The role of ionic liquids in Photoelectrochemical Water Splitting systems.
SEP 4, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Liquids in PEC Water Splitting: Background and Objectives
Photoelectrochemical (PEC) water splitting represents a promising approach for sustainable hydrogen production, harnessing solar energy to drive the decomposition of water into hydrogen and oxygen. The integration of ionic liquids (ILs) into PEC systems has emerged as a significant research direction over the past decade, offering potential solutions to longstanding challenges in this field. Historically, conventional PEC systems have faced limitations related to stability, efficiency, and scalability, prompting researchers to explore alternative electrolyte systems beyond traditional aqueous solutions.
Ionic liquids, defined as salts with melting points below 100°C, possess unique physicochemical properties including negligible vapor pressure, high thermal stability, wide electrochemical windows, and tunable structures. These characteristics make them particularly attractive for electrochemical applications, including water splitting. The evolution of IL research in PEC systems can be traced from initial exploratory studies in the early 2000s to more sophisticated applications in recent years, with significant acceleration in research output since 2015.
The fundamental objective of incorporating ionic liquids into PEC water splitting systems is to enhance overall system performance through multiple mechanisms. These include improving charge transfer at semiconductor-electrolyte interfaces, extending the stability of photoelectrode materials, expanding the operational temperature range, and facilitating more efficient separation of produced gases. Additionally, the unique solvation environment provided by ILs can potentially alter reaction pathways and energetics in ways that promote hydrogen evolution.
Current technological trends indicate growing interest in task-specific ionic liquids designed explicitly for PEC applications, with molecular engineering approaches enabling precise control over properties relevant to water splitting. The development of protic ionic liquids, which can participate directly in proton transfer processes, represents a particularly promising direction. Simultaneously, research is advancing toward hybrid electrolyte systems that combine the advantages of ionic liquids with conventional aqueous electrolytes.
The anticipated technological goals in this field include achieving solar-to-hydrogen conversion efficiencies exceeding 10% in IL-based systems, developing IL electrolytes compatible with earth-abundant photoelectrode materials, and designing systems capable of continuous operation for thousands of hours without significant degradation. Long-term objectives extend to the integration of IL-based PEC systems with existing renewable energy infrastructure and the development of scalable manufacturing processes suitable for commercial deployment.
Understanding the role of ionic liquids in PEC water splitting necessitates a multidisciplinary approach, bridging electrochemistry, materials science, photophysics, and chemical engineering. This convergence of disciplines reflects the complexity of the challenges being addressed and the transformative potential of successful solutions for sustainable energy production.
Ionic liquids, defined as salts with melting points below 100°C, possess unique physicochemical properties including negligible vapor pressure, high thermal stability, wide electrochemical windows, and tunable structures. These characteristics make them particularly attractive for electrochemical applications, including water splitting. The evolution of IL research in PEC systems can be traced from initial exploratory studies in the early 2000s to more sophisticated applications in recent years, with significant acceleration in research output since 2015.
The fundamental objective of incorporating ionic liquids into PEC water splitting systems is to enhance overall system performance through multiple mechanisms. These include improving charge transfer at semiconductor-electrolyte interfaces, extending the stability of photoelectrode materials, expanding the operational temperature range, and facilitating more efficient separation of produced gases. Additionally, the unique solvation environment provided by ILs can potentially alter reaction pathways and energetics in ways that promote hydrogen evolution.
Current technological trends indicate growing interest in task-specific ionic liquids designed explicitly for PEC applications, with molecular engineering approaches enabling precise control over properties relevant to water splitting. The development of protic ionic liquids, which can participate directly in proton transfer processes, represents a particularly promising direction. Simultaneously, research is advancing toward hybrid electrolyte systems that combine the advantages of ionic liquids with conventional aqueous electrolytes.
The anticipated technological goals in this field include achieving solar-to-hydrogen conversion efficiencies exceeding 10% in IL-based systems, developing IL electrolytes compatible with earth-abundant photoelectrode materials, and designing systems capable of continuous operation for thousands of hours without significant degradation. Long-term objectives extend to the integration of IL-based PEC systems with existing renewable energy infrastructure and the development of scalable manufacturing processes suitable for commercial deployment.
