Strategies to minimize photoelectrode degradation in water splitting.
SEP 4, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Photoelectrochemical Water Splitting Background and Objectives
Photoelectrochemical (PEC) water splitting represents a promising approach for sustainable hydrogen production, utilizing solar energy to directly convert water into hydrogen and oxygen. This technology has evolved significantly since its inception in the 1970s with the groundbreaking work of Fujishima and Honda, who demonstrated the photocatalytic splitting of water using titanium dioxide electrodes under ultraviolet light.
The evolution of PEC water splitting technology has been marked by continuous improvements in photoelectrode materials, system designs, and fundamental understanding of the underlying photochemical processes. Early research focused primarily on simple semiconductor materials, while recent advances have explored complex nanostructured materials, heterojunctions, and surface modifications to enhance efficiency and stability.
A critical challenge in this field has been the persistent issue of photoelectrode degradation during operation. This degradation manifests through various mechanisms including photocorrosion, surface passivation, and structural changes that collectively diminish the performance and longevity of PEC systems. The severity of this issue has positioned electrode stability as a key barrier to commercial viability of PEC water splitting technology.
The primary technical objective in addressing photoelectrode degradation is to develop strategies that can significantly extend operational lifetimes while maintaining high solar-to-hydrogen conversion efficiencies. This involves understanding degradation mechanisms at the molecular and atomic levels, and developing innovative approaches to mitigate these effects without compromising performance.
Current research trends indicate a multifaceted approach to this challenge, incorporating advances in materials science, surface chemistry, and electrochemical engineering. Promising directions include the development of protective coatings, novel semiconductor architectures, and strategic doping strategies that enhance stability without significantly impeding charge transfer processes.
The global research landscape shows accelerating interest in this field, with significant contributions from research institutions across North America, Europe, and East Asia. Publication trends indicate a shift from purely efficiency-focused research to a more balanced approach that equally values stability and performance.
Looking forward, the technical trajectory suggests potential breakthroughs in hybrid materials, self-healing mechanisms, and in-situ regeneration techniques that could fundamentally transform the stability profile of photoelectrodes. These advances would directly address the critical barriers to widespread adoption of PEC water splitting as a viable hydrogen production technology.
The evolution of PEC water splitting technology has been marked by continuous improvements in photoelectrode materials, system designs, and fundamental understanding of the underlying photochemical processes. Early research focused primarily on simple semiconductor materials, while recent advances have explored complex nanostructured materials, heterojunctions, and surface modifications to enhance efficiency and stability.
A critical challenge in this field has been the persistent issue of photoelectrode degradation during operation. This degradation manifests through various mechanisms including photocorrosion, surface passivation, and structural changes that collectively diminish the performance and longevity of PEC systems. The severity of this issue has positioned electrode stability as a key barrier to commercial viability of PEC water splitting technology.
The primary technical objective in addressing photoelectrode degradation is to develop strategies that can significantly extend operational lifetimes while maintaining high solar-to-hydrogen conversion efficiencies. This involves understanding degradation mechanisms at the molecular and atomic levels, and developing innovative approaches to mitigate these effects without compromising performance.
Current research trends indicate a multifaceted approach to this challenge, incorporating advances in materials science, surface chemistry, and electrochemical engineering. Promising directions include the development of protective coatings, novel semiconductor architectures, and strategic doping strategies that enhance stability without significantly impeding charge transfer processes.
The global research landscape shows accelerating interest in this field, with significant contributions from research institutions across North America, Europe, and East Asia. Publication trends indicate a shift from purely efficiency-focused research to a more balanced approach that equally values stability and performance.
Looking forward, the technical trajectory suggests potential breakthroughs in hybrid materials, self-healing mechanisms, and in-situ regeneration techniques that could fundamentally transform the stability profile of photoelectrodes. These advances would directly address the critical barriers to widespread adoption of PEC water splitting as a viable hydrogen production technology.
Market Analysis for Hydrogen Production Technologies
The global hydrogen production market is experiencing significant growth, driven by increasing demand for clean energy solutions and decarbonization efforts across industries. Currently valued at approximately $130 billion, the market is projected to reach $220 billion by 2030, with a compound annual growth rate of 6.8%. Water splitting technologies, particularly photoelectrochemical (PEC) methods, represent an emerging segment within this expanding market.
Traditional hydrogen production methods dominate the current market landscape, with steam methane reforming accounting for nearly 76% of global production. However, green hydrogen production technologies, including water electrolysis and photoelectrochemical water splitting, are gaining traction due to their environmental benefits and alignment with global sustainability goals.
The photoelectrochemical water splitting segment, while currently occupying less than 1% of the hydrogen production market, is expected to grow at a faster rate of 15-20% annually through 2030. This growth is contingent upon addressing key technical challenges, particularly photoelectrode degradation issues that currently limit commercial viability.
Regional analysis reveals varying market dynamics. Asia-Pacific leads in hydrogen production capacity, with China investing heavily in research and development of advanced water splitting technologies. Europe follows closely, driven by ambitious climate targets and supportive regulatory frameworks. The European Hydrogen Strategy aims to install at least 40 GW of renewable hydrogen electrolyzers by 2030, creating substantial market opportunities for degradation-resistant photoelectrode technologies.
End-user industries demonstrate diverse demand patterns. The industrial sector, particularly chemical manufacturing and refining, remains the largest consumer of hydrogen. However, transportation and power generation sectors are emerging as high-growth segments, with fuel cell vehicles and hydrogen energy storage solutions gaining momentum.
Market barriers for photoelectrochemical water splitting technologies include high initial capital costs, efficiency limitations, and durability concerns. The cost of producing hydrogen via PEC methods currently ranges from $5-10 per kilogram, significantly higher than conventional methods ($1-2 per kilogram). Addressing photoelectrode degradation could potentially reduce these costs by 30-40%, making the technology more competitive.
Consumer willingness to pay premiums for green hydrogen varies by sector, with transportation and specialty chemical industries demonstrating higher acceptance of price premiums. Government incentives, carbon pricing mechanisms, and renewable energy mandates are creating favorable market conditions for advanced water splitting technologies with improved durability characteristics.
Traditional hydrogen production methods dominate the current market landscape, with steam methane reforming accounting for nearly 76% of global production. However, green hydrogen production technologies, including water electrolysis and photoelectrochemical water splitting, are gaining traction due to their environmental benefits and alignment with global sustainability goals.
The photoelectrochemical water splitting segment, while currently occupying less than 1% of the hydrogen production market, is expected to grow at a faster rate of 15-20% annually through 2030. This growth is contingent upon addressing key technical challenges, particularly photoelectrode degradation issues that currently limit commercial viability.
Regional analysis reveals varying market dynamics. Asia-Pacific leads in hydrogen production capacity, with China investing heavily in research and development of advanced water splitting technologies. Europe follows closely, driven by ambitious climate targets and supportive regulatory frameworks. The European Hydrogen Strategy aims to install at least 40 GW of renewable hydrogen electrolyzers by 2030, creating substantial market opportunities for degradation-resistant photoelectrode technologies.
End-user industries demonstrate diverse demand patterns. The industrial sector, particularly chemical manufacturing and refining, remains the largest consumer of hydrogen. However, transportation and power generation sectors are emerging as high-growth segments, with fuel cell vehicles and hydrogen energy storage solutions gaining momentum.
Market barriers for photoelectrochemical water splitting technologies include high initial capital costs, efficiency limitations, and durability concerns. The cost of producing hydrogen via PEC methods currently ranges from $5-10 per kilogram, significantly higher than conventional methods ($1-2 per kilogram). Addressing photoelectrode degradation could potentially reduce these costs by 30-40%, making the technology more competitive.
