Advancing PEC water splitting through selective catalytic processes.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
PEC Water Splitting Background and Objectives
Photoelectrochemical (PEC) water splitting represents a promising approach for sustainable hydrogen production, leveraging solar energy to directly convert water into hydrogen and oxygen. This technology has evolved significantly since its inception in the 1970s with Fujishima and Honda's groundbreaking demonstration of water photolysis using titanium dioxide electrodes. The field has since expanded to encompass diverse semiconductor materials, novel catalyst designs, and innovative device architectures.
The fundamental principle of PEC water splitting involves the absorption of photons by semiconductor materials, generating electron-hole pairs that drive water oxidation and reduction reactions. This process mimics natural photosynthesis but aims to achieve higher solar-to-hydrogen conversion efficiencies. Current research focuses on overcoming efficiency limitations, enhancing stability, and reducing costs to make PEC systems commercially viable.
Selective catalytic processes have emerged as a critical frontier in advancing PEC water splitting. These processes involve the development of catalysts that can selectively promote desired reaction pathways while suppressing competing reactions. By enhancing reaction selectivity, these catalysts can significantly improve overall system efficiency and reduce energy losses associated with side reactions.
The global transition toward renewable energy sources has intensified interest in hydrogen as an energy carrier, positioning PEC water splitting as a key technology for future energy systems. Unlike conventional electrolysis, which requires separate electricity generation and water splitting steps, PEC systems integrate both functions into a single device, potentially offering higher overall efficiencies and lower costs.
The primary objectives in this field include achieving solar-to-hydrogen conversion efficiencies exceeding 10% (the threshold for commercial viability), developing materials with operational lifetimes of 10+ years, and reducing system costs to below $2/kg H₂. These targets align with the U.S. Department of Energy's Hydrogen Energy Earthshot initiative, which aims to reduce clean hydrogen costs by 80% within a decade.
Recent technological advances have focused on developing tandem photoelectrode configurations, surface modification strategies, and novel catalytic materials. Particularly promising are earth-abundant catalysts that can replace precious metals while maintaining high activity and selectivity. The integration of computational modeling with experimental approaches has accelerated materials discovery and optimization processes.
Looking forward, the field is moving toward integrated systems that combine selective catalysis with advanced light management strategies and robust protection layers. These developments aim to address the persistent challenges of efficiency, stability, and scalability that have limited widespread adoption of PEC water splitting technology.
The fundamental principle of PEC water splitting involves the absorption of photons by semiconductor materials, generating electron-hole pairs that drive water oxidation and reduction reactions. This process mimics natural photosynthesis but aims to achieve higher solar-to-hydrogen conversion efficiencies. Current research focuses on overcoming efficiency limitations, enhancing stability, and reducing costs to make PEC systems commercially viable.
Selective catalytic processes have emerged as a critical frontier in advancing PEC water splitting. These processes involve the development of catalysts that can selectively promote desired reaction pathways while suppressing competing reactions. By enhancing reaction selectivity, these catalysts can significantly improve overall system efficiency and reduce energy losses associated with side reactions.
The global transition toward renewable energy sources has intensified interest in hydrogen as an energy carrier, positioning PEC water splitting as a key technology for future energy systems. Unlike conventional electrolysis, which requires separate electricity generation and water splitting steps, PEC systems integrate both functions into a single device, potentially offering higher overall efficiencies and lower costs.
The primary objectives in this field include achieving solar-to-hydrogen conversion efficiencies exceeding 10% (the threshold for commercial viability), developing materials with operational lifetimes of 10+ years, and reducing system costs to below $2/kg H₂. These targets align with the U.S. Department of Energy's Hydrogen Energy Earthshot initiative, which aims to reduce clean hydrogen costs by 80% within a decade.
Recent technological advances have focused on developing tandem photoelectrode configurations, surface modification strategies, and novel catalytic materials. Particularly promising are earth-abundant catalysts that can replace precious metals while maintaining high activity and selectivity. The integration of computational modeling with experimental approaches has accelerated materials discovery and optimization processes.
Looking forward, the field is moving toward integrated systems that combine selective catalysis with advanced light management strategies and robust protection layers. These developments aim to address the persistent challenges of efficiency, stability, and scalability that have limited widespread adoption of PEC water splitting technology.