Understanding the role of ionic liquids in PEC water splitting necessitates a multidisciplinary approach, bridging electrochemistry, materials science, photophysics, and chemical engineering. This convergence of disciplines reflects the complexity of the challenges being addressed and the transformative potential of successful solutions for sustainable energy production.
Market Analysis of PEC Hydrogen Production Technologies
The global market for photoelectrochemical (PEC) hydrogen production technologies is experiencing significant growth, driven by increasing demand for clean energy solutions and the global push towards decarbonization. Current market valuations estimate the PEC hydrogen sector to reach approximately $5.4 billion by 2030, with a compound annual growth rate of 14.2% from 2023 to 2030.
The market segmentation for PEC hydrogen production technologies can be divided into three primary categories: research and development, commercial demonstration projects, and early commercial deployments. Currently, the R&D segment dominates the market share, accounting for over 65% of investments, reflecting the nascent nature of this technology.
Geographically, North America and Europe lead in PEC hydrogen research investments, with the United States, Germany, and Japan being the primary markets. However, China is rapidly increasing its market presence through substantial government funding initiatives aimed at renewable hydrogen technologies, including PEC systems.
Key market drivers include declining renewable energy costs, stringent carbon emission regulations, and increasing government subsidies for clean hydrogen production. The integration of ionic liquids in PEC systems represents a growing niche within this market, with specialized applications showing particular promise in enhancing system efficiency and durability.
Market challenges remain significant, primarily centered around high production costs compared to conventional hydrogen production methods. Current levelized cost of hydrogen (LCOH) from PEC systems ranges between $8-15/kg, substantially higher than the $1-3/kg target needed for widespread commercial viability. The incorporation of ionic liquids adds complexity to this cost structure, though long-term benefits may offset initial investments.
Industry analysts project that technological breakthroughs in PEC systems, particularly those involving advanced electrolytes like ionic liquids, could reduce production costs by up to 40% by 2028, potentially accelerating market adoption. The market for specialized ionic liquid formulations for PEC applications is expected to grow at 18% annually, outpacing the broader PEC market.
End-user industries showing the strongest interest include chemical manufacturing, transportation, and energy storage sectors. Strategic partnerships between technology developers and industrial end-users are becoming increasingly common, with over 30 major collaboration agreements announced in the past two years focusing on scaling PEC hydrogen production technologies.
The competitive landscape features both established energy companies diversifying into hydrogen technologies and specialized startups focused exclusively on PEC innovations. Venture capital funding for PEC technology startups has reached $1.2 billion in 2022, with approximately 22% directed toward projects involving advanced electrolyte systems including ionic liquid applications.
The market segmentation for PEC hydrogen production technologies can be divided into three primary categories: research and development, commercial demonstration projects, and early commercial deployments. Currently, the R&D segment dominates the market share, accounting for over 65% of investments, reflecting the nascent nature of this technology.
Geographically, North America and Europe lead in PEC hydrogen research investments, with the United States, Germany, and Japan being the primary markets. However, China is rapidly increasing its market presence through substantial government funding initiatives aimed at renewable hydrogen technologies, including PEC systems.
Key market drivers include declining renewable energy costs, stringent carbon emission regulations, and increasing government subsidies for clean hydrogen production. The integration of ionic liquids in PEC systems represents a growing niche within this market, with specialized applications showing particular promise in enhancing system efficiency and durability.
Market challenges remain significant, primarily centered around high production costs compared to conventional hydrogen production methods. Current levelized cost of hydrogen (LCOH) from PEC systems ranges between $8-15/kg, substantially higher than the $1-3/kg target needed for widespread commercial viability. The incorporation of ionic liquids adds complexity to this cost structure, though long-term benefits may offset initial investments.
Industry analysts project that technological breakthroughs in PEC systems, particularly those involving advanced electrolytes like ionic liquids, could reduce production costs by up to 40% by 2028, potentially accelerating market adoption. The market for specialized ionic liquid formulations for PEC applications is expected to grow at 18% annually, outpacing the broader PEC market.
End-user industries showing the strongest interest include chemical manufacturing, transportation, and energy storage sectors. Strategic partnerships between technology developers and industrial end-users are becoming increasingly common, with over 30 major collaboration agreements announced in the past two years focusing on scaling PEC hydrogen production technologies.
The competitive landscape features both established energy companies diversifying into hydrogen technologies and specialized startups focused exclusively on PEC innovations. Venture capital funding for PEC technology startups has reached $1.2 billion in 2022, with approximately 22% directed toward projects involving advanced electrolyte systems including ionic liquid applications.
Current Status and Challenges of Ionic Liquids in PEC Systems
The integration of ionic liquids (ILs) in photoelectrochemical (PEC) water splitting systems has gained significant attention in recent years. Currently, ILs are being explored as electrolytes, solvents, and modifiers for photoelectrode materials. Their unique properties, including high ionic conductivity, wide electrochemical windows, and negligible vapor pressure, make them promising candidates for enhancing PEC performance. Research indicates that ILs can improve charge transfer efficiency at semiconductor-electrolyte interfaces and stabilize photoactive materials against photocorrosion.
Despite these advantages, the widespread implementation of ILs in PEC systems faces several challenges. The high viscosity of many ILs limits mass transport and consequently affects the overall efficiency of water splitting reactions. Additionally, the cost of synthesizing high-purity ILs remains prohibitively high for large-scale applications, hindering their commercial viability in renewable energy technologies.
Another significant challenge is the limited understanding of the complex interactions between ILs and semiconductor surfaces. The interfacial chemistry, which is crucial for efficient charge separation and transfer, is not fully elucidated. This knowledge gap impedes the rational design of IL-based PEC systems with optimized performance. Furthermore, the long-term stability of IL-containing PEC cells under continuous operation and exposure to light remains questionable, with degradation pathways not thoroughly investigated.
From a geographical perspective, research on IL applications in PEC water splitting is concentrated primarily in developed nations, with significant contributions from research institutions in the United States, European Union, Japan, and increasingly China. This uneven distribution of research capabilities creates disparities in technological advancement and potential implementation.
The environmental impact of ILs also presents a paradoxical challenge. While they are often touted as "green" solvents due to their non-volatility, some ILs exhibit toxicity and poor biodegradability. This contradicts the fundamental sustainability goals of PEC water splitting technology, which aims to produce clean hydrogen fuel without adverse environmental consequences.
Technical standardization represents another hurdle, as there is currently no consensus on optimal IL compositions or testing protocols for PEC applications. This lack of standardization complicates comparative analyses between different research findings and slows progress toward practical implementations. Additionally, the scalability of IL synthesis and purification processes needs significant improvement to meet the potential demand for large-scale PEC systems.
Despite these advantages, the widespread implementation of ILs in PEC systems faces several challenges. The high viscosity of many ILs limits mass transport and consequently affects the overall efficiency of water splitting reactions. Additionally, the cost of synthesizing high-purity ILs remains prohibitively high for large-scale applications, hindering their commercial viability in renewable energy technologies.
Another significant challenge is the limited understanding of the complex interactions between ILs and semiconductor surfaces. The interfacial chemistry, which is crucial for efficient charge separation and transfer, is not fully elucidated. This knowledge gap impedes the rational design of IL-based PEC systems with optimized performance. Furthermore, the long-term stability of IL-containing PEC cells under continuous operation and exposure to light remains questionable, with degradation pathways not thoroughly investigated.
From a geographical perspective, research on IL applications in PEC water splitting is concentrated primarily in developed nations, with significant contributions from research institutions in the United States, European Union, Japan, and increasingly China. This uneven distribution of research capabilities creates disparities in technological advancement and potential implementation.
The environmental impact of ILs also presents a paradoxical challenge. While they are often touted as "green" solvents due to their non-volatility, some ILs exhibit toxicity and poor biodegradability. This contradicts the fundamental sustainability goals of PEC water splitting technology, which aims to produce clean hydrogen fuel without adverse environmental consequences.
Technical standardization represents another hurdle, as there is currently no consensus on optimal IL compositions or testing protocols for PEC applications. This lack of standardization complicates comparative analyses between different research findings and slows progress toward practical implementations. Additionally, the scalability of IL synthesis and purification processes needs significant improvement to meet the potential demand for large-scale PEC systems.
Current Implementation Strategies of Ionic Liquids in PEC Systems
01 Synthesis and preparation of ionic liquids
Various methods for synthesizing and preparing ionic liquids are described, including novel reaction pathways and purification techniques. These methods aim to produce ionic liquids with specific properties, such as low melting points, high thermal stability, and low viscosity. The synthesis typically involves the combination of organic cations with various anions to create room-temperature ionic liquids suitable for different applications.- Synthesis and preparation of ionic liquids: Various methods for synthesizing and preparing ionic liquids are described, including novel approaches to create ionic liquids with specific properties. These methods involve chemical reactions to form ionic compounds that remain liquid at room temperature. The synthesis processes often focus on creating ionic liquids with tailored characteristics for specific applications, such as improved conductivity, stability, or solubility properties.
- Ionic liquids in electrochemical applications: Ionic liquids are utilized in various electrochemical applications due to their excellent conductivity and wide electrochemical windows. They serve as electrolytes in batteries, fuel cells, and other energy storage devices. Their non-volatility and thermal stability make them superior alternatives to conventional electrolytes, enhancing the performance and safety of electrochemical systems.
- Ionic liquids for separation and purification processes: Ionic liquids are employed in separation and purification processes across various industries. Their unique solvation properties allow for selective extraction of target compounds from mixtures. These liquids can be designed to have specific affinities for certain molecules, enabling efficient separation of components in complex mixtures, such as in petrochemical processing or pharmaceutical purification.
- Ionic liquids as catalysts and reaction media: Ionic liquids function as both catalysts and reaction media in various chemical processes. Their unique properties allow them to facilitate reactions that might be difficult in conventional solvents. They can enhance reaction rates, improve selectivity, and enable novel reaction pathways. Additionally, their recyclability makes them environmentally friendly alternatives to traditional catalysts and solvents.
- Ionic liquids in material science and engineering: Ionic liquids are increasingly used in material science and engineering applications. They serve as components in advanced materials such as polymers, coatings, and lubricants. Their incorporation can impart unique properties to materials, including enhanced thermal stability, improved mechanical properties, and specialized surface characteristics. These applications extend to areas such as aerospace, automotive, and electronics industries.
02 Ionic liquids for electrochemical applications
Ionic liquids are utilized in various electrochemical applications, including batteries, fuel cells, and supercapacitors. Their unique properties, such as wide electrochemical windows, high ionic conductivity, and negligible vapor pressure, make them excellent electrolytes. These ionic liquid-based electrolytes can enhance the performance, safety, and longevity of electrochemical devices compared to conventional electrolyte systems.Expand Specific Solutions03 Ionic liquids for separation and extraction processes
Ionic liquids serve as effective solvents for separation and extraction processes in various industries. Their tunable properties allow for selective extraction of target compounds from complex mixtures. Applications include the separation of metals, removal of contaminants from fuels, gas separation, and extraction of valuable compounds from biomass. These processes often demonstrate higher efficiency and environmental benefits compared to conventional separation methods.Expand Specific Solutions04 Functionalized ionic liquids and task-specific designs
Functionalized ionic liquids are designed with specific chemical groups to perform targeted tasks. These task-specific ionic liquids can be tailored for catalysis, CO2 capture, biomass processing, or other specialized applications. By incorporating functional groups into either the cation or anion, these ionic liquids can achieve enhanced selectivity, reactivity, or affinity for particular substrates, making them valuable tools in green chemistry and sustainable processes.Expand Specific Solutions05 Ionic liquids in polymer and material science
Ionic liquids are incorporated into polymers and materials to create composites with enhanced properties. These applications include polymer electrolytes, conductive materials, coatings, and advanced functional materials. The integration of ionic liquids can improve mechanical strength, thermal stability, conductivity, or introduce stimuli-responsive behavior to materials. These ionic liquid-based materials find applications in sensors, actuators, membranes, and smart devices.Expand Specific Solutions
Leading Research Groups and Companies in PEC Ionic Liquid Applications
The photoelectrochemical water splitting market is currently in an early growth phase, with increasing research interest driven by renewable energy demands. The global market size remains relatively modest but is projected to expand significantly as hydrogen economy initiatives gain momentum. Technologically, ionic liquids in water splitting systems are at the emerging stage of development, with key players demonstrating varied levels of maturity. Academic institutions like King Abdullah University of Science & Technology, University of Tokyo, and Chinese Academy of Sciences are leading fundamental research, while companies including BASF, Toyota, and Siemens are advancing practical applications. Specialized firms like Linzhou Ke Neng Material Science & Technology have developed proprietary ionic liquid technologies specifically targeting electrochemical applications, indicating growing commercial interest in this promising green technology field.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering (IPE) at the Chinese Academy of Sciences has developed innovative ionic liquid-based electrolytes for photoelectrochemical (PEC) water splitting systems. Their approach focuses on using room temperature ionic liquids (RTILs) as both electrolytes and stabilizing agents for semiconductor photoelectrodes. IPE researchers have engineered task-specific ionic liquids with functional groups that enhance charge transfer at the electrode-electrolyte interface while minimizing recombination losses. Their proprietary ionic liquid formulations have demonstrated improved stability of metal oxide photoanodes (particularly BiVO4 and Fe2O3) by forming protective layers that prevent photocorrosion while maintaining efficient charge transport. Recent work has shown that imidazolium-based ionic liquids with phosphate functionalization can increase photocurrent densities by up to 40% compared to conventional aqueous electrolytes, while extending electrode operational lifetime from hours to weeks.
Strengths: Superior electrode stability in harsh oxidative environments; enhanced charge separation efficiency; tunable viscosity and conductivity properties for optimized performance. Weaknesses: Higher cost compared to conventional aqueous electrolytes; potential mass transport limitations due to higher viscosity; scaling challenges for large-area photoelectrodes.
École Polytechnique Fédérale de Lausanne
Technical Solution: École Polytechnique Fédérale de Lausanne (EPFL) has pioneered the development of ionic liquid-based systems for photoelectrochemical water splitting through their Laboratory of Photonics and Interfaces. Their approach centers on using ionic liquids as both electrolytes and structural components in dye-sensitized photoelectrochemical cells (DS-PECs). EPFL researchers have developed proprietary formulations of imidazolium-based ionic liquids that serve as redox mediators while providing exceptional stability against water and oxygen. Their most significant innovation involves the creation of water-in-ionic-liquid microemulsions that maintain the benefits of ionic liquids while enabling water splitting reactions. These systems have demonstrated solar-to-hydrogen efficiencies exceeding 5% with remarkable stability over thousands of hours of operation. EPFL has also developed novel ionic liquid-based gel electrolytes incorporating nanostructured materials that enhance charge transport while maintaining the structural integrity of photoelectrodes. Their recent work has focused on integrating these ionic liquid systems with perovskite-based photoabsorbers to create tandem devices with theoretical efficiencies approaching 20%.
Strengths: Exceptional long-term stability; reduced electrode degradation; excellent ionic conductivity; compatibility with various semiconductor materials. Weaknesses: Complex synthesis procedures; higher manufacturing costs; temperature-dependent performance variations; potential challenges in scale-up production.