Consumer willingness to pay premiums for green hydrogen varies by sector, with transportation and specialty chemical industries demonstrating higher acceptance of price premiums. Government incentives, carbon pricing mechanisms, and renewable energy mandates are creating favorable market conditions for advanced water splitting technologies with improved durability characteristics.
Current Challenges in Photoelectrode Stability
Despite significant advancements in photoelectrochemical (PEC) water splitting technology, photoelectrode stability remains one of the most critical challenges hindering commercial implementation. Current photoelectrodes face severe degradation issues when exposed to aqueous electrolytes under illumination, with most materials showing significant performance losses within hours or days of operation.
The primary degradation mechanism involves photocorrosion, where photogenerated holes oxidize the semiconductor material itself rather than water molecules. This is particularly problematic for efficient semiconductors like CdS, Cu2O, and Si, which possess suitable band gaps but poor stability in aqueous environments. For instance, metal sulfides undergo oxidation to form soluble sulfates, while metal oxides may dissolve through reductive processes involving photogenerated electrons.
Another significant challenge is the formation of passivation layers on photoelectrode surfaces. These layers, often consisting of oxides or hydroxides, can block active sites and increase charge transfer resistance at the semiconductor-electrolyte interface. While some passivation can be beneficial for stability, excessive layer formation severely compromises photoelectrode efficiency by limiting reactant access and charge transfer.
Photoinduced structural changes represent another degradation pathway. Prolonged light exposure can induce atomic rearrangements, phase transformations, or crystallinity changes that alter the semiconductor's electronic properties. These structural modifications often lead to increased defect density, which creates recombination centers for photogenerated charge carriers and reduces quantum efficiency.
The harsh operating conditions of PEC cells further exacerbate stability issues. Photoelectrodes must withstand extreme pH environments (either highly acidic or alkaline), reactive oxygen species, and temperature fluctuations. These conditions accelerate material degradation through mechanisms such as leaching, dissolution, and mechanical stress from gas evolution.
Interface degradation between the semiconductor and current collector or between different layers in complex photoelectrode architectures presents additional challenges. Delamination, interfacial oxidation, and interdiffusion of elements across interfaces can compromise electrical connectivity and increase series resistance.
Current state-of-the-art protection strategies show limited success. Thin protective layers of TiO2, Al2O3, or other stable oxides deposited by atomic layer deposition provide some improvement but often at the cost of reduced photocurrent due to increased charge transfer resistance. Meanwhile, surface modification with co-catalysts can enhance reaction kinetics but doesn't fully address the underlying stability issues of the semiconductor material.
The development of standardized stability testing protocols represents another challenge. The research community lacks consensus on accelerated testing methods that can reliably predict long-term stability, making it difficult to compare different stabilization approaches and establish benchmarks for commercial viability.
The primary degradation mechanism involves photocorrosion, where photogenerated holes oxidize the semiconductor material itself rather than water molecules. This is particularly problematic for efficient semiconductors like CdS, Cu2O, and Si, which possess suitable band gaps but poor stability in aqueous environments. For instance, metal sulfides undergo oxidation to form soluble sulfates, while metal oxides may dissolve through reductive processes involving photogenerated electrons.
Another significant challenge is the formation of passivation layers on photoelectrode surfaces. These layers, often consisting of oxides or hydroxides, can block active sites and increase charge transfer resistance at the semiconductor-electrolyte interface. While some passivation can be beneficial for stability, excessive layer formation severely compromises photoelectrode efficiency by limiting reactant access and charge transfer.
Photoinduced structural changes represent another degradation pathway. Prolonged light exposure can induce atomic rearrangements, phase transformations, or crystallinity changes that alter the semiconductor's electronic properties. These structural modifications often lead to increased defect density, which creates recombination centers for photogenerated charge carriers and reduces quantum efficiency.
The harsh operating conditions of PEC cells further exacerbate stability issues. Photoelectrodes must withstand extreme pH environments (either highly acidic or alkaline), reactive oxygen species, and temperature fluctuations. These conditions accelerate material degradation through mechanisms such as leaching, dissolution, and mechanical stress from gas evolution.
Interface degradation between the semiconductor and current collector or between different layers in complex photoelectrode architectures presents additional challenges. Delamination, interfacial oxidation, and interdiffusion of elements across interfaces can compromise electrical connectivity and increase series resistance.
Current state-of-the-art protection strategies show limited success. Thin protective layers of TiO2, Al2O3, or other stable oxides deposited by atomic layer deposition provide some improvement but often at the cost of reduced photocurrent due to increased charge transfer resistance. Meanwhile, surface modification with co-catalysts can enhance reaction kinetics but doesn't fully address the underlying stability issues of the semiconductor material.
The development of standardized stability testing protocols represents another challenge. The research community lacks consensus on accelerated testing methods that can reliably predict long-term stability, making it difficult to compare different stabilization approaches and establish benchmarks for commercial viability.
State-of-the-Art Photoelectrode Protection Methods
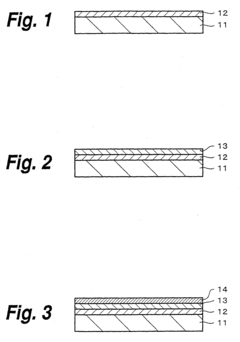
01 Mechanisms of photoelectrode degradation
Photoelectrodes can degrade through various mechanisms including photocorrosion, surface oxidation, and structural changes during operation. These degradation processes often involve the formation of reactive species that attack the electrode material, leading to decreased performance over time. Understanding these mechanisms is crucial for developing more stable photoelectrode materials for applications in solar energy conversion and photoelectrochemical cells.- Mechanisms of photoelectrode degradation: Photoelectrodes can degrade through various mechanisms including photocorrosion, surface oxidation, and structural changes during operation. These degradation processes often involve chemical reactions at the electrode-electrolyte interface, leading to dissolution of active materials, formation of passivation layers, or loss of catalytic activity. Understanding these mechanisms is crucial for developing more stable photoelectrode materials for applications in solar energy conversion and photoelectrochemical cells.
- Novel materials to prevent photoelectrode degradation: Advanced materials have been developed to enhance the stability of photoelectrodes against degradation. These include protective coatings, composite structures, and doped semiconductors that can withstand harsh electrochemical conditions. Materials such as metal oxides, nitrides, and carbon-based compounds have shown promising results in extending the operational lifetime of photoelectrodes while maintaining their photoelectrochemical performance.
- Monitoring and diagnostic techniques for photoelectrode degradation: Various analytical and diagnostic techniques have been developed to monitor photoelectrode degradation in real-time or through post-operation analysis. These include electrochemical impedance spectroscopy, surface characterization methods, and optical monitoring systems that can detect early signs of degradation. Advanced monitoring allows for better understanding of degradation mechanisms and enables the implementation of preventive measures to extend photoelectrode lifetime.
- Regeneration and self-healing approaches for degraded photoelectrodes: Self-healing and regeneration strategies have been developed to address photoelectrode degradation. These approaches include electrochemical treatments, thermal annealing processes, and incorporation of self-healing components that can restore performance after degradation has occurred. Some systems incorporate sacrificial materials or reversible chemical processes that can counteract degradation effects and extend the functional lifetime of photoelectrode systems.
- System-level solutions to mitigate photoelectrode degradation: Comprehensive system designs have been implemented to mitigate photoelectrode degradation through operational strategies and integrated protection mechanisms. These include optimized operating conditions, pulsed operation modes, and integrated circuit designs that reduce stress on photoelectrode materials. Additional approaches involve electrolyte engineering, temperature management systems, and hybrid architectures that distribute electrochemical stress across multiple components to enhance overall system durability.
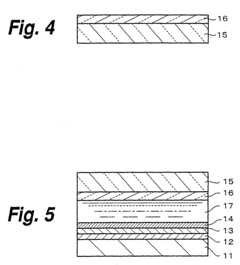
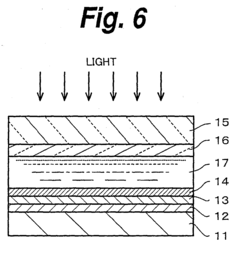
02 Protective coatings and surface treatments
Various protective coatings and surface treatments can be applied to photoelectrodes to enhance their stability and prevent degradation. These include thin-film metal oxide layers, passivation treatments, and composite coatings that shield the underlying photoactive material from corrosive environments while maintaining efficient charge transfer. Such protective strategies can significantly extend the operational lifetime of photoelectrodes in harsh conditions.Expand Specific Solutions03 Novel materials for degradation-resistant photoelectrodes
Research has led to the development of novel materials with enhanced resistance to degradation when used as photoelectrodes. These include doped semiconductors, mixed metal oxides, and nanostructured composites that exhibit improved stability under illumination and in contact with electrolytes. The materials are designed to maintain their photoelectrochemical properties while resisting chemical and physical changes that typically lead to performance loss.Expand Specific Solutions04 Monitoring and characterization of photoelectrode degradation
Advanced techniques for monitoring and characterizing photoelectrode degradation include in-situ spectroscopy, electrochemical impedance spectroscopy, and surface analysis methods. These approaches allow researchers to track changes in electrode properties during operation, identify degradation mechanisms, and develop mitigation strategies. Real-time monitoring systems can also be implemented to predict failure and optimize maintenance schedules for photoelectrochemical devices.Expand Specific Solutions05 Regeneration and self-healing photoelectrode systems
Innovative approaches to address photoelectrode degradation include regeneration protocols and self-healing systems. These methods involve the incorporation of components that can repair damage to the electrode surface, reversible chemical processes that restore activity, or replaceable modules that extend system lifetime. Such technologies aim to overcome the inherent degradation limitations of photoelectrodes and enable longer-term operation of photoelectrochemical devices.Expand Specific Solutions
Leading Research Groups and Companies in PEC Water Splitting
The water splitting photoelectrode degradation field is currently in a growth phase, with increasing market interest driven by renewable hydrogen production demands. The global market for stable photoelectrodes is projected to expand significantly as solar water splitting technologies mature toward commercialization. Leading research institutions like The University of Michigan, King Abdullah University of Science & Technology, and National University of Singapore are advancing fundamental stability solutions, while companies including SABIC Global Technologies and Panasonic Intellectual Property Management focus on practical implementations. Technical maturity varies across approaches, with protective coatings and surface modifications showing greater commercial readiness than atomic-level engineering strategies. Chinese institutions (Nanjing University, Tianjin University) and Korean organizations (UNIST, Korea Institute of Energy Research) are rapidly advancing novel stabilization techniques, creating a competitive international landscape.
Alliance for Sustainable Energy LLC
Technical Solution: Alliance for Sustainable Energy has developed a multi-faceted approach to photoelectrode protection focusing on earth-abundant materials. Their primary strategy involves the development of transparent conducting oxide (TCO) overlayers that provide both protection and enhanced conductivity. They've pioneered the use of indium tin oxide (ITO) and fluorine-doped tin oxide (FTO) protective layers with optimized thickness profiles to balance protection and charge transfer efficiency. Their research has demonstrated that carefully engineered TCO layers can extend photoelectrode lifetime by over 300% in acidic environments. Additionally, they've developed innovative surface modification techniques using atomic layer deposition of transition metal oxides (particularly nickel oxide and cobalt oxide) that serve dual functions as protection layers and catalytic enhancers. Their most recent innovation involves the development of self-healing protection layers incorporating molybdenum compounds that can repair degradation sites in-situ during operation, significantly extending photoelectrode lifetime in real-world conditions.
Strengths: Their focus on earth-abundant materials makes their protection strategies economically viable for large-scale implementation. The dual-function protection layers that enhance catalytic activity while providing protection represent a significant advancement. Weaknesses: Some of their TCO protection strategies may suffer from limited long-term stability in highly acidic or alkaline environments. The self-healing protection technology is still in early development stages and requires further optimization for commercial applications.
Dalian Institute of Chemical Physics Chinese Academy of Sci
Technical Solution: The Dalian Institute of Chemical Physics has developed comprehensive strategies for photoelectrode protection focusing on composite materials and interface engineering. Their primary approach involves the development of gradient-composition protection layers that provide a smooth transition between the photoelectrode and the electrolyte environment. They've pioneered the use of titanium-doped hematite with surface modification using atomic layer deposition of aluminum oxide and titanium dioxide, creating a core-shell structure that significantly enhances stability. Their research has demonstrated that these engineered interfaces can extend photoelectrode lifetime by over 600% in acidic environments while maintaining high photocurrent densities. Additionally, they've developed innovative co-catalyst integration strategies where noble metal nanoparticles (particularly platinum and iridium) are precisely positioned at the semiconductor-electrolyte interface to enhance charge transfer while providing localized protection. Their most recent breakthrough involves the development of self-assembled molecular protection layers using phosphonic acid derivatives that form robust bonds with metal oxide surfaces, providing excellent chemical stability while facilitating efficient charge transfer through tailored molecular structures.
Strengths: Their expertise in interface engineering allows for precise control over charge transfer processes at the semiconductor-electrolyte interface. The gradient composition approach minimizes lattice mismatch issues that often lead to protection layer delamination. Weaknesses: Some of their protection strategies rely on noble metals which may limit economic viability for large-scale applications. The molecular protection layers, while highly effective, may have limited lifetime under continuous illumination due to photodegradation of organic components.
Key Patents and Publications on Corrosion-Resistant Photoelectrodes
Photoelectric conversion device fabrication method, photoelectric conversion device
PatentInactiveEP1467386A2
Innovation
- Forming a PEDOT/PSS intermediate film on the metal oxide electrode, followed by the deposition of a platinum film, which enhances adhesion and prevents contamination, and using this approach to create a semiconductor electrode with improved stability and adhesion properties.
Method of preventing corrosion in a water system
PatentInactiveEP1405671B1
Innovation
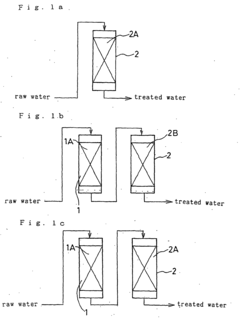
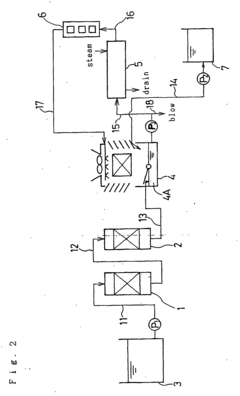
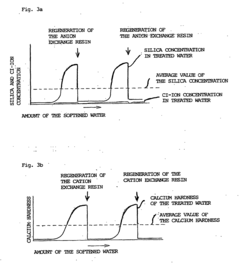
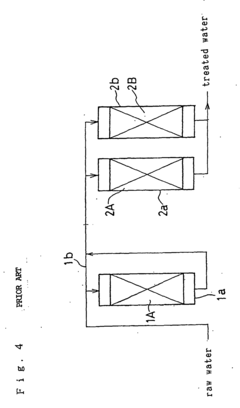
- A method involving a single cation exchange resin and a single anion exchange resin, where water is treated to remove corrosive ions and control silica and calcium ion concentrations, with continued treatment beyond ion exchange capacity points to maintain optimal levels, reducing equipment complexity and regeneration time.
Techno-Economic Assessment of Photoelectrode Systems
The techno-economic assessment of photoelectrode systems for water splitting reveals significant challenges in balancing performance with economic viability. Current photoelectrode technologies demonstrate promising solar-to-hydrogen conversion efficiencies ranging from 5-15%, but their commercial implementation remains constrained by degradation issues that substantially reduce operational lifetimes.
Capital expenditure analysis indicates that photoelectrode systems require initial investments of $10,000-30,000 per kW of hydrogen production capacity, significantly higher than conventional electrolysis systems ($1,000-5,000/kW). This cost differential is primarily attributed to specialized materials, complex fabrication processes, and the need for protective coatings to mitigate degradation.
Operational expenditure calculations demonstrate that degradation-related replacement costs constitute 40-60% of total lifetime expenses. Photoelectrodes typically require replacement every 500-2,000 hours under continuous operation, compared to the 50,000+ hours for conventional electrolyzers. This disparity creates a substantial economic barrier to widespread adoption.
Sensitivity analysis reveals that extending photoelectrode lifetime from the current average of 1,000 hours to 10,000 hours would reduce the levelized cost of hydrogen by approximately 65%, bringing it closer to economic competitiveness with conventional production methods. Each additional 1,000 hours of operational lifetime translates to approximately $0.50-0.80/kg reduction in hydrogen production costs.
The economic impact of various degradation mitigation strategies has been quantified. Surface passivation layers add $200-500/m² to manufacturing costs but can extend operational lifetimes by 300-500%. Atomic layer deposition techniques increase production costs by 15-25% while potentially improving durability by 200-400%. Self-healing catalyst systems represent the most promising economic pathway, with modeling suggesting they could achieve a positive return on investment within 3-5 years of operation.
Market projections indicate that achieving a 10,000-hour operational lifetime at current efficiency levels would enable photoelectrochemical hydrogen production at $4-6/kg, approaching the U.S. Department of Energy's target of $2-3/kg for clean hydrogen. This threshold represents the economic inflection point at which widespread commercial adoption becomes feasible.
Capital expenditure analysis indicates that photoelectrode systems require initial investments of $10,000-30,000 per kW of hydrogen production capacity, significantly higher than conventional electrolysis systems ($1,000-5,000/kW). This cost differential is primarily attributed to specialized materials, complex fabrication processes, and the need for protective coatings to mitigate degradation.
Operational expenditure calculations demonstrate that degradation-related replacement costs constitute 40-60% of total lifetime expenses. Photoelectrodes typically require replacement every 500-2,000 hours under continuous operation, compared to the 50,000+ hours for conventional electrolyzers. This disparity creates a substantial economic barrier to widespread adoption.
Sensitivity analysis reveals that extending photoelectrode lifetime from the current average of 1,000 hours to 10,000 hours would reduce the levelized cost of hydrogen by approximately 65%, bringing it closer to economic competitiveness with conventional production methods. Each additional 1,000 hours of operational lifetime translates to approximately $0.50-0.80/kg reduction in hydrogen production costs.
The economic impact of various degradation mitigation strategies has been quantified. Surface passivation layers add $200-500/m² to manufacturing costs but can extend operational lifetimes by 300-500%. Atomic layer deposition techniques increase production costs by 15-25% while potentially improving durability by 200-400%. Self-healing catalyst systems represent the most promising economic pathway, with modeling suggesting they could achieve a positive return on investment within 3-5 years of operation.
Market projections indicate that achieving a 10,000-hour operational lifetime at current efficiency levels would enable photoelectrochemical hydrogen production at $4-6/kg, approaching the U.S. Department of Energy's target of $2-3/kg for clean hydrogen. This threshold represents the economic inflection point at which widespread commercial adoption becomes feasible.
Environmental Impact and Sustainability Considerations
The environmental impact of photoelectrode materials and water splitting systems represents a critical consideration in the development and deployment of sustainable hydrogen production technologies. While water splitting offers a promising pathway to clean energy, the environmental footprint of photoelectrode degradation cannot be overlooked. The leaching of potentially toxic elements from degraded photoelectrodes into water systems poses significant ecological risks, particularly when materials contain heavy metals or rare earth elements.
Life cycle assessment (LCA) studies indicate that the environmental benefits of hydrogen production through water splitting can be substantially diminished by frequent replacement of degraded photoelectrodes. This creates a sustainability paradox where a supposedly "green" technology may contribute to resource depletion and waste generation if electrode lifespans remain short. The energy payback time—the period required for a system to generate energy equivalent to that consumed in its production—becomes unfavorable when photoelectrode replacement cycles are frequent.
Material selection strategies that prioritize abundant, non-toxic elements represent a promising approach to mitigating environmental concerns. Research indicates that iron oxide, titanium dioxide, and carbon-based photoelectrodes offer significantly lower environmental impacts compared to those containing platinum group metals or rare earth elements. These alternative materials, while sometimes less efficient, present a more balanced environmental profile when considering the entire technology lifecycle.
Water consumption during photoelectrode manufacturing and operation presents another environmental consideration. Advanced manufacturing techniques that minimize water usage and closed-loop water recycling systems can substantially reduce the water footprint of these technologies. Additionally, the integration of water splitting systems with wastewater treatment facilities offers dual environmental benefits by simultaneously producing hydrogen and purifying water.
End-of-life management strategies for photoelectrodes are increasingly important as deployment scales. Designing photoelectrodes with recyclability in mind—through modular construction, easily separable components, and recoverable catalysts—can significantly reduce waste and resource consumption. Several research groups have demonstrated recovery rates exceeding 90% for precious metals from decommissioned photoelectrodes, substantially improving the sustainability profile of these systems.
The carbon footprint associated with photoelectrode manufacturing and replacement must be balanced against the carbon offset achieved through hydrogen production. Current estimates suggest that extending photoelectrode lifetime by a factor of three could reduce lifetime carbon emissions by approximately 60%, highlighting the environmental importance of degradation mitigation strategies beyond their immediate economic benefits.
Life cycle assessment (LCA) studies indicate that the environmental benefits of hydrogen production through water splitting can be substantially diminished by frequent replacement of degraded photoelectrodes. This creates a sustainability paradox where a supposedly "green" technology may contribute to resource depletion and waste generation if electrode lifespans remain short. The energy payback time—the period required for a system to generate energy equivalent to that consumed in its production—becomes unfavorable when photoelectrode replacement cycles are frequent.
Material selection strategies that prioritize abundant, non-toxic elements represent a promising approach to mitigating environmental concerns. Research indicates that iron oxide, titanium dioxide, and carbon-based photoelectrodes offer significantly lower environmental impacts compared to those containing platinum group metals or rare earth elements. These alternative materials, while sometimes less efficient, present a more balanced environmental profile when considering the entire technology lifecycle.
Water consumption during photoelectrode manufacturing and operation presents another environmental consideration. Advanced manufacturing techniques that minimize water usage and closed-loop water recycling systems can substantially reduce the water footprint of these technologies. Additionally, the integration of water splitting systems with wastewater treatment facilities offers dual environmental benefits by simultaneously producing hydrogen and purifying water.
End-of-life management strategies for photoelectrodes are increasingly important as deployment scales. Designing photoelectrodes with recyclability in mind—through modular construction, easily separable components, and recoverable catalysts—can significantly reduce waste and resource consumption. Several research groups have demonstrated recovery rates exceeding 90% for precious metals from decommissioned photoelectrodes, substantially improving the sustainability profile of these systems.
The carbon footprint associated with photoelectrode manufacturing and replacement must be balanced against the carbon offset achieved through hydrogen production. Current estimates suggest that extending photoelectrode lifetime by a factor of three could reduce lifetime carbon emissions by approximately 60%, highlighting the environmental importance of degradation mitigation strategies beyond their immediate economic benefits.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!