Market Analysis for Hydrogen Production Technologies
The global hydrogen market is experiencing significant growth, driven by increasing focus on clean energy solutions and decarbonization efforts across industries. Currently valued at approximately $130 billion, the hydrogen market is projected to reach $500 billion by 2030, with a compound annual growth rate exceeding 9.2% through the decade. Green hydrogen production methods, including photoelectrochemical (PEC) water splitting, are positioned to capture an expanding share of this market.
Traditional hydrogen production remains dominated by fossil fuel-based methods, with steam methane reforming accounting for roughly 76% of global production. However, this landscape is rapidly evolving as environmental regulations tighten and renewable energy costs decline. Electrolysis currently represents about 4% of hydrogen production but is experiencing the fastest growth rate among all production technologies at 22% annually.
PEC water splitting technology, while still emerging commercially, shows promising economic potential. Current production costs for hydrogen via PEC systems range between $8-15/kg, significantly higher than conventional methods ($1-3/kg) and standard electrolysis ($4-6/kg). However, technological advancements in selective catalytic processes could reduce PEC production costs to $3-5/kg by 2030, making it increasingly competitive.
Demand drivers for advanced hydrogen production technologies like selective catalytic PEC systems include industrial applications (refining, ammonia production), transportation (fuel cells), power generation, and emerging applications in steel manufacturing and synthetic fuel production. The transportation sector represents the fastest-growing demand segment, with a projected 35% annual growth rate through 2030.
Regional market analysis reveals Asia-Pacific as the dominant market for hydrogen technologies, followed by Europe and North America. Europe leads in green hydrogen initiatives with substantial government support, while Japan and South Korea focus heavily on hydrogen for transportation and power generation. China is rapidly scaling hydrogen production capacity with significant investments in both conventional and renewable methods.
Investment trends show accelerating capital flows into hydrogen technologies, with over $70 billion committed globally between 2020-2022. Venture capital funding for innovative hydrogen production startups reached $2.5 billion in 2022 alone, with catalytic technologies receiving particular attention. Major industrial players are forming strategic partnerships and joint ventures to commercialize advanced hydrogen production methods, including selective catalytic PEC water splitting technologies.
Traditional hydrogen production remains dominated by fossil fuel-based methods, with steam methane reforming accounting for roughly 76% of global production. However, this landscape is rapidly evolving as environmental regulations tighten and renewable energy costs decline. Electrolysis currently represents about 4% of hydrogen production but is experiencing the fastest growth rate among all production technologies at 22% annually.
PEC water splitting technology, while still emerging commercially, shows promising economic potential. Current production costs for hydrogen via PEC systems range between $8-15/kg, significantly higher than conventional methods ($1-3/kg) and standard electrolysis ($4-6/kg). However, technological advancements in selective catalytic processes could reduce PEC production costs to $3-5/kg by 2030, making it increasingly competitive.
Demand drivers for advanced hydrogen production technologies like selective catalytic PEC systems include industrial applications (refining, ammonia production), transportation (fuel cells), power generation, and emerging applications in steel manufacturing and synthetic fuel production. The transportation sector represents the fastest-growing demand segment, with a projected 35% annual growth rate through 2030.
Regional market analysis reveals Asia-Pacific as the dominant market for hydrogen technologies, followed by Europe and North America. Europe leads in green hydrogen initiatives with substantial government support, while Japan and South Korea focus heavily on hydrogen for transportation and power generation. China is rapidly scaling hydrogen production capacity with significant investments in both conventional and renewable methods.
Investment trends show accelerating capital flows into hydrogen technologies, with over $70 billion committed globally between 2020-2022. Venture capital funding for innovative hydrogen production startups reached $2.5 billion in 2022 alone, with catalytic technologies receiving particular attention. Major industrial players are forming strategic partnerships and joint ventures to commercialize advanced hydrogen production methods, including selective catalytic PEC water splitting technologies.