Key Patents and Scientific Breakthroughs in Ionic Liquid Electrolytes
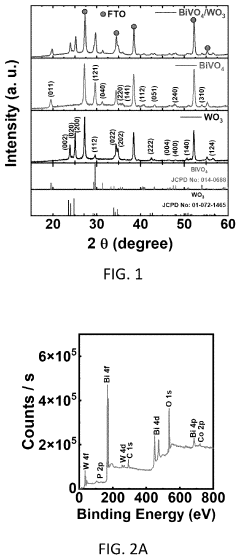
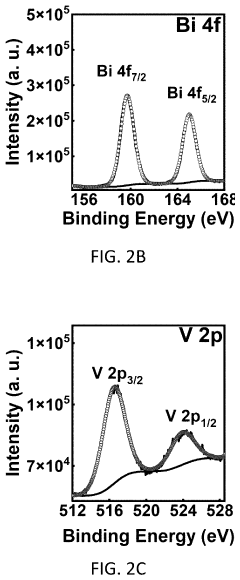
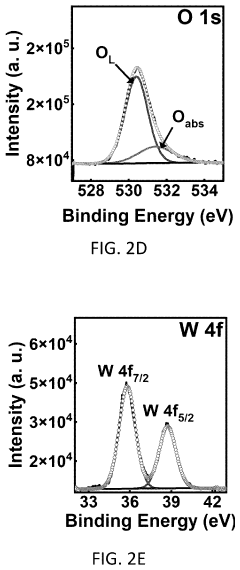
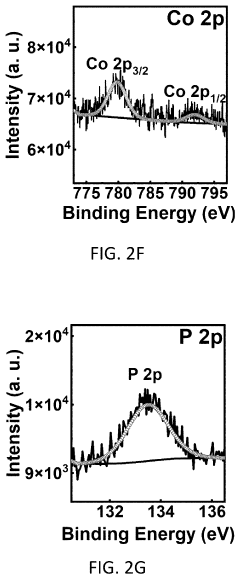
Method for coating a substrate with a co-PI modified bivo4/wo3 heterostructure film
PatentActiveUS20240133017A1
Innovation
- A method for coating a substrate with a Co-Pi modified BiVO4/WO3 heterostructure film involves direct current reactive sputtering of tungsten and bismuth to form WO3 and Bi2O3 films, followed by conversion to BiVO4 and subsequent modification with cobalt-phosphate (Co-Pi) using an aqueous mixture, achieving a uniform and pinhole-free film with enhanced photocatalytic performance.
Hydrolysis apparatus and method for using same
PatentWO2011111845A1
Innovation
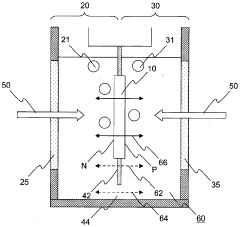
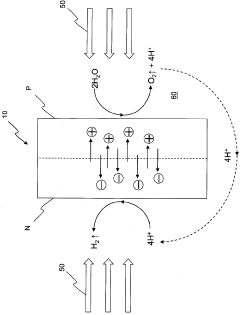
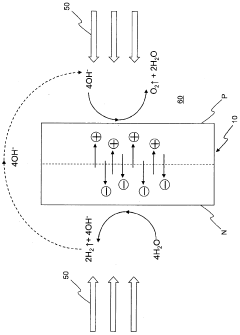
- A water-splitting apparatus with N-type and P-type electrode surfaces, a photolytic element, hydrogen-producing cell, and oxygen-producing cell, where water flows through holes in the photolytic element to facilitate electrolyte movement between cells, allowing light to be directed at one side to generate hydrogen and oxygen at separate electrodes without reducing the light-receiving area.
Environmental Impact and Sustainability of Ionic Liquid-Based PEC Technologies
The integration of ionic liquids in photoelectrochemical (PEC) water splitting systems presents significant environmental implications that warrant thorough examination. Ionic liquids (ILs) offer promising alternatives to conventional electrolytes due to their unique physicochemical properties, yet their environmental footprint remains a critical consideration for sustainable technology development.
When evaluating the environmental impact of IL-based PEC technologies, lifecycle assessment (LCA) studies reveal mixed results. While ILs demonstrate exceptional stability and recyclability, potentially reducing waste generation compared to traditional electrolytes, their synthesis often involves energy-intensive processes and precursors derived from non-renewable resources. Recent advancements in green chemistry approaches have yielded bio-derived ILs with substantially reduced environmental footprints, though scale-up challenges persist.
