC systems are significantly lower than those for biofuel-based hydrogen production pathways, with estimates suggesting 70-85% reduction in water consumption per kilogram of hydrogen produced.
Land use impacts vary considerably depending on system design and deployment scale. Distributed PEC systems integrated into existing infrastructure demonstrate minimal additional land requirements, whereas centralized production facilities necessitate dedicated land allocation. The scalability of advanced catalyst technologies will determine their ultimate land use efficiency, with current projections suggesting potential hydrogen production of 200-300 kg/hectare/year for optimized systems.
From a circular economy perspective, catalyst recyclability presents both challenges and opportunities. Precious metal catalysts offer economic incentives for recovery and reuse, with established recycling pathways achieving recovery rates exceeding 90%. However, composite and nanostructured catalysts often complicate separation processes. Emerging technologies for selective leaching and electrochemical recovery show promise for improving end-of-life management of complex catalyst systems.
The net environmental benefit of advanced PEC catalysts ultimately depends on their efficiency, durability, and production methods. Life cycle assessments indicate that high-efficiency catalysts with lifespans exceeding 5 years can achieve carbon payback periods of 1-2 years when powered by renewable electricity, representing a significant improvement over fossil-based hydrogen production pathways that emit 9-12 kg CO₂/kg H₂.
The manufacturing processes for advanced catalysts involve multiple chemical treatments and high-temperature syntheses that contribute to their carbon footprint. Nanostructured catalysts, while enhancing performance through increased surface area, often require specialized fabrication techniques with additional environmental costs. Recent advancements in green synthesis methods, including biomass-derived precursors and ambient-temperature processes, have shown potential to reduce these impacts by up to 40% compared to conventional methods.
Water consumption represents another critical environmental consideration. While PEC systems directly utilize water as a feedstock, the water footprint extends to catalyst production and system manufacturing. Encouragingly, the operational water requirements for PEC systems are significantly lower than those for biofuel-based hydrogen production pathways, with estimates suggesting 70-85% reduction in water consumption per kilogram of hydrogen produced.
Land use impacts vary considerably depending on system design and deployment scale. Distributed PEC systems integrated into existing infrastructure demonstrate minimal additional land requirements, whereas centralized production facilities necessitate dedicated land allocation. The scalability of advanced catalyst technologies will determine their ultimate land use efficiency, with current projections suggesting potential hydrogen production of 200-300 kg/hectare/year for optimized systems.
From a circular economy perspective, catalyst recyclability presents both challenges and opportunities. Precious metal catalysts offer economic incentives for recovery and reuse, with established recycling pathways achieving recovery rates exceeding 90%. However, composite and nanostructured catalysts often complicate separation processes. Emerging technologies for selective leaching and electrochemical recovery show promise for improving end-of-life management of complex catalyst systems.
The net environmental benefit of advanced PEC catalysts ultimately depends on their efficiency, durability, and production methods. Life cycle assessments indicate that high-efficiency catalysts with lifespans exceeding 5 years can achieve carbon payback periods of 1-2 years when powered by renewable electricity, representing a significant improvement over fossil-based hydrogen production pathways that emit 9-12 kg CO₂/kg H₂.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!