Current Challenges in Selective Catalysis for PEC Systems
Despite significant advancements in photoelectrochemical (PEC) water splitting technology, selective catalysis remains a critical bottleneck limiting commercial viability. Current catalytic systems face substantial challenges in achieving both high selectivity and efficiency under practical operating conditions. The fundamental issue lies in the complex interplay between light absorption, charge separation, and catalytic reactions occurring at semiconductor-electrolyte interfaces.
A primary challenge is catalyst stability under the harsh oxidative and reductive environments present during water splitting. Most high-performance catalysts for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) degrade rapidly, particularly when exposed to fluctuating pH conditions and reactive oxygen species generated during operation. Noble metal catalysts like platinum and iridium oxide demonstrate superior performance but remain prohibitively expensive for large-scale implementation.
Selectivity issues present another significant hurdle. Current catalytic systems often produce unwanted side reactions, reducing Faradaic efficiency and generating potentially harmful byproducts. For instance, carbon-based photoanodes frequently produce carbon dioxide instead of oxygen, while some metal oxide photocatalysts facilitate undesired redox reactions with solution components rather than water molecules.
The interface engineering between semiconductor photoabsorbers and catalytic materials presents persistent difficulties. Poor electronic coupling leads to charge recombination and energy losses at these critical junctions. Additionally, many catalyst deposition methods compromise the underlying semiconductor's light absorption properties, creating an inherent trade-off between catalytic activity and photoabsorption efficiency.
Scale-up challenges further complicate selective catalysis for PEC systems. Laboratory-scale catalysts often demonstrate dramatically reduced performance when implemented in larger devices due to mass transport limitations, uneven catalyst distribution, and increased resistance across larger electrode surfaces.
Mechanistic understanding remains incomplete for many catalytic processes in PEC environments. The dynamic nature of catalyst surfaces under illumination and applied potential creates transient active sites that are difficult to characterize using conventional analytical techniques. This knowledge gap hinders rational catalyst design approaches.
Earth-abundant alternatives to precious metal catalysts typically suffer from lower intrinsic activity, requiring higher catalyst loadings that block incident light and reduce overall system efficiency. While significant research focuses on developing non-noble metal catalysts, most still fall short of the activity and stability benchmarks needed for commercial viability.
The integration of selective catalysts with emerging tandem photoabsorber architectures presents additional compatibility challenges, as optimal operating conditions for catalysts may not align with the electrical characteristics of multi-junction semiconductor systems.
A primary challenge is catalyst stability under the harsh oxidative and reductive environments present during water splitting. Most high-performance catalysts for hydrogen evolution reaction (HER) and oxygen evolution reaction (OER) degrade rapidly, particularly when exposed to fluctuating pH conditions and reactive oxygen species generated during operation. Noble metal catalysts like platinum and iridium oxide demonstrate superior performance but remain prohibitively expensive for large-scale implementation.
Selectivity issues present another significant hurdle. Current catalytic systems often produce unwanted side reactions, reducing Faradaic efficiency and generating potentially harmful byproducts. For instance, carbon-based photoanodes frequently produce carbon dioxide instead of oxygen, while some metal oxide photocatalysts facilitate undesired redox reactions with solution components rather than water molecules.
The interface engineering between semiconductor photoabsorbers and catalytic materials presents persistent difficulties. Poor electronic coupling leads to charge recombination and energy losses at these critical junctions. Additionally, many catalyst deposition methods compromise the underlying semiconductor's light absorption properties, creating an inherent trade-off between catalytic activity and photoabsorption efficiency.
Scale-up challenges further complicate selective catalysis for PEC systems. Laboratory-scale catalysts often demonstrate dramatically reduced performance when implemented in larger devices due to mass transport limitations, uneven catalyst distribution, and increased resistance across larger electrode surfaces.
Mechanistic understanding remains incomplete for many catalytic processes in PEC environments. The dynamic nature of catalyst surfaces under illumination and applied potential creates transient active sites that are difficult to characterize using conventional analytical techniques. This knowledge gap hinders rational catalyst design approaches.
Earth-abundant alternatives to precious metal catalysts typically suffer from lower intrinsic activity, requiring higher catalyst loadings that block incident light and reduce overall system efficiency. While significant research focuses on developing non-noble metal catalysts, most still fall short of the activity and stability benchmarks needed for commercial viability.
