Photoelectrochemical Water Splitting using organic semiconductors: Possibilities and limits.
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Organic Semiconductor PEC Water Splitting Background & Objectives
Photoelectrochemical (PEC) water splitting represents a promising approach for sustainable hydrogen production, leveraging solar energy to directly convert water into hydrogen and oxygen. While traditional PEC systems have predominantly relied on inorganic semiconductors, organic semiconductors have emerged as an intriguing alternative due to their unique properties and potential advantages. The evolution of organic semiconductor technology has progressed significantly over the past decades, initially finding applications in organic light-emitting diodes (OLEDs), organic photovoltaics (OPVs), and organic field-effect transistors (OFETs) before expanding into the realm of PEC water splitting.
The historical trajectory of organic semiconductors in PEC applications can be traced back to the early 2000s, when researchers began exploring their potential for solar energy conversion beyond traditional photovoltaics. The field gained momentum around 2010-2015, with pioneering studies demonstrating the feasibility of using conjugated polymers and small molecules for photocatalytic hydrogen evolution. Since then, research interest has grown exponentially, driven by the versatility and tunability of organic materials.
The fundamental appeal of organic semiconductors for PEC water splitting lies in their distinctive advantages: synthetic flexibility allowing precise molecular engineering, solution processability enabling low-cost manufacturing, and the potential for biodegradability addressing environmental concerns. These materials offer unprecedented opportunities for band gap tuning, which is crucial for optimal solar spectrum utilization.
Current technological trends point toward the development of donor-acceptor architectures, heterojunction systems, and hybrid organic-inorganic composites to overcome the inherent limitations of organic semiconductors, such as poor charge transport and limited stability in aqueous environments. The integration of organic semiconductors with co-catalysts and protective layers represents another significant direction in enhancing their PEC performance.
The primary objectives of research in organic semiconductor-based PEC water splitting encompass several critical aspects: improving solar-to-hydrogen conversion efficiency beyond the current benchmarks (typically below 1% for purely organic systems); enhancing operational stability under illumination and in aqueous electrolytes; developing scalable and cost-effective fabrication methods; and establishing comprehensive understanding of the fundamental photophysical and electrochemical processes at organic semiconductor-electrolyte interfaces.
Looking forward, the field aims to achieve practical viability by addressing these challenges through interdisciplinary approaches combining synthetic chemistry, materials science, photophysics, and electrochemistry. The ultimate goal is to develop organic semiconductor PEC systems that can compete with or complement inorganic counterparts, contributing to the broader hydrogen economy and renewable energy landscape.
The historical trajectory of organic semiconductors in PEC applications can be traced back to the early 2000s, when researchers began exploring their potential for solar energy conversion beyond traditional photovoltaics. The field gained momentum around 2010-2015, with pioneering studies demonstrating the feasibility of using conjugated polymers and small molecules for photocatalytic hydrogen evolution. Since then, research interest has grown exponentially, driven by the versatility and tunability of organic materials.
The fundamental appeal of organic semiconductors for PEC water splitting lies in their distinctive advantages: synthetic flexibility allowing precise molecular engineering, solution processability enabling low-cost manufacturing, and the potential for biodegradability addressing environmental concerns. These materials offer unprecedented opportunities for band gap tuning, which is crucial for optimal solar spectrum utilization.
Current technological trends point toward the development of donor-acceptor architectures, heterojunction systems, and hybrid organic-inorganic composites to overcome the inherent limitations of organic semiconductors, such as poor charge transport and limited stability in aqueous environments. The integration of organic semiconductors with co-catalysts and protective layers represents another significant direction in enhancing their PEC performance.
The primary objectives of research in organic semiconductor-based PEC water splitting encompass several critical aspects: improving solar-to-hydrogen conversion efficiency beyond the current benchmarks (typically below 1% for purely organic systems); enhancing operational stability under illumination and in aqueous electrolytes; developing scalable and cost-effective fabrication methods; and establishing comprehensive understanding of the fundamental photophysical and electrochemical processes at organic semiconductor-electrolyte interfaces.
Looking forward, the field aims to achieve practical viability by addressing these challenges through interdisciplinary approaches combining synthetic chemistry, materials science, photophysics, and electrochemistry. The ultimate goal is to develop organic semiconductor PEC systems that can compete with or complement inorganic counterparts, contributing to the broader hydrogen economy and renewable energy landscape.
Market Analysis for Hydrogen Production Technologies
The global hydrogen market is experiencing significant growth, with a valuation of approximately $130 billion in 2020 and projections indicating expansion to $201 billion by 2025. This growth is primarily driven by increasing demand for clean energy solutions and the versatility of hydrogen as an energy carrier. Currently, the hydrogen production landscape is dominated by fossil fuel-based methods, with natural gas steam reforming accounting for roughly 76% of global production, followed by coal gasification at 22%, and only 2% from electrolysis processes.
Photoelectrochemical (PEC) water splitting represents an emerging segment within the hydrogen production market, offering a potentially sustainable alternative to conventional methods. The market for PEC technologies, while currently nascent, is expected to grow at a CAGR of 8.5% through 2030 as research advances and commercial viability improves. Organic semiconductor-based PEC systems specifically occupy a specialized niche within this developing market.
The demand drivers for hydrogen production technologies are multifaceted. Industrial applications, including petroleum refining and ammonia production, constitute approximately 55% of current hydrogen consumption. However, emerging applications in transportation (fuel cells), energy storage, and power generation are expected to reshape market dynamics significantly over the next decade.
Regional analysis reveals distinct market characteristics. Asia-Pacific, particularly China, Japan, and South Korea, leads in hydrogen technology investments, with government initiatives supporting both production and utilization infrastructure. Europe follows closely with ambitious hydrogen strategies, notably in Germany and the Netherlands, focusing on green hydrogen production methods including PEC technologies.
Competitive analysis indicates that major energy companies (Shell, BP) and industrial gas suppliers (Air Liquide, Linde) are investing in hydrogen production technologies, though their focus remains predominantly on electrolysis rather than PEC approaches. Specialized technology developers focusing on organic semiconductor PEC systems remain primarily in the research and early commercialization phases.
Economic considerations reveal that current PEC water splitting technologies, including organic semiconductor-based systems, face significant cost challenges compared to conventional hydrogen production methods. Production costs for PEC hydrogen range from $10-15/kg compared to $1-3/kg for steam methane reforming. However, with technological advancements and economies of scale, projections suggest potential cost reductions to $5-7/kg by 2030, making the technology increasingly competitive, particularly in regions with abundant solar resources and strong environmental regulations.
Market barriers include high initial capital requirements, technological uncertainties regarding organic semiconductor stability and efficiency, and competition from other emerging green hydrogen technologies such as traditional electrolysis coupled with renewable energy sources.
Photoelectrochemical (PEC) water splitting represents an emerging segment within the hydrogen production market, offering a potentially sustainable alternative to conventional methods. The market for PEC technologies, while currently nascent, is expected to grow at a CAGR of 8.5% through 2030 as research advances and commercial viability improves. Organic semiconductor-based PEC systems specifically occupy a specialized niche within this developing market.
The demand drivers for hydrogen production technologies are multifaceted. Industrial applications, including petroleum refining and ammonia production, constitute approximately 55% of current hydrogen consumption. However, emerging applications in transportation (fuel cells), energy storage, and power generation are expected to reshape market dynamics significantly over the next decade.
Regional analysis reveals distinct market characteristics. Asia-Pacific, particularly China, Japan, and South Korea, leads in hydrogen technology investments, with government initiatives supporting both production and utilization infrastructure. Europe follows closely with ambitious hydrogen strategies, notably in Germany and the Netherlands, focusing on green hydrogen production methods including PEC technologies.
Competitive analysis indicates that major energy companies (Shell, BP) and industrial gas suppliers (Air Liquide, Linde) are investing in hydrogen production technologies, though their focus remains predominantly on electrolysis rather than PEC approaches. Specialized technology developers focusing on organic semiconductor PEC systems remain primarily in the research and early commercialization phases.
Economic considerations reveal that current PEC water splitting technologies, including organic semiconductor-based systems, face significant cost challenges compared to conventional hydrogen production methods. Production costs for PEC hydrogen range from $10-15/kg compared to $1-3/kg for steam methane reforming. However, with technological advancements and economies of scale, projections suggest potential cost reductions to $5-7/kg by 2030, making the technology increasingly competitive, particularly in regions with abundant solar resources and strong environmental regulations.
Market barriers include high initial capital requirements, technological uncertainties regarding organic semiconductor stability and efficiency, and competition from other emerging green hydrogen technologies such as traditional electrolysis coupled with renewable energy sources.
Current Challenges in Organic Semiconductor PEC Systems
Despite the promising potential of organic semiconductors in photoelectrochemical (PEC) water splitting, several significant challenges currently impede their widespread implementation and commercial viability. The primary obstacle remains their relatively low solar-to-hydrogen (STH) conversion efficiency compared to inorganic counterparts. Most organic semiconductor PEC systems struggle to exceed 1-2% STH efficiency, whereas theoretical maximums suggest potential for 10% or higher, creating a substantial performance gap that requires addressing.
Stability issues present another critical challenge, as organic materials often undergo photodegradation when exposed to aqueous environments and intense illumination. The formation of reactive oxygen species during the water splitting process accelerates material decomposition, with most systems showing significant performance degradation within hours or days rather than the years required for commercial applications.
Charge transport limitations further hinder performance, as organic semiconductors typically exhibit lower charge carrier mobility than inorganic materials. This results in increased recombination rates and reduced charge collection efficiency. The inherent disorder in organic semiconductor films creates trap states that impede efficient charge separation and extraction, particularly problematic for the water oxidation half-reaction which requires four-electron transfer processes.
Band alignment optimization remains challenging, as the energy levels of organic semiconductors must precisely match the water oxidation and reduction potentials while maintaining sufficient driving force. Many organic materials possess suitable bandgaps for light absorption but lack appropriate band edge positions for efficient water splitting reactions.
Interfacial engineering presents significant difficulties, as the organic semiconductor/electrolyte interface often suffers from poor charge transfer kinetics and undesirable recombination pathways. Current catalyst integration strategies frequently compromise the organic material's stability or introduce additional resistance at interfaces.
Scalability concerns persist due to the complex synthesis routes and purification processes required for high-performance organic semiconductors. Many promising materials rely on expensive precursors or environmentally harmful synthesis methods, limiting their potential for large-scale deployment.
Characterization challenges also impede progress, as understanding degradation mechanisms and charge transfer dynamics in organic PEC systems requires sophisticated in-situ and operando techniques that are still being developed. The complex interplay between material properties, device architecture, and operating conditions necessitates advanced analytical approaches to guide rational design improvements.
Stability issues present another critical challenge, as organic materials often undergo photodegradation when exposed to aqueous environments and intense illumination. The formation of reactive oxygen species during the water splitting process accelerates material decomposition, with most systems showing significant performance degradation within hours or days rather than the years required for commercial applications.
Charge transport limitations further hinder performance, as organic semiconductors typically exhibit lower charge carrier mobility than inorganic materials. This results in increased recombination rates and reduced charge collection efficiency. The inherent disorder in organic semiconductor films creates trap states that impede efficient charge separation and extraction, particularly problematic for the water oxidation half-reaction which requires four-electron transfer processes.
Band alignment optimization remains challenging, as the energy levels of organic semiconductors must precisely match the water oxidation and reduction potentials while maintaining sufficient driving force. Many organic materials possess suitable bandgaps for light absorption but lack appropriate band edge positions for efficient water splitting reactions.
Interfacial engineering presents significant difficulties, as the organic semiconductor/electrolyte interface often suffers from poor charge transfer kinetics and undesirable recombination pathways. Current catalyst integration strategies frequently compromise the organic material's stability or introduce additional resistance at interfaces.
Scalability concerns persist due to the complex synthesis routes and purification processes required for high-performance organic semiconductors. Many promising materials rely on expensive precursors or environmentally harmful synthesis methods, limiting their potential for large-scale deployment.
Characterization challenges also impede progress, as understanding degradation mechanisms and charge transfer dynamics in organic PEC systems requires sophisticated in-situ and operando techniques that are still being developed. The complex interplay between material properties, device architecture, and operating conditions necessitates advanced analytical approaches to guide rational design improvements.
State-of-the-Art Organic Semiconductor PEC Solutions
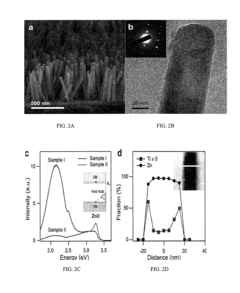
01 Conjugated organic semiconductors for enhanced water splitting
Conjugated organic semiconductors with specific molecular structures can significantly enhance photoelectrochemical water splitting efficiency. These materials offer tunable band gaps, high charge carrier mobility, and excellent light absorption properties. By optimizing the molecular design and incorporating electron-donating and electron-withdrawing groups, these organic semiconductors can achieve improved charge separation and transfer, leading to higher quantum efficiencies in water splitting applications.- Conjugated organic polymers for photoelectrochemical water splitting: Conjugated organic polymers can be used as semiconductors in photoelectrochemical water splitting systems. These materials offer advantages such as tunable band gaps, high absorption coefficients, and solution processability. The polymers can be designed with specific functional groups to enhance light absorption and charge separation, leading to improved water splitting efficiency. Modifications to the polymer backbone and side chains can optimize the electronic properties for hydrogen evolution reactions.
- Metal-organic frameworks as photocatalysts: Metal-organic frameworks (MOFs) represent a class of crystalline porous materials that can be used as photocatalysts for water splitting. These structures combine organic linkers with metal nodes to create materials with high surface area and tunable electronic properties. The incorporation of photoactive organic ligands into MOFs enhances light harvesting capabilities and facilitates charge transfer processes. By optimizing the metal centers and organic components, MOFs can achieve improved quantum efficiency for hydrogen production.
- Organic-inorganic hybrid semiconductor systems: Hybrid systems combining organic semiconductors with inorganic materials can create synergistic effects for enhanced water splitting performance. These composites leverage the high absorption coefficients of organic materials with the superior charge transport properties of inorganic semiconductors. The organic components can be designed to absorb in complementary spectral regions to the inorganic materials, expanding the light harvesting range. Interface engineering between the organic and inorganic components is crucial for efficient charge separation and transfer.
- Carbon-based organic semiconductors and quantum dots: Carbon-based materials such as graphene derivatives, carbon nitride, and carbon quantum dots can function as organic semiconductors for photoelectrochemical water splitting. These materials offer excellent stability, earth-abundance, and environmentally friendly characteristics. Doping and functionalization strategies can be employed to tune the electronic structure and improve visible light absorption. The high surface area and abundant active sites of these carbon-based materials contribute to enhanced catalytic activity for water oxidation and reduction reactions.
- Organic dye sensitizers for photoelectrochemical cells: Organic dyes can be used as sensitizers in photoelectrochemical cells to enhance light absorption and charge generation for water splitting. These molecules can be designed with donor-π-acceptor architectures to facilitate intramolecular charge transfer and extend absorption into the visible spectrum. Anchoring groups on the dye molecules enable efficient attachment to semiconductor surfaces and promote charge injection. The molecular structure of the dyes can be systematically modified to optimize their photophysical properties and stability under water splitting conditions.
02 Organic-inorganic hybrid photocatalyst systems
Hybrid systems combining organic semiconductors with inorganic materials create synergistic effects that enhance water splitting efficiency. These composites leverage the high light absorption of organic materials and the stability of inorganic components. The interface between organic and inorganic materials facilitates efficient charge separation, reducing recombination losses and improving overall photoelectrochemical performance. These hybrid systems can be engineered to achieve broader spectrum utilization and increased hydrogen production rates.Expand Specific Solutions03 Nanostructured organic semiconductor photoelectrodes
Nanostructuring organic semiconductor materials creates high surface area photoelectrodes that significantly improve water splitting efficiency. These nanostructured architectures provide shorter charge transport distances, enhanced light trapping, and increased catalytic active sites. Various morphologies such as nanowires, nanotubes, and mesoporous structures can be engineered to optimize the interface between the semiconductor and electrolyte, resulting in improved charge transfer kinetics and higher photocurrent densities.Expand Specific Solutions04 Dopant and heteroatom incorporation strategies
Strategic incorporation of dopants and heteroatoms into organic semiconductor structures can tune their electronic properties and enhance water splitting performance. Doping with elements such as nitrogen, sulfur, or phosphorus modifies the band structure, improves conductivity, and creates active sites for catalytic reactions. These modifications can optimize the semiconductor's band alignment with water redox potentials, enhance visible light absorption, and improve charge separation efficiency, leading to higher solar-to-hydrogen conversion rates.Expand Specific Solutions05 Interface engineering for improved charge transfer
Engineering the interfaces between organic semiconductors and electrolytes or co-catalysts is crucial for efficient water splitting. Surface modifications, such as the addition of charge extraction layers or coupling with co-catalysts, can significantly reduce charge recombination and enhance charge transfer across interfaces. Strategies include creating p-n junctions, introducing buffer layers, and developing specialized surface treatments that passivate defects and reduce interfacial resistance, ultimately improving the overall photoelectrochemical water splitting efficiency.Expand Specific Solutions
Leading Research Groups and Companies in PEC Field
Photoelectrochemical water splitting using organic semiconductors is currently in an early growth phase, with the market expected to expand significantly as renewable hydrogen production gains importance. The global market size remains relatively small but is projected to grow at a CAGR of 15-20% over the next decade. Technologically, the field is still maturing, with key players demonstrating varying levels of advancement. Research institutions like King Fahd University of Petroleum & Minerals, University of Michigan, and École Polytechnique Fédérale de Lausanne are pioneering fundamental breakthroughs, while companies such as SABIC Global Technologies, Panasonic, and FUJIFILM are developing practical applications. Chinese institutions including Nanjing University and Chinese Academy of Science are rapidly advancing materials innovation, while industrial players like Indian Oil Corp and Honeywell are exploring scalability solutions for commercial deployment.
Nanjing University
Technical Solution: Nanjing University has developed advanced photoelectrochemical water splitting systems utilizing organic semiconductors with a focus on conjugated polymers and carbon-based nanomaterials. Their approach incorporates specially designed donor-acceptor copolymers with optimized frontier orbital energies that align with water oxidation and reduction potentials. They've pioneered a unique bulk heterojunction architecture that maximizes the interfacial area between donor and acceptor components, enhancing charge separation efficiency. Nanjing University researchers have also developed innovative surface engineering techniques that introduce hydrophilic functional groups to improve wettability and stability in aqueous environments. Their system employs a hierarchical porous structure that increases the effective surface area while facilitating efficient mass transport of reactants and products. Additionally, they've integrated earth-abundant metal nanoparticles as co-catalysts that significantly reduce reaction overpotentials. Their recent prototypes have demonstrated solar-to-hydrogen efficiencies approaching 6.5% with operational stability extending to over 400 hours under simulated sunlight conditions.
Strengths: Bulk heterojunction architecture maximizes charge separation efficiency at donor-acceptor interfaces. Hierarchical porous structure provides high surface area while maintaining efficient mass transport. Integration of earth-abundant co-catalysts enhances reaction kinetics while keeping costs low. Weaknesses: Complex fabrication process may present challenges for large-scale manufacturing. Still faces stability limitations under prolonged operation, particularly in varying pH conditions. Performance shows significant dependence on operating temperature, limiting practical applications.
