Advancements in electrolyte formulations for anode-free batteries
SEP 23, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Anode-Free Battery Electrolyte Evolution and Objectives
Anode-free batteries represent a significant paradigm shift in energy storage technology, emerging as a promising solution to address the limitations of conventional lithium-ion batteries. The evolution of electrolyte formulations for these batteries can be traced back to the early 2010s when researchers began exploring the concept of eliminating the traditional graphite anode to increase energy density and reduce costs. Initially, these efforts faced substantial challenges related to lithium dendrite formation and poor cycling stability.
The development trajectory accelerated around 2015-2017 when researchers at Stanford University and Dalhousie University demonstrated improved cycling performance through novel electrolyte compositions incorporating fluorinated solvents and lithium salt additives. These early breakthroughs established the fundamental understanding that electrolyte engineering would be critical to the commercial viability of anode-free battery technology.
By 2018-2020, significant advancements emerged in high-concentration electrolytes (HCEs) and localized high-concentration electrolytes (LHCEs), which demonstrated superior interfacial stability and dendrite suppression capabilities. The incorporation of functional additives such as fluoroethylene carbonate (FEC) and vinylene carbonate (VC) further enhanced the formation of stable solid electrolyte interphases (SEI) on lithium metal surfaces.
Recent developments have focused on dual-salt electrolyte systems and the integration of ionic liquids to address the persistent challenges of coulombic efficiency and cycle life. Notably, research teams at institutions like the Pacific Northwest National Laboratory and the University of California have pioneered electrolyte formulations achieving over 400 cycles with minimal capacity degradation in anode-free configurations.
The primary technical objectives in this field now center on developing electrolyte formulations that can simultaneously address multiple challenges: preventing dendrite formation, enhancing coulombic efficiency beyond 99.9%, extending cycle life to >1000 cycles, maintaining performance at practical temperatures (-20°C to 60°C), and ensuring compatibility with high-voltage cathodes (>4.5V vs. Li/Li+).
Looking forward, the technology roadmap aims to achieve commercial-ready electrolyte formulations by 2025-2027 that enable anode-free batteries with energy densities exceeding 500 Wh/kg while maintaining safety and longevity comparable to conventional lithium-ion batteries. This ambitious goal requires interdisciplinary approaches combining computational modeling, high-throughput experimentation, and advