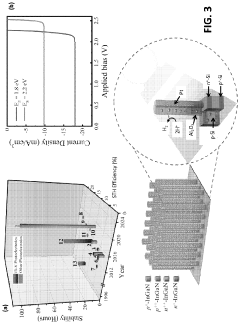
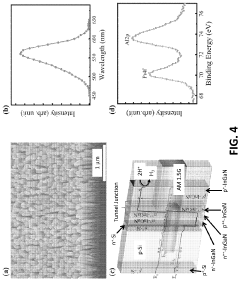
Role of conductive polymers in PEC water splitting advancements.
SEP 5, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Conductive Polymers in PEC Water Splitting: Background & Objectives
Photoelectrochemical (PEC) water splitting has emerged as a promising approach for sustainable hydrogen production, leveraging solar energy to drive the decomposition of water into hydrogen and oxygen. The evolution of this technology spans several decades, beginning with Fujishima and Honda's groundbreaking demonstration of photocatalytic water splitting using titanium dioxide electrodes in 1972. Since then, the field has witnessed significant advancements in materials science and engineering to enhance efficiency and stability.
Conductive polymers represent a revolutionary class of materials that combine the electrical properties of metals with the processing advantages of polymers. Their discovery in the late 1970s by Alan Heeger, Alan MacDiarmid, and Hideki Shirakawa—work that earned them the Nobel Prize in Chemistry in 2000—marked a paradigm shift in material science. These polymers, characterized by their conjugated backbone structures, facilitate electron movement along their chains, making them ideal candidates for various electrochemical applications.
The integration of conductive polymers into PEC water splitting systems has gained momentum over the past decade. This convergence addresses critical challenges in traditional semiconductor-based PEC systems, particularly regarding charge carrier mobility, light absorption, and stability in aqueous environments. Polymers such as polyaniline (PANI), polypyrrole (PPy), and poly(3,4-ethylenedioxythiophene) (PEDOT) have demonstrated promising capabilities in enhancing photoanode and photocathode performance.
Recent technological trends indicate a shift toward hybrid systems that combine conductive polymers with inorganic semiconductors, creating synergistic effects that overcome limitations of individual components. Additionally, advancements in polymer engineering have enabled precise control over bandgap, conductivity, and morphology, allowing tailored designs for specific PEC requirements.
The primary technical objectives in this field include enhancing solar-to-hydrogen conversion efficiency beyond the current benchmarks (typically below 10% for most systems), improving long-term stability under operational conditions, and developing scalable fabrication methods suitable for industrial implementation. Researchers aim to understand fundamental charge transfer mechanisms at polymer-semiconductor interfaces and optimize these interactions to minimize recombination losses.
Furthermore, there is growing interest in developing earth-abundant, environmentally benign materials to replace rare or toxic elements currently used in high-performance PEC systems. Conductive polymers, primarily composed of carbon, nitrogen, and other abundant elements, align well with this sustainability objective.
As we advance toward a hydrogen-based economy, the role of conductive polymers in PEC water splitting represents a critical research frontier with significant implications for renewable energy technologies and environmental sustainability.
Conductive polymers represent a revolutionary class of materials that combine the electrical properties of metals with the processing advantages of polymers. Their discovery in the late 1970s by Alan Heeger, Alan MacDiarmid, and Hideki Shirakawa—work that earned them the Nobel Prize in Chemistry in 2000—marked a paradigm shift in material science. These polymers, characterized by their conjugated backbone structures, facilitate electron movement along their chains, making them ideal candidates for various electrochemical applications.
The integration of conductive polymers into PEC water splitting systems has gained momentum over the past decade. This convergence addresses critical challenges in traditional semiconductor-based PEC systems, particularly regarding charge carrier mobility, light absorption, and stability in aqueous environments. Polymers such as polyaniline (PANI), polypyrrole (PPy), and poly(3,4-ethylenedioxythiophene) (PEDOT) have demonstrated promising capabilities in enhancing photoanode and photocathode performance.
Recent technological trends indicate a shift toward hybrid systems that combine conductive polymers with inorganic semiconductors, creating synergistic effects that overcome limitations of individual components. Additionally, advancements in polymer engineering have enabled precise control over bandgap, conductivity, and morphology, allowing tailored designs for specific PEC requirements.
The primary technical objectives in this field include enhancing solar-to-hydrogen conversion efficiency beyond the current benchmarks (typically below 10% for most systems), improving long-term stability under operational conditions, and developing scalable fabrication methods suitable for industrial implementation. Researchers aim to understand fundamental charge transfer mechanisms at polymer-semiconductor interfaces and optimize these interactions to minimize recombination losses.
Furthermore, there is growing interest in developing earth-abundant, environmentally benign materials to replace rare or toxic elements currently used in high-performance PEC systems. Conductive polymers, primarily composed of carbon, nitrogen, and other abundant elements, align well with this sustainability objective.
As we advance toward a hydrogen-based economy, the role of conductive polymers in PEC water splitting represents a critical research frontier with significant implications for renewable energy technologies and environmental sustainability.
Market Analysis for PEC Hydrogen Production Technologies
The global market for photoelectrochemical (PEC) hydrogen production technologies is experiencing significant growth, driven by increasing demand for clean energy solutions and the global push towards decarbonization. Current market valuations indicate that the PEC water splitting sector is positioned to reach approximately $2.5 billion by 2030, with a compound annual growth rate of 14.7% from 2023 to 2030.
The integration of conductive polymers in PEC systems represents a particularly promising segment within this market. These materials offer cost advantages over traditional noble metal catalysts, with production costs potentially 40-60% lower while maintaining comparable efficiency levels. This economic advantage is creating substantial market opportunities, especially in regions with established renewable energy infrastructures.
Regional market analysis reveals that North America and Europe currently lead in PEC technology development and commercialization, accounting for approximately 65% of global research investments. However, the Asia-Pacific region, particularly China, Japan, and South Korea, is demonstrating the fastest growth rate at 17.3% annually, fueled by aggressive government renewable energy policies and substantial industrial investments.
Market segmentation shows that conductive polymer-based PEC systems are gaining traction in both centralized and distributed hydrogen production applications. The distributed segment is projected to grow more rapidly due to increasing interest in localized energy solutions and the inherent scalability advantages of polymer-based systems.
End-user analysis indicates diversification across multiple sectors. Industrial applications currently represent the largest market share at 42%, followed by transportation (28%), power generation (18%), and residential applications (12%). The transportation sector, particularly hydrogen fuel cell vehicles, is expected to become the fastest-growing application segment with projected annual growth of 19.2%.
Competitive landscape assessment reveals that established energy companies are increasingly partnering with materials science startups to accelerate commercialization of conductive polymer PEC technologies. This trend is creating a dynamic market environment characterized by strategic alliances and technology transfer agreements.
Market barriers include scaling challenges, durability concerns in real-world conditions, and competition from alternative hydrogen production methods such as electrolysis. However, the unique advantages of conductive polymers—including earth-abundant material composition, tunable properties, and simplified manufacturing processes—position them favorably within the broader hydrogen production technology landscape.
The integration of conductive polymers in PEC systems represents a particularly promising segment within this market. These materials offer cost advantages over traditional noble metal catalysts, with production costs potentially 40-60% lower while maintaining comparable efficiency levels. This economic advantage is creating substantial market opportunities, especially in regions with established renewable energy infrastructures.
Regional market analysis reveals that North America and Europe currently lead in PEC technology development and commercialization, accounting for approximately 65% of global research investments. However, the Asia-Pacific region, particularly China, Japan, and South Korea, is demonstrating the fastest growth rate at 17.3% annually, fueled by aggressive government renewable energy policies and substantial industrial investments.
Market segmentation shows that conductive polymer-based PEC systems are gaining traction in both centralized and distributed hydrogen production applications. The distributed segment is projected to grow more rapidly due to increasing interest in localized energy solutions and the inherent scalability advantages of polymer-based systems.
End-user analysis indicates diversification across multiple sectors. Industrial applications currently represent the largest market share at 42%, followed by transportation (28%), power generation (18%), and residential applications (12%). The transportation sector, particularly hydrogen fuel cell vehicles, is expected to become the fastest-growing application segment with projected annual growth of 19.2%.
Competitive landscape assessment reveals that established energy companies are increasingly partnering with materials science startups to accelerate commercialization of conductive polymer PEC technologies. This trend is creating a dynamic market environment characterized by strategic alliances and technology transfer agreements.
Market barriers include scaling challenges, durability concerns in real-world conditions, and competition from alternative hydrogen production methods such as electrolysis. However, the unique advantages of conductive polymers—including earth-abundant material composition, tunable properties, and simplified manufacturing processes—position them favorably within the broader hydrogen production technology landscape.
Current Status and Challenges of Conductive Polymers in PEC Systems
The global landscape of conductive polymers in photoelectrochemical (PEC) water splitting has witnessed significant advancements in recent years. Currently, several conductive polymers including polyaniline (PANI), polypyrrole (PPy), poly(3,4-ethylenedioxythiophene) (PEDOT), and polythiophene derivatives have emerged as promising materials for PEC applications. These polymers exhibit favorable properties such as tunable bandgaps, high conductivity, and excellent stability in aqueous environments, making them suitable candidates for integration into photoelectrodes.
Research institutions across North America, Europe, and East Asia have established strong footholds in this domain, with China, the United States, and South Korea leading in publication output. The technological readiness level (TRL) of conductive polymer-based PEC systems currently ranges between 3-5, indicating that while laboratory demonstrations have been successful, commercial viability remains distant.
Despite promising developments, several critical challenges impede the widespread implementation of conductive polymers in PEC water splitting. Foremost among these is the limited photocatalytic efficiency, with most systems achieving solar-to-hydrogen conversion efficiencies below 5%, significantly lower than the theoretical maximum and commercial viability threshold of 10%. This efficiency gap stems from rapid charge recombination processes and insufficient light absorption in the visible spectrum.
Stability presents another major hurdle, as many conductive polymers undergo degradation under prolonged illumination and in oxidative environments. Current research indicates that most polymer-based photoelectrodes maintain optimal performance for only 10-100 hours, whereas commercial applications require thousands of hours of stable operation.
Scalability concerns also persist, as laboratory synthesis methods often involve complex procedures and environmentally harmful reagents. The transition from milligram-scale production to industrial quantities introduces challenges in maintaining uniform properties and performance metrics.
Interface engineering between conductive polymers and other components in PEC systems remains underdeveloped. Poor interfacial contact leads to increased charge transfer resistance and reduced overall system efficiency. Current research focuses on developing novel coupling agents and fabrication techniques to optimize these interfaces.
The cost-effectiveness of conductive polymer-based systems presents a complex challenge. While the raw materials for polymer synthesis are relatively inexpensive compared to noble metal catalysts, the processing costs and limited durability offset these initial advantages. Economic analyses suggest that the levelized cost of hydrogen from current polymer-based PEC systems exceeds $10/kg, significantly higher than the DOE target of $2/kg by 2030.
Research institutions across North America, Europe, and East Asia have established strong footholds in this domain, with China, the United States, and South Korea leading in publication output. The technological readiness level (TRL) of conductive polymer-based PEC systems currently ranges between 3-5, indicating that while laboratory demonstrations have been successful, commercial viability remains distant.
Despite promising developments, several critical challenges impede the widespread implementation of conductive polymers in PEC water splitting. Foremost among these is the limited photocatalytic efficiency, with most systems achieving solar-to-hydrogen conversion efficiencies below 5%, significantly lower than the theoretical maximum and commercial viability threshold of 10%. This efficiency gap stems from rapid charge recombination processes and insufficient light absorption in the visible spectrum.
Stability presents another major hurdle, as many conductive polymers undergo degradation under prolonged illumination and in oxidative environments. Current research indicates that most polymer-based photoelectrodes maintain optimal performance for only 10-100 hours, whereas commercial applications require thousands of hours of stable operation.
Scalability concerns also persist, as laboratory synthesis methods often involve complex procedures and environmentally harmful reagents. The transition from milligram-scale production to industrial quantities introduces challenges in maintaining uniform properties and performance metrics.
Interface engineering between conductive polymers and other components in PEC systems remains underdeveloped. Poor interfacial contact leads to increased charge transfer resistance and reduced overall system efficiency. Current research focuses on developing novel coupling agents and fabrication techniques to optimize these interfaces.
The cost-effectiveness of conductive polymer-based systems presents a complex challenge. While the raw materials for polymer synthesis are relatively inexpensive compared to noble metal catalysts, the processing costs and limited durability offset these initial advantages. Economic analyses suggest that the levelized cost of hydrogen from current polymer-based PEC systems exceeds $10/kg, significantly higher than the DOE target of $2/kg by 2030.
Current Conductive Polymer Integration Strategies for PEC Cells
01 Polyaniline-based conductive polymers
Polyaniline is a widely studied conductive polymer due to its environmental stability, simple synthesis, and tunable electrical properties. These polymers can be doped with various acids to enhance conductivity and can be processed into films, fibers, and coatings. Polyaniline-based materials find applications in batteries, sensors, electromagnetic shielding, and antistatic coatings due to their unique combination of electrical conductivity and processability.- Synthesis and preparation of conductive polymers: Various methods for synthesizing and preparing conductive polymers are disclosed. These include chemical and electrochemical polymerization techniques to create polymers with high electrical conductivity. The processes often involve oxidative polymerization of monomers such as pyrrole, thiophene, or aniline derivatives. Different catalysts and reaction conditions can be employed to control the molecular structure and resulting electrical properties of the conductive polymers.
- Doping techniques to enhance conductivity: Doping processes are essential for improving the electrical conductivity of polymers. Various dopants including halogens, metal salts, and organic compounds can be incorporated into polymer structures to create charge carriers. The doping level and type of dopant significantly influence the conductivity, stability, and processability of the resulting materials. Both p-type and n-type doping strategies are employed to achieve desired electronic properties in different applications.
- Conductive polymer composites and blends: Conductive polymer composites combine polymeric materials with conductive fillers or other polymers to create materials with tailored electrical properties. These composites often incorporate carbon-based materials, metal particles, or inherently conductive polymers into conventional polymer matrices. The resulting materials benefit from both the processability of polymers and enhanced electrical conductivity. Various blending techniques and compatibilization methods are used to ensure uniform dispersion and optimal interfacial interactions.
- Structure-property relationships in conductive polymers: The molecular structure of conductive polymers directly influences their electrical, optical, and mechanical properties. Factors such as conjugation length, chain alignment, crystallinity, and molecular weight significantly affect conductivity. Understanding these structure-property relationships enables the design of polymers with optimized performance characteristics. Various analytical techniques are employed to characterize these relationships and establish design principles for new conductive polymer systems.
- Applications of conductive polymers: Conductive polymers find applications across numerous fields including electronics, energy storage, sensors, and biomedical devices. They are used in organic light-emitting diodes, solar cells, batteries, supercapacitors, electromagnetic shielding, antistatic coatings, and biosensors. Their unique combination of electrical conductivity with polymer properties such as flexibility, low weight, and processability makes them valuable in emerging technologies. Specific formulations and processing methods are developed to optimize performance in each application area.
02 Polythiophene derivatives for electronic applications
Polythiophene and its derivatives represent an important class of conductive polymers with excellent thermal stability and electrical properties. These materials can be functionalized with various side chains to improve solubility and processability. Polythiophene-based conductive polymers are particularly valuable in organic electronics, including organic field-effect transistors, organic photovoltaics, and flexible displays due to their semiconductor properties and ability to form ordered structures.Expand Specific Solutions03 Conductive polymer composites with fillers
Conductive polymer composites incorporate conductive fillers such as carbon nanotubes, graphene, or metal particles into polymer matrices to enhance electrical conductivity. These composites combine the processability of polymers with the electrical properties of the fillers. The resulting materials offer tunable conductivity based on filler concentration and distribution, making them suitable for applications in electromagnetic interference shielding, antistatic packaging, and flexible electronics.Expand Specific Solutions04 Water-soluble conductive polymers
Water-soluble conductive polymers address environmental concerns and expand application possibilities by eliminating the need for organic solvents during processing. These polymers typically incorporate hydrophilic functional groups or are synthesized as polyelectrolytes. Water-soluble conductive polymers are particularly valuable in biomedical applications, including biosensors, tissue engineering, and drug delivery systems, as well as in environmentally friendly electronic devices.Expand Specific Solutions05 Processing methods for conductive polymers
Various processing techniques have been developed to fabricate conductive polymer materials into usable forms. These methods include solution processing, electrochemical deposition, vapor phase polymerization, and melt processing. Each technique offers different advantages in terms of film quality, pattern definition, and scalability. Advanced processing methods enable the creation of structured conductive polymer materials with controlled morphology, which is critical for optimizing performance in applications such as sensors, actuators, and energy storage devices.Expand Specific Solutions
Leading Research Groups and Companies in Conductive Polymer PEC
The conductive polymers market in PEC water splitting is currently in a growth phase, characterized by increasing research activities and commercial applications. The market size is expanding due to rising demand for sustainable hydrogen production technologies, with projections indicating significant growth over the next decade. Technologically, the field is advancing rapidly but remains in mid-maturity, with key players demonstrating varying levels of expertise. LG Chem and DuPont lead commercial polymer development, while research institutions like Nanjing University, University of Michigan, and Tokyo Institute of Technology drive fundamental innovations. Specialized companies such as Cambridge Display Technology and Shin-Etsu Polymer contribute niche expertise in conductive materials. The competitive landscape shows a healthy balance between established chemical corporations and emerging research-driven entities, suggesting a dynamic ecosystem poised for breakthrough advancements.
LG Chem Ltd.
Technical Solution: LG Chem has developed advanced conductive polymer-based photoelectrodes for PEC water splitting, focusing on PEDOT:PSS (poly(3,4-ethylenedioxythiophene):polystyrene sulfonate) composites with metal oxide semiconductors. Their approach involves creating core-shell nanostructures where conductive polymers form a protective layer around semiconductors like BiVO4 and TiO2. This configuration enhances charge separation efficiency by facilitating hole transport to the electrolyte interface while protecting the semiconductor from photocorrosion. LG Chem's proprietary synthesis methods achieve uniform polymer coating with controlled thickness (5-20 nm), optimizing the balance between light absorption and charge transport. Their systems demonstrate solar-to-hydrogen conversion efficiencies of up to 5.2% under simulated sunlight, with remarkable stability exceeding 100 hours of continuous operation without significant performance degradation.
Strengths: Superior charge separation efficiency and excellent stability against photocorrosion. The polymer coatings provide protection while maintaining high catalytic activity. Weaknesses: Higher production costs compared to pure inorganic systems and potential degradation under extreme pH conditions or prolonged UV exposure.
Samsung Display Co., Ltd.
Technical Solution: Samsung Display has pioneered hybrid photoelectrochemical systems incorporating polythiophene derivatives and polyaniline-based conductive polymers for water splitting applications. Their technology utilizes a multi-layer electrode design where conductive polymers serve as both charge transport mediators and protective barriers. Samsung's approach features precisely controlled electropolymerization techniques to create nanopatterned polymer films with optimized morphology for maximum surface area and light absorption. These electrodes incorporate strategic dopants to tune the polymer's electronic properties, achieving band alignment with common semiconductor materials. The company has demonstrated tandem PEC cells combining n-type semiconductors with p-type conductive polymers, creating built-in electric fields that enhance charge separation. Their latest prototypes achieve hydrogen production rates of approximately 10 μmol/h/cm² under standard testing conditions, with operational stability maintained for over 80 hours.
Strengths: Excellent scalability potential through established manufacturing processes and superior optical properties allowing for efficient light harvesting across the visible spectrum. Weaknesses: Relatively high cost of specialized polymer materials and some performance limitations in alkaline electrolytes.
Key Patents and Scientific Breakthroughs in Polymer-Based PEC
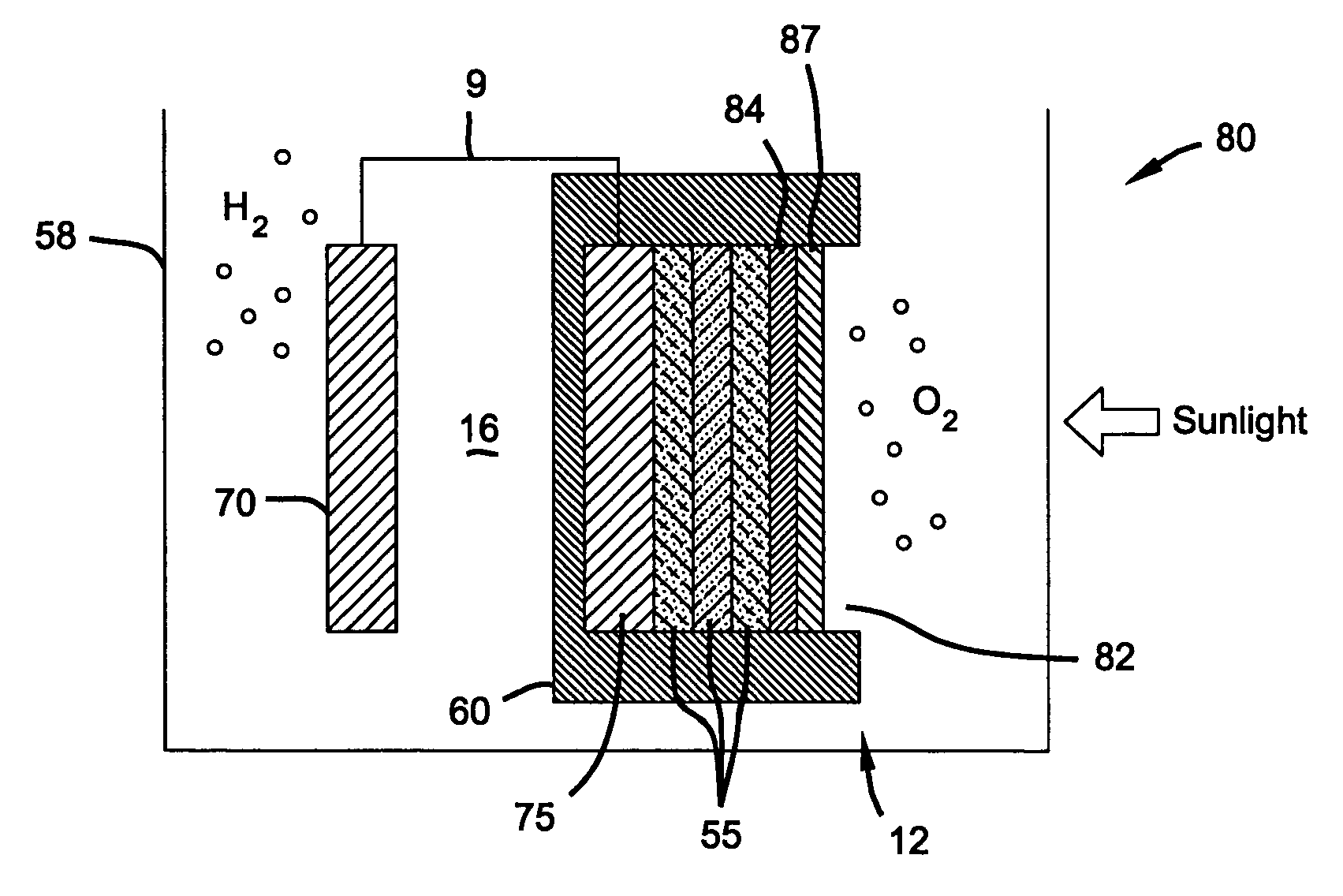
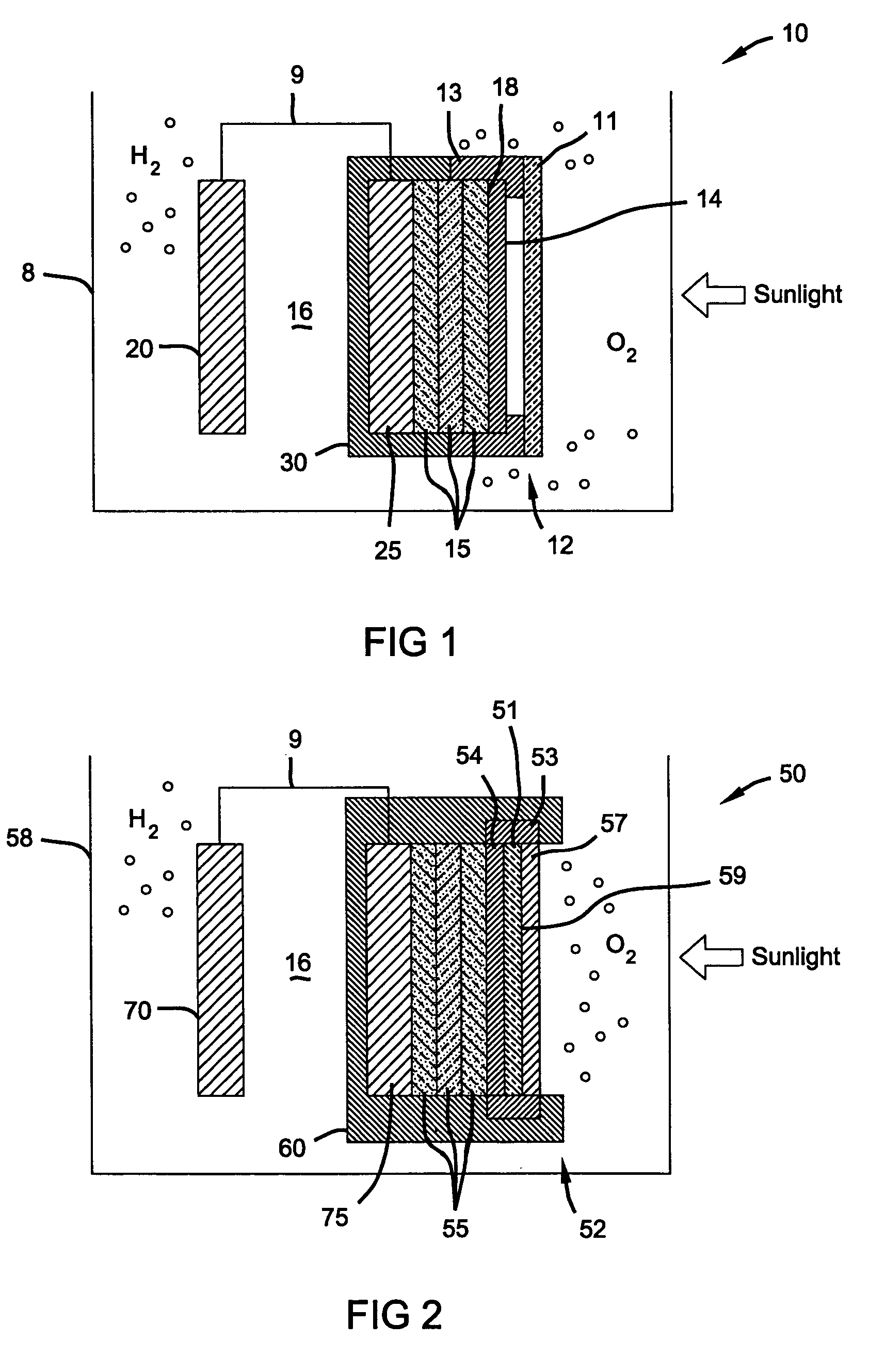
Photoelectrochemical device and electrode
PatentInactiveUS7052587B2
Innovation
- A photoelectrochemical (PEC) device with a doped tin oxide layer and a triple-junction amorphous silicon solar cell, protected by a transparent, anti-reflective, and conductive metal oxide layer, and a non-conductive glass or polymer shield, which provides a robust and corrosion-resistant interface for water splitting.
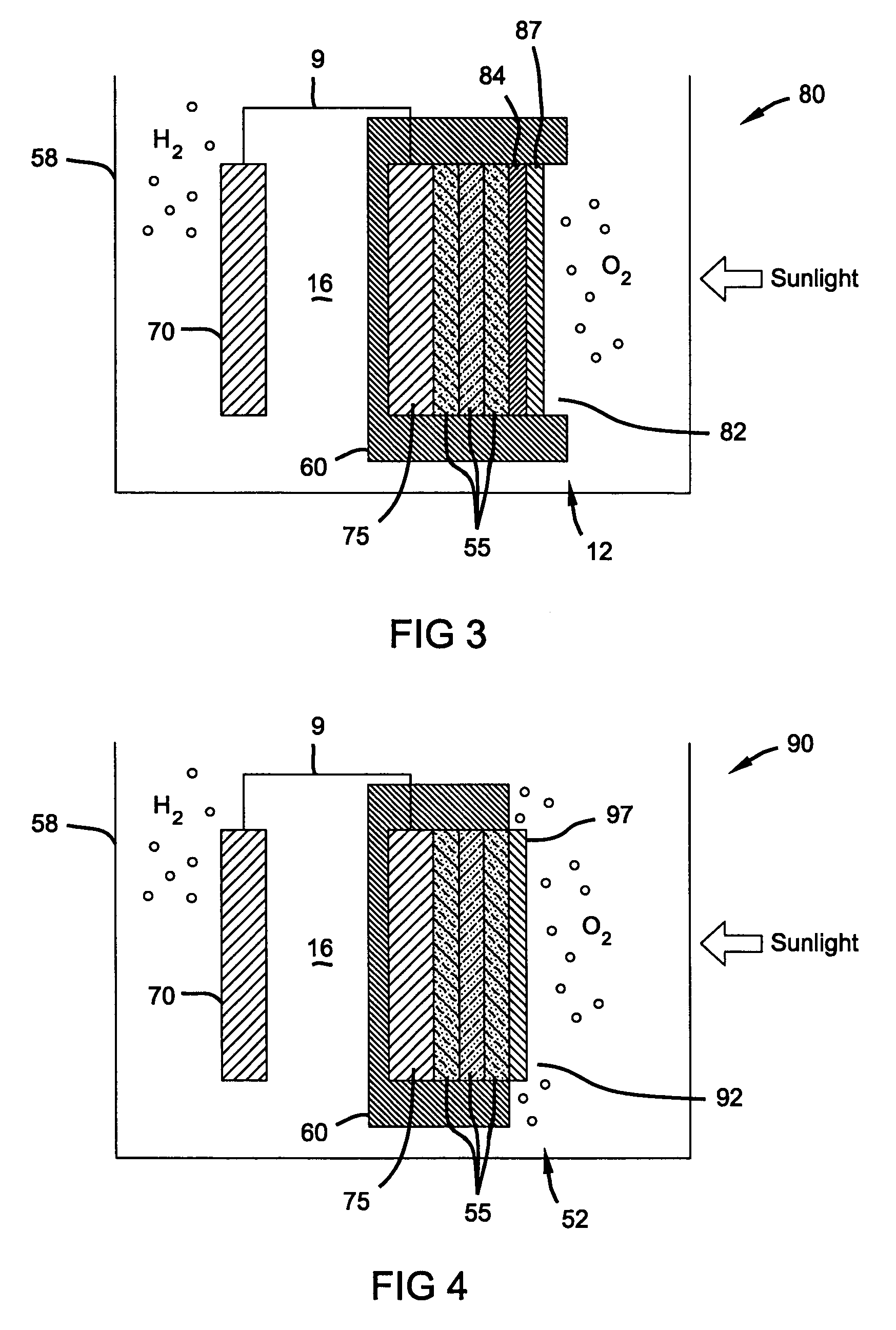
Water splitting device protection
PatentPendingUS20230407498A1
Innovation
- The development of InGaN/Si double-junction photocathodes with a defect-free nanowire tunnel junction and an ultrathin Al2O3 surface passivation layer, integrated with Pt catalysts, enhances charge carrier extraction and stability, allowing for efficient and long-term unassisted solar water splitting.
Scalability and Cost Analysis of Polymer-Enhanced PEC Systems
The economic viability of polymer-enhanced photoelectrochemical (PEC) water splitting systems remains a critical factor determining their widespread adoption. Current cost analyses indicate that conductive polymer integration in PEC systems offers significant advantages over traditional semiconductor-based approaches, with potential material cost reductions of 30-45% depending on the specific polymers employed.
Manufacturing scalability presents both opportunities and challenges. Conductive polymers like PEDOT:PSS and polyaniline can be processed using solution-based techniques including spray coating, inkjet printing, and roll-to-roll manufacturing, enabling large-scale production with reduced capital equipment requirements compared to vacuum deposition methods used for inorganic semiconductors. These processing advantages translate to approximately 25% lower production costs at commercial scales.
However, several economic barriers persist. The synthesis of specialized conductive polymers with optimized properties for PEC applications currently involves complex procedures that are difficult to scale. Laboratory-grade polymers can cost 5-10 times more than their potential commercial price points, creating a significant gap between research demonstrations and commercial feasibility.
Lifetime considerations significantly impact the levelized cost of hydrogen production. While inorganic PEC systems typically demonstrate stability measured in thousands of hours, polymer-enhanced systems currently average 500-1500 hours before significant degradation occurs. This shorter operational lifetime necessitates more frequent replacement, offsetting some of the initial cost advantages.
Energy return on investment (EROI) calculations reveal that polymer-enhanced PEC systems must achieve solar-to-hydrogen efficiencies of at least 8-10% with operational lifetimes exceeding 5 years to become economically competitive with conventional hydrogen production methods. Current systems achieve 5-7% efficiency with substantially shorter lifetimes.
Supply chain analysis indicates potential bottlenecks in scaling production of specialty monomers required for high-performance conductive polymers. Diversification of precursor sources and development of synthetic routes using more abundant starting materials will be essential for large-scale deployment.
Recent techno-economic assessments project that with continued improvements in efficiency, stability, and manufacturing processes, polymer-enhanced PEC systems could achieve hydrogen production costs of $3-4/kg by 2030, approaching the $2/kg target set by various hydrogen roadmaps. This would position them as viable contributors to the renewable hydrogen economy, particularly in distributed production scenarios where their lower capital costs provide competitive advantages.
Manufacturing scalability presents both opportunities and challenges. Conductive polymers like PEDOT:PSS and polyaniline can be processed using solution-based techniques including spray coating, inkjet printing, and roll-to-roll manufacturing, enabling large-scale production with reduced capital equipment requirements compared to vacuum deposition methods used for inorganic semiconductors. These processing advantages translate to approximately 25% lower production costs at commercial scales.
However, several economic barriers persist. The synthesis of specialized conductive polymers with optimized properties for PEC applications currently involves complex procedures that are difficult to scale. Laboratory-grade polymers can cost 5-10 times more than their potential commercial price points, creating a significant gap between research demonstrations and commercial feasibility.
Lifetime considerations significantly impact the levelized cost of hydrogen production. While inorganic PEC systems typically demonstrate stability measured in thousands of hours, polymer-enhanced systems currently average 500-1500 hours before significant degradation occurs. This shorter operational lifetime necessitates more frequent replacement, offsetting some of the initial cost advantages.
Energy return on investment (EROI) calculations reveal that polymer-enhanced PEC systems must achieve solar-to-hydrogen efficiencies of at least 8-10% with operational lifetimes exceeding 5 years to become economically competitive with conventional hydrogen production methods. Current systems achieve 5-7% efficiency with substantially shorter lifetimes.
Supply chain analysis indicates potential bottlenecks in scaling production of specialty monomers required for high-performance conductive polymers. Diversification of precursor sources and development of synthetic routes using more abundant starting materials will be essential for large-scale deployment.
Recent techno-economic assessments project that with continued improvements in efficiency, stability, and manufacturing processes, polymer-enhanced PEC systems could achieve hydrogen production costs of $3-4/kg by 2030, approaching the $2/kg target set by various hydrogen roadmaps. This would position them as viable contributors to the renewable hydrogen economy, particularly in distributed production scenarios where their lower capital costs provide competitive advantages.
Environmental Impact and Sustainability of Polymer-Based PEC Technology
The integration of conductive polymers in photoelectrochemical (PEC) water splitting systems represents a significant advancement in renewable energy technology, but its environmental implications warrant careful consideration. Polymer-based PEC technologies offer substantial environmental benefits compared to traditional energy sources, primarily through their contribution to clean hydrogen production without carbon emissions during operation. This represents a critical pathway toward decarbonizing energy systems and reducing dependence on fossil fuels.
The sustainability profile of polymer-based PEC systems is enhanced by their relatively low material intensity compared to conventional semiconductor-based alternatives. Many conductive polymers can be synthesized from abundant precursors, potentially reducing reliance on rare or geographically concentrated resources. This characteristic addresses supply chain vulnerabilities and extraction-related environmental impacts associated with certain metal-based catalysts and photoactive materials.
Life cycle assessment (LCA) studies indicate that polymer-based PEC systems generally demonstrate favorable environmental performance metrics, particularly regarding embodied energy and carbon footprint during manufacturing. However, challenges remain in the synthesis processes of some conductive polymers, which may involve toxic solvents or reagents. Recent research has focused on developing greener synthesis routes, including aqueous processing methods and bio-based precursors, to mitigate these concerns.
The durability and degradation characteristics of conductive polymers present both environmental challenges and opportunities. While some polymers exhibit vulnerability to photo-degradation and chemical deterioration in aqueous environments, this biodegradability can reduce end-of-life environmental impacts compared to more persistent materials. Emerging research on stabilization strategies, including encapsulation techniques and molecular engineering approaches, aims to optimize the balance between operational longevity and environmental fate.
Water consumption and quality impacts represent another important sustainability dimension. PEC water splitting inherently requires water as a feedstock, raising questions about resource competition in water-stressed regions. However, polymer-based systems potentially offer advantages through their compatibility with impure water sources, including certain wastewaters, creating opportunities for integrated water treatment and energy production systems.
The scalability of polymer-based PEC technologies carries significant implications for their ultimate environmental impact. Current laboratory-scale demonstrations must transition to industrial implementation with careful attention to material efficiency, manufacturing energy intensity, and system integration. Emerging circular economy approaches, including polymer recycling and recovery strategies, show promise for further enhancing the sustainability profile of these technologies as they mature toward commercial deployment.
The sustainability profile of polymer-based PEC systems is enhanced by their relatively low material intensity compared to conventional semiconductor-based alternatives. Many conductive polymers can be synthesized from abundant precursors, potentially reducing reliance on rare or geographically concentrated resources. This characteristic addresses supply chain vulnerabilities and extraction-related environmental impacts associated with certain metal-based catalysts and photoactive materials.
Life cycle assessment (LCA) studies indicate that polymer-based PEC systems generally demonstrate favorable environmental performance metrics, particularly regarding embodied energy and carbon footprint during manufacturing. However, challenges remain in the synthesis processes of some conductive polymers, which may involve toxic solvents or reagents. Recent research has focused on developing greener synthesis routes, including aqueous processing methods and bio-based precursors, to mitigate these concerns.
The durability and degradation characteristics of conductive polymers present both environmental challenges and opportunities. While some polymers exhibit vulnerability to photo-degradation and chemical deterioration in aqueous environments, this biodegradability can reduce end-of-life environmental impacts compared to more persistent materials. Emerging research on stabilization strategies, including encapsulation techniques and molecular engineering approaches, aims to optimize the balance between operational longevity and environmental fate.
Water consumption and quality impacts represent another important sustainability dimension. PEC water splitting inherently requires water as a feedstock, raising questions about resource competition in water-stressed regions. However, polymer-based systems potentially offer advantages through their compatibility with impure water sources, including certain wastewaters, creating opportunities for integrated water treatment and energy production systems.
The scalability of polymer-based PEC technologies carries significant implications for their ultimate environmental impact. Current laboratory-scale demonstrations must transition to industrial implementation with careful attention to material efficiency, manufacturing energy intensity, and system integration. Emerging circular economy approaches, including polymer recycling and recovery strategies, show promise for further enhancing the sustainability profile of these technologies as they mature toward commercial deployment.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!