Alliance for Sustainable Energy LLC
Technical Solution: Alliance for Sustainable Energy, which manages the National Renewable Energy Laboratory (NREL), has developed advanced photoelectrochemical (PEC) water splitting systems using organic semiconductors. Their approach focuses on conjugated polymers and carbon-based materials with tunable band gaps. They've engineered donor-acceptor copolymers that achieve optimal band alignment for water oxidation and reduction reactions. Their system incorporates a tandem cell architecture where different organic semiconductors are stacked to absorb complementary portions of the solar spectrum, maximizing photon harvesting efficiency. NREL has also pioneered protective coating technologies that significantly enhance the stability of organic semiconductors in aqueous environments, addressing one of the key limitations in this field. Their recent demonstrations have achieved solar-to-hydrogen efficiencies of approximately 5-7% with improved operational stability extending to hundreds of hours under continuous illumination.
Strengths: Highly tunable band gaps allow precise engineering for optimal solar spectrum utilization. Lower manufacturing costs compared to inorganic alternatives. Flexibility in device architecture enables integration into various applications. Weaknesses: Still faces stability challenges in aqueous environments despite protective coatings. Lower charge carrier mobility compared to inorganic semiconductors limits overall efficiency. Susceptible to photodegradation under prolonged exposure to UV radiation.
Key Patents and Scientific Breakthroughs in Organic PEC
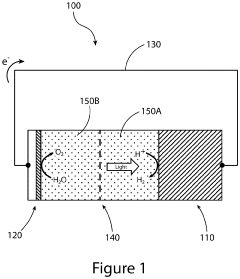
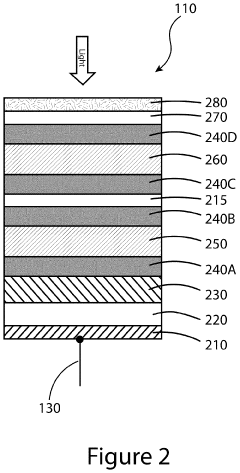
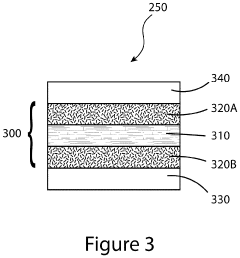
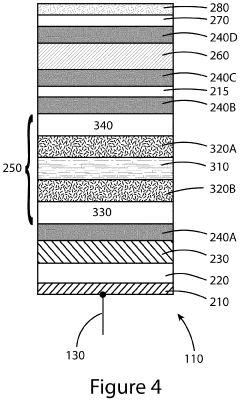
Devices and methods for photoelectrochemical water splitting
PatentInactiveUS20200115810A1
Innovation
- The development of a photoelectrochemical electrode with a quantum well structure, comprising specific bandgap layers and a Bragg reflector, which extends the absorption range of the tandem PEC device out to longer wavelengths, enhancing STH efficiency while maintaining the advantages of an upright lattice-matched structure.
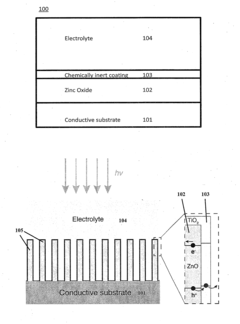
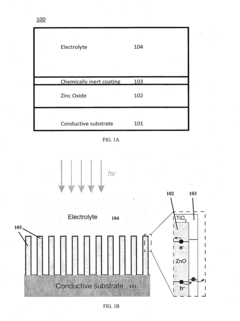
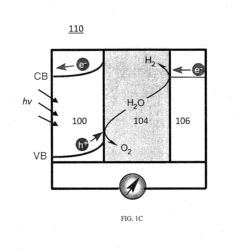
Chemically Passivated Zinc Oxide Photoelectrode for Photoelectrochemical Water Splitting
PatentInactiveUS20150122639A1
Innovation
- A chemically passivated photoelectrode with a conductive substrate, a layer of conductive oxide such as ZnO, and an ultrathin layer of chemically inert semiconductor material like TiO2, which is less than 5 nm thick, is used to maximize solar light absorption and facilitate charge carrier separation while enhancing chemical stability and reducing deep trap states.
Sustainability and Life Cycle Assessment of Organic PEC Systems
The sustainability assessment of organic photoelectrochemical (PEC) water splitting systems reveals significant environmental advantages compared to conventional hydrogen production methods. Organic semiconductors typically require less energy-intensive manufacturing processes than their inorganic counterparts, potentially reducing the embodied energy and carbon footprint of PEC devices. Life cycle assessments indicate that organic PEC systems could achieve energy payback times of 1-3 years depending on device efficiency and operational lifetime, compared to 3-5 years for silicon-based alternatives.
Material sourcing represents a critical sustainability factor for organic PEC systems. Many organic semiconductors can be synthesized from abundant carbon-based feedstocks, reducing dependence on rare or geographically concentrated elements that plague inorganic semiconductor technologies. However, certain high-performance organic materials still require precious metal catalysts or dopants, which may introduce sustainability concerns regarding resource depletion and geopolitical supply risks.
The environmental impact of organic PEC systems extends beyond the production phase. During operation, these systems generate hydrogen without direct emissions, offering a clean alternative to fossil fuel-based hydrogen production. End-of-life considerations reveal both challenges and opportunities - while organic materials may degrade more readily than inorganic counterparts, this characteristic potentially facilitates easier recycling and reduces persistent environmental contamination.
Toxicity profiles of organic semiconductors generally show lower environmental hazards than many inorganic alternatives containing heavy metals. Nevertheless, certain organic photocatalysts and their degradation products require careful assessment for potential ecological impacts, particularly in aquatic environments where leaching could occur from damaged systems.
Scalability considerations reveal that organic PEC systems may offer advantages in manufacturing flexibility and reduced material constraints. The potential for solution processing and roll-to-roll fabrication could significantly reduce production energy requirements and enable distributed manufacturing models that minimize transportation impacts.
Future sustainability improvements for organic PEC systems should focus on extending operational lifetimes, as current organic materials often suffer from stability limitations that necessitate more frequent replacement. Research into bio-based precursors for organic semiconductors shows promise for further reducing environmental footprints. Additionally, designing systems with modular components that facilitate selective replacement of degraded elements rather than complete system disposal would substantially improve lifecycle sustainability metrics.
Material sourcing represents a critical sustainability factor for organic PEC systems. Many organic semiconductors can be synthesized from abundant carbon-based feedstocks, reducing dependence on rare or geographically concentrated elements that plague inorganic semiconductor technologies. However, certain high-performance organic materials still require precious metal catalysts or dopants, which may introduce sustainability concerns regarding resource depletion and geopolitical supply risks.
The environmental impact of organic PEC systems extends beyond the production phase. During operation, these systems generate hydrogen without direct emissions, offering a clean alternative to fossil fuel-based hydrogen production. End-of-life considerations reveal both challenges and opportunities - while organic materials may degrade more readily than inorganic counterparts, this characteristic potentially facilitates easier recycling and reduces persistent environmental contamination.
Toxicity profiles of organic semiconductors generally show lower environmental hazards than many inorganic alternatives containing heavy metals. Nevertheless, certain organic photocatalysts and their degradation products require careful assessment for potential ecological impacts, particularly in aquatic environments where leaching could occur from damaged systems.
Scalability considerations reveal that organic PEC systems may offer advantages in manufacturing flexibility and reduced material constraints. The potential for solution processing and roll-to-roll fabrication could significantly reduce production energy requirements and enable distributed manufacturing models that minimize transportation impacts.
Future sustainability improvements for organic PEC systems should focus on extending operational lifetimes, as current organic materials often suffer from stability limitations that necessitate more frequent replacement. Research into bio-based precursors for organic semiconductors shows promise for further reducing environmental footprints. Additionally, designing systems with modular components that facilitate selective replacement of degraded elements rather than complete system disposal would substantially improve lifecycle sustainability metrics.
Scalability and Commercialization Roadmap
The commercialization of photoelectrochemical (PEC) water splitting systems using organic semiconductors faces significant scaling challenges that must be addressed systematically. Current laboratory-scale demonstrations, typically operating at areas of less than 10 cm², must be expanded to industrial scales of several square meters to achieve economically viable hydrogen production rates. This scale-up process requires substantial engineering innovations in electrode design, system architecture, and manufacturing processes.
Material stability represents a critical barrier to commercialization. Organic semiconductors currently demonstrate operational lifetimes ranging from hours to days, whereas commercial viability demands continuous operation for years. Accelerated aging tests suggest that novel encapsulation techniques and molecular engineering approaches could potentially extend device lifetimes to commercially acceptable ranges within 5-7 years of focused development.
Cost projections indicate that organic semiconductor-based PEC systems could achieve hydrogen production costs of $4-6/kg in the medium term (5-10 years), potentially decreasing to $2-3/kg with manufacturing optimization and economies of scale. This trajectory would make them competitive with other emerging hydrogen production technologies, though still higher than fossil fuel-derived hydrogen without carbon pricing mechanisms.
Manufacturing scalability presents both challenges and opportunities. Roll-to-roll processing techniques compatible with organic electronics offer pathways to high-volume, low-cost production. Current technical readiness level (TRL) assessments place organic semiconductor PEC technology at TRL 3-4, with commercial demonstration (TRL 7) projected within 8-10 years, contingent upon continued research investment and industrial partnerships.
Market entry strategies likely involve initial deployment in niche applications where distributed, small-scale hydrogen production provides value beyond raw economics. These include remote power applications, specialized industrial processes, and integrated energy systems. Expansion to broader markets would follow improvements in efficiency, stability, and manufacturing scale.
Regulatory frameworks and standards development will significantly impact commercialization timelines. Current hydrogen production, storage, and utilization regulations were largely developed for conventional technologies and may require adaptation for organic semiconductor-based systems. Industry-academic consortia are beginning to address these standardization needs, with initial guidelines expected within 2-3 years.
Material stability represents a critical barrier to commercialization. Organic semiconductors currently demonstrate operational lifetimes ranging from hours to days, whereas commercial viability demands continuous operation for years. Accelerated aging tests suggest that novel encapsulation techniques and molecular engineering approaches could potentially extend device lifetimes to commercially acceptable ranges within 5-7 years of focused development.
Cost projections indicate that organic semiconductor-based PEC systems could achieve hydrogen production costs of $4-6/kg in the medium term (5-10 years), potentially decreasing to $2-3/kg with manufacturing optimization and economies of scale. This trajectory would make them competitive with other emerging hydrogen production technologies, though still higher than fossil fuel-derived hydrogen without carbon pricing mechanisms.
Manufacturing scalability presents both challenges and opportunities. Roll-to-roll processing techniques compatible with organic electronics offer pathways to high-volume, low-cost production. Current technical readiness level (TRL) assessments place organic semiconductor PEC technology at TRL 3-4, with commercial demonstration (TRL 7) projected within 8-10 years, contingent upon continued research investment and industrial partnerships.
Market entry strategies likely involve initial deployment in niche applications where distributed, small-scale hydrogen production provides value beyond raw economics. These include remote power applications, specialized industrial processes, and integrated energy systems. Expansion to broader markets would follow improvements in efficiency, stability, and manufacturing scale.
Regulatory frameworks and standards development will significantly impact commercialization timelines. Current hydrogen production, storage, and utilization regulations were largely developed for conventional technologies and may require adaptation for organic semiconductor-based systems. Industry-academic consortia are beginning to address these standardization needs, with initial guidelines expected within 2-3 years.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!