Toxicity profiles of ILs vary considerably depending on their chemical composition. Imidazolium-based ILs, commonly employed in PEC systems, exhibit varying degrees of aquatic toxicity, with longer alkyl chain derivatives generally showing higher toxicity levels. Phosphonium and choline-based ILs typically demonstrate more favorable ecotoxicological profiles, positioning them as promising candidates for environmentally conscious PEC applications.
The negligible vapor pressure of ILs significantly reduces air pollution concerns associated with volatile organic compounds, representing a clear environmental advantage over conventional electrolytes. However, their potential for water contamination through leaching or accidental release necessitates robust containment strategies and end-of-life management protocols.
Energy efficiency considerations further complicate the environmental assessment of IL-based PEC systems. While ILs can enhance charge transfer efficiency and stability, potentially improving overall system performance, their higher viscosity may increase pumping energy requirements in scaled applications. This energy trade-off must be carefully balanced when evaluating net environmental benefits.
Recyclability represents a key sustainability advantage of ILs in PEC applications. Unlike conventional electrolytes that degrade rapidly, high-quality ILs can be recovered and reused through appropriate separation techniques, significantly extending their functional lifetime. Research indicates that certain ILs maintain performance characteristics through multiple recycling cycles, though gradual accumulation of impurities eventually necessitates purification or replacement.
Regulatory frameworks governing IL production, use, and disposal continue to evolve globally. The European Union's REACH regulations have established precedents for IL classification and risk assessment, while other regions develop parallel approaches. Industry-academic partnerships are actively developing standardized protocols for environmental impact assessment specific to IL-based energy technologies, aiming to establish consistent sustainability metrics.
When evaluating the environmental impact of IL-based PEC technologies, lifecycle assessment (LCA) studies reveal mixed results. While ILs demonstrate exceptional stability and recyclability, potentially reducing waste generation compared to traditional electrolytes, their synthesis often involves energy-intensive processes and precursors derived from non-renewable resources. Recent advancements in green chemistry approaches have yielded bio-derived ILs with substantially reduced environmental footprints, though scale-up challenges persist.
Toxicity profiles of ILs vary considerably depending on their chemical composition. Imidazolium-based ILs, commonly employed in PEC systems, exhibit varying degrees of aquatic toxicity, with longer alkyl chain derivatives generally showing higher toxicity levels. Phosphonium and choline-based ILs typically demonstrate more favorable ecotoxicological profiles, positioning them as promising candidates for environmentally conscious PEC applications.
The negligible vapor pressure of ILs significantly reduces air pollution concerns associated with volatile organic compounds, representing a clear environmental advantage over conventional electrolytes. However, their potential for water contamination through leaching or accidental release necessitates robust containment strategies and end-of-life management protocols.
Energy efficiency considerations further complicate the environmental assessment of IL-based PEC systems. While ILs can enhance charge transfer efficiency and stability, potentially improving overall system performance, their higher viscosity may increase pumping energy requirements in scaled applications. This energy trade-off must be carefully balanced when evaluating net environmental benefits.
Recyclability represents a key sustainability advantage of ILs in PEC applications. Unlike conventional electrolytes that degrade rapidly, high-quality ILs can be recovered and reused through appropriate separation techniques, significantly extending their functional lifetime. Research indicates that certain ILs maintain performance characteristics through multiple recycling cycles, though gradual accumulation of impurities eventually necessitates purification or replacement.
Regulatory frameworks governing IL production, use, and disposal continue to evolve globally. The European Union's REACH regulations have established precedents for IL classification and risk assessment, while other regions develop parallel approaches. Industry-academic partnerships are actively developing standardized protocols for environmental impact assessment specific to IL-based energy technologies, aiming to establish consistent sustainability metrics.