The integration of selective catalysts with emerging tandem photoabsorber architectures presents additional compatibility challenges, as optimal operating conditions for catalysts may not align with the electrical characteristics of multi-junction semiconductor systems.
Current Catalytic Approaches for Enhanced PEC Efficiency
01 Catalyst design for selective PEC water splitting
Specific catalyst materials and designs can significantly enhance the selectivity of photoelectrochemical (PEC) water splitting processes. These catalysts are engineered to preferentially drive either hydrogen or oxygen evolution reactions while suppressing competing reactions. Advanced catalyst architectures including core-shell structures, nanoparticles, and composite materials can be tailored to optimize electron transfer pathways and reaction kinetics, thereby improving product selectivity and overall efficiency in PEC systems.- Catalyst materials for selective PEC water splitting: Various catalyst materials can be used to enhance the selectivity of photoelectrochemical (PEC) water splitting. These materials include metal oxides, noble metals, and composite structures that can selectively promote either hydrogen or oxygen evolution reactions. The catalysts can be designed with specific surface properties to improve charge separation and transfer, thereby increasing the efficiency and selectivity of the water splitting process.
- Semiconductor photoelectrode modifications for improved selectivity: Modifications to semiconductor photoelectrodes can significantly enhance the selectivity of PEC water splitting. These modifications include surface treatments, doping with specific elements, and creating heterojunctions that facilitate directional charge transfer. By engineering the band structure and surface properties of photoelectrodes, the selectivity towards hydrogen or oxygen evolution can be controlled, leading to more efficient water splitting processes.
- Nanostructured materials for selective PEC reactions: Nanostructured materials offer unique advantages for selective PEC water splitting due to their high surface area and tunable properties. These materials include nanowires, nanotubes, and nanoparticles that can be designed to have specific catalytic sites for targeted reactions. The controlled morphology and composition of these nanostructures can enhance charge separation and transfer, leading to improved selectivity in the water splitting process.
- Reaction environment control for enhanced selectivity: Controlling the reaction environment is crucial for enhancing the selectivity of PEC water splitting. This includes adjusting parameters such as pH, electrolyte composition, temperature, and light intensity. By optimizing these conditions, the kinetics of competing reactions can be manipulated to favor the desired pathway, thereby improving the selectivity of the water splitting process and reducing unwanted side reactions.
- Novel device architectures for selective PEC systems: Innovative device architectures can significantly improve the selectivity of PEC water splitting systems. These designs include tandem cell configurations, Z-scheme systems, and integrated devices that spatially separate the hydrogen and oxygen evolution reactions. By optimizing the arrangement of photoelectrodes, membranes, and catalysts, these architectures can enhance charge separation and product collection, leading to higher selectivity and efficiency in water splitting.
02 Semiconductor photoelectrode modifications for selective water splitting
Modifications to semiconductor photoelectrodes can enhance selectivity in PEC water splitting. These modifications include surface treatments, doping strategies, and bandgap engineering to optimize light absorption and charge separation. By tuning the electronic properties of photoelectrodes, the energy levels can be aligned to favor specific redox reactions, improving selectivity toward hydrogen or oxygen production while minimizing side reactions and recombination losses.Expand Specific Solutions03 Electrolyte composition and pH control for selective PEC reactions
The composition of the electrolyte solution and pH control play crucial roles in determining the selectivity of PEC water splitting. By adjusting electrolyte components, concentration, and pH levels, the thermodynamics and kinetics of competing reactions can be manipulated to favor desired pathways. Specific additives can suppress unwanted side reactions while promoting hydrogen or oxygen evolution, thereby enhancing overall process selectivity and efficiency.Expand Specific Solutions04 Novel device architectures for selective PEC systems
Innovative PEC device architectures can significantly improve reaction selectivity in water splitting applications. These designs include tandem cell configurations, Z-scheme systems, and compartmentalized reactors that physically separate hydrogen and oxygen evolution reactions. Advanced membrane technologies and specialized flow systems can further enhance product separation and collection, minimizing back-reactions and improving overall system selectivity and efficiency.Expand Specific Solutions05 Light management strategies for selective PEC processes
Strategic light management approaches can enhance selectivity in PEC water splitting by optimizing photon utilization. These strategies include spectral splitting techniques, plasmonic enhancement, photonic crystals, and specialized light-trapping structures. By directing specific wavelengths to appropriate photoactive components and enhancing light absorption at catalytically active sites, these methods can improve charge carrier generation and transfer to favor desired reaction pathways, thereby increasing process selectivity.Expand Specific Solutions
Leading Research Groups and Companies in PEC Catalysis
The photoelectrochemical (PEC) water splitting market is currently in an early growth phase, characterized by intensive R&D activities and emerging commercial applications. The global market size is projected to expand significantly as renewable hydrogen production becomes increasingly critical for decarbonization efforts. Technologically, selective catalytic processes for PEC water splitting are advancing rapidly but remain at moderate maturity levels. Leading academic institutions including Northwestern University, Monash University, and Technical University of Berlin are driving fundamental research, while companies like SABIC Global Technologies, DENSO Corp, and EDAC Labs are focusing on commercial applications. Research organizations such as Max Planck Society and Alliance for Sustainable Energy are bridging the gap between theoretical advances and practical implementation, creating a competitive landscape where collaboration between industry and academia is essential for market advancement.
Alliance for Sustainable Energy LLC
Technical Solution: Alliance for Sustainable Energy, which manages the National Renewable Energy Laboratory (NREL), has developed comprehensive solutions for advancing PEC water splitting through selective catalytic processes. Their approach focuses on tandem photoelectrochemical cells that maximize solar-to-hydrogen conversion efficiency by utilizing complementary light absorption. They've engineered innovative semiconductor materials with precisely tuned band gaps to optimize solar spectrum utilization. Their technology employs advanced surface modification techniques including atomic layer deposition to create protective layers that enhance stability while maintaining catalytic activity. NREL researchers have developed earth-abundant catalysts based on transition metal phosphides, nitrides, and carbides that offer performance comparable to precious metal catalysts at significantly lower costs[9]. Their systems incorporate innovative device architectures that minimize resistive losses and optimize mass transport. Recent breakthroughs include the development of III-V semiconductor-based photoelectrodes with record-setting solar-to-hydrogen efficiencies exceeding 16% and the integration of selective membranes that enable efficient product separation[10]. Their technology also features advanced modeling capabilities that guide materials discovery and device optimization through high-throughput computational screening.
Strengths: Industry-leading solar-to-hydrogen conversion efficiencies; comprehensive approach addressing materials, catalysts, and device integration; strong focus on practical implementation and scalability. Weaknesses: Higher costs associated with high-efficiency III-V semiconductor systems; challenges in maintaining performance during scale-up; potential durability issues under real-world operating conditions.
Dalian University of Technology
Technical Solution: Dalian University of Technology has pioneered innovative approaches to PEC water splitting through selective catalytic processes. Their research focuses on developing highly efficient and stable photocatalysts based on earth-abundant materials. They've created novel composite photoanodes combining metal oxides with carbon-based materials to enhance charge separation and transfer. Their technology employs a unique co-catalyst loading strategy that selectively promotes water oxidation while suppressing side reactions. The university has developed a series of hierarchical nanostructured catalysts with optimized morphology for maximum active site exposure and light harvesting capability[2]. Their systems incorporate Z-scheme heterojunctions that effectively separate photogenerated electron-hole pairs, significantly improving quantum efficiency. Recent breakthroughs include the development of defect-engineered semiconductors with tailored band structures for visible light absorption and the integration of plasmonic nanoparticles to enhance light absorption through surface plasmon resonance effects[5].
Strengths: Cost-effective materials selection focusing on earth-abundant elements; innovative heterojunction designs with superior charge separation; excellent stability under operating conditions. Weaknesses: Lower overall efficiency compared to noble metal-based systems; potential challenges in maintaining performance during scale-up; limited performance in the infrared region of the solar spectrum.
Key Innovations in Selective Catalytic Materials
Photo electrochemical cell for water splitting
PatentActiveUS11859295B2
Innovation
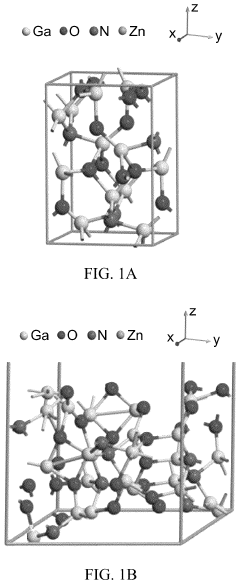
- A GaON/ZnO photoelectrode is developed with gallium oxynitride nanoparticles interspersed in zinc oxide nanoparticles, deposited on a metal oxide conducting substrate, optimizing the nanoarchitectured photocatalytic material for improved visible light absorption and charge separation.
Scalability and Economic Viability Assessment
The scalability of photoelectrochemical (PEC) water splitting technologies utilizing selective catalytic processes faces significant challenges when transitioning from laboratory-scale demonstrations to industrial applications. Current PEC systems typically operate at efficiencies between 5-15% in controlled laboratory environments, but these figures often decrease substantially when scaled up due to non-uniform light distribution, mass transport limitations, and catalyst degradation over larger surface areas.
Economic viability remains a critical barrier to widespread adoption. Capital expenditure for PEC infrastructure currently ranges from $300-500/kW, significantly higher than conventional hydrogen production methods such as steam methane reforming ($600-900/kW) but potentially competitive with electrolysis ($1000-1500/kW) when considering integrated solar harvesting capabilities. Operating costs are primarily influenced by system durability, with current catalyst lifespans typically limited to 1000-2000 hours before significant performance degradation occurs.
Manufacturing scalability presents another dimension of challenges. While many selective catalysts demonstrate promising performance using precious metals like platinum and iridium, their limited global supply and high costs (currently $30,000-50,000/kg) restrict large-scale implementation. Earth-abundant alternatives based on transition metal compounds show promise but currently exhibit 30-50% lower catalytic efficiency and accelerated degradation rates.
Infrastructure requirements for scaled PEC systems include considerations for water purification, product separation, and storage facilities. The integration with existing energy systems requires substantial investment in hydrogen transport and storage infrastructure, estimated at $1-2 million per mile of hydrogen pipeline. Regional variations in solar irradiance further complicate economic assessments, with optimal locations potentially achieving levelized hydrogen costs of $4-6/kg compared to $8-12/kg in less favorable regions.
Recent techno-economic analyses suggest that selective catalytic PEC systems could achieve hydrogen production costs of $3-5/kg by 2030 with continued research advances and manufacturing scale-up, approaching the U.S. Department of Energy target of $2/kg by 2035. This would require improvements in catalyst selectivity to achieve faradaic efficiencies consistently above 95%, system lifetimes exceeding 10,000 hours, and significant reductions in balance-of-system costs through standardized manufacturing and installation processes.
Economic viability remains a critical barrier to widespread adoption. Capital expenditure for PEC infrastructure currently ranges from $300-500/kW, significantly higher than conventional hydrogen production methods such as steam methane reforming ($600-900/kW) but potentially competitive with electrolysis ($1000-1500/kW) when considering integrated solar harvesting capabilities. Operating costs are primarily influenced by system durability, with current catalyst lifespans typically limited to 1000-2000 hours before significant performance degradation occurs.
Manufacturing scalability presents another dimension of challenges. While many selective catalysts demonstrate promising performance using precious metals like platinum and iridium, their limited global supply and high costs (currently $30,000-50,000/kg) restrict large-scale implementation. Earth-abundant alternatives based on transition metal compounds show promise but currently exhibit 30-50% lower catalytic efficiency and accelerated degradation rates.
Infrastructure requirements for scaled PEC systems include considerations for water purification, product separation, and storage facilities. The integration with existing energy systems requires substantial investment in hydrogen transport and storage infrastructure, estimated at $1-2 million per mile of hydrogen pipeline. Regional variations in solar irradiance further complicate economic assessments, with optimal locations potentially achieving levelized hydrogen costs of $4-6/kg compared to $8-12/kg in less favorable regions.
Recent techno-economic analyses suggest that selective catalytic PEC systems could achieve hydrogen production costs of $3-5/kg by 2030 with continued research advances and manufacturing scale-up, approaching the U.S. Department of Energy target of $2/kg by 2035. This would require improvements in catalyst selectivity to achieve faradaic efficiencies consistently above 95%, system lifetimes exceeding 10,000 hours, and significant reductions in balance-of-system costs through standardized manufacturing and installation processes.
Environmental Impact and Sustainability Considerations
Photoelectrochemical (PEC) water splitting through selective catalytic processes represents a promising pathway toward sustainable hydrogen production, yet its environmental implications warrant careful consideration. The environmental footprint of PEC systems extends beyond operational phases to encompass manufacturing, deployment, and end-of-life management. Materials used in photocatalysts and electrodes—often including rare earth elements, noble metals, and semiconductor compounds—raise sustainability concerns regarding resource depletion and extraction impacts. Current manufacturing processes for high-efficiency catalysts frequently involve energy-intensive methods and potentially hazardous chemicals, creating environmental trade-offs that must be balanced against the clean energy benefits.
Water consumption presents another critical environmental consideration. While water serves as the primary feedstock for hydrogen production, PEC systems must be designed to minimize freshwater usage, particularly in water-stressed regions. The integration of seawater or wastewater as alternative sources shows promise but introduces additional technical challenges related to catalyst durability and system performance under non-ideal conditions. Furthermore, the potential release of catalyst nanoparticles or degradation products into aquatic ecosystems necessitates robust containment strategies and lifecycle monitoring protocols.
Energy payback periods—the time required for a PEC system to generate the equivalent energy used in its production—vary significantly based on catalyst composition and manufacturing methods. Selective catalytic processes that utilize earth-abundant materials and low-energy synthesis routes offer substantially improved sustainability profiles compared to conventional approaches. Life cycle assessment (LCA) studies indicate that optimized PEC systems can achieve carbon payback within 1-3 years of operation, though this varies with geographical location, system configuration, and operational parameters.
Land use implications also merit attention, particularly for scaled deployment scenarios. While distributed PEC systems can leverage existing infrastructure, utility-scale implementations require dedicated land area with appropriate solar exposure. Dual-use approaches, such as integrating PEC systems with agricultural activities or building surfaces, present opportunities to minimize land-use conflicts while maximizing value generation from available space.
Regulatory frameworks governing chemical usage, emissions standards, and waste management significantly influence the environmental viability of advanced PEC technologies. Emerging international standards for sustainable hydrogen production increasingly incorporate full lifecycle environmental considerations, creating both challenges and opportunities for technology developers. Forward-looking PEC research must therefore prioritize not only catalytic efficiency but also environmental compatibility through green chemistry principles, circular design approaches, and responsible materials selection.
Water consumption presents another critical environmental consideration. While water serves as the primary feedstock for hydrogen production, PEC systems must be designed to minimize freshwater usage, particularly in water-stressed regions. The integration of seawater or wastewater as alternative sources shows promise but introduces additional technical challenges related to catalyst durability and system performance under non-ideal conditions. Furthermore, the potential release of catalyst nanoparticles or degradation products into aquatic ecosystems necessitates robust containment strategies and lifecycle monitoring protocols.
Energy payback periods—the time required for a PEC system to generate the equivalent energy used in its production—vary significantly based on catalyst composition and manufacturing methods. Selective catalytic processes that utilize earth-abundant materials and low-energy synthesis routes offer substantially improved sustainability profiles compared to conventional approaches. Life cycle assessment (LCA) studies indicate that optimized PEC systems can achieve carbon payback within 1-3 years of operation, though this varies with geographical location, system configuration, and operational parameters.
Land use implications also merit attention, particularly for scaled deployment scenarios. While distributed PEC systems can leverage existing infrastructure, utility-scale implementations require dedicated land area with appropriate solar exposure. Dual-use approaches, such as integrating PEC systems with agricultural activities or building surfaces, present opportunities to minimize land-use conflicts while maximizing value generation from available space.
Regulatory frameworks governing chemical usage, emissions standards, and waste management significantly influence the environmental viability of advanced PEC technologies. Emerging international standards for sustainable hydrogen production increasingly incorporate full lifecycle environmental considerations, creating both challenges and opportunities for technology developers. Forward-looking PEC research must therefore prioritize not only catalytic efficiency but also environmental compatibility through green chemistry principles, circular design approaches, and responsible materials selection.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!