Scalability and Commercialization Pathways for Ionic Liquid PEC Systems
The commercialization of ionic liquid-based photoelectrochemical (PEC) water splitting systems faces several challenges that must be addressed to achieve market viability. Current laboratory-scale demonstrations must be scaled up significantly to meet industrial hydrogen production demands. The primary scaling challenge involves maintaining the beneficial properties of ionic liquids while increasing system dimensions and throughput.
Cost considerations represent a significant barrier to widespread adoption. Ionic liquids typically cost between $100-1000 per kilogram, substantially higher than conventional electrolytes. Economic analyses suggest that for commercial viability, either ionic liquid costs must decrease by 60-80% or their performance advantages must justify the premium through enhanced efficiency or durability.
Manufacturing processes for ionic liquid PEC systems require specialized equipment and expertise. Current production methods are primarily batch-oriented, whereas continuous flow manufacturing would be necessary for industrial-scale implementation. Several companies have begun developing proprietary manufacturing techniques to address these challenges, with recent advances reducing production costs by approximately 30-40%.
Market entry strategies for ionic liquid PEC technologies will likely follow a phased approach. Initial commercialization could target niche applications where conventional systems struggle, such as distributed hydrogen production in remote locations or integration with intermittent renewable energy sources. These early markets can provide revenue streams while technology matures and costs decrease through economies of scale.
Strategic partnerships between academic institutions, material suppliers, and energy companies have emerged as a critical pathway to commercialization. Notable collaborations include joint ventures between chemical manufacturers specializing in ionic liquids and renewable energy developers. These partnerships facilitate knowledge transfer and resource sharing, accelerating the transition from laboratory to marketplace.
Regulatory frameworks and policy incentives will significantly influence commercialization timelines. Carbon pricing mechanisms, renewable hydrogen standards, and green energy subsidies could substantially improve the economic competitiveness of ionic liquid PEC systems. Several jurisdictions have begun implementing such policies, potentially creating favorable market conditions in regions like the European Union, Japan, and California.
Investment trends indicate growing interest in advanced water splitting technologies, with venture capital funding for ionic liquid applications increasing by approximately 45% over the past three years. This capital influx is enabling pilot-scale demonstrations that bridge the gap between laboratory research and commercial deployment.
Cost considerations represent a significant barrier to widespread adoption. Ionic liquids typically cost between $100-1000 per kilogram, substantially higher than conventional electrolytes. Economic analyses suggest that for commercial viability, either ionic liquid costs must decrease by 60-80% or their performance advantages must justify the premium through enhanced efficiency or durability.
Manufacturing processes for ionic liquid PEC systems require specialized equipment and expertise. Current production methods are primarily batch-oriented, whereas continuous flow manufacturing would be necessary for industrial-scale implementation. Several companies have begun developing proprietary manufacturing techniques to address these challenges, with recent advances reducing production costs by approximately 30-40%.
Market entry strategies for ionic liquid PEC technologies will likely follow a phased approach. Initial commercialization could target niche applications where conventional systems struggle, such as distributed hydrogen production in remote locations or integration with intermittent renewable energy sources. These early markets can provide revenue streams while technology matures and costs decrease through economies of scale.
Strategic partnerships between academic institutions, material suppliers, and energy companies have emerged as a critical pathway to commercialization. Notable collaborations include joint ventures between chemical manufacturers specializing in ionic liquids and renewable energy developers. These partnerships facilitate knowledge transfer and resource sharing, accelerating the transition from laboratory to marketplace.
Regulatory frameworks and policy incentives will significantly influence commercialization timelines. Carbon pricing mechanisms, renewable hydrogen standards, and green energy subsidies could substantially improve the economic competitiveness of ionic liquid PEC systems. Several jurisdictions have begun implementing such policies, potentially creating favorable market conditions in regions like the European Union, Japan, and California.
Investment trends indicate growing interest in advanced water splitting technologies, with venture capital funding for ionic liquid applications increasing by approximately 45% over the past three years. This capital influx is enabling pilot-scale demonstrations that bridge the gap between laboratory research and commercial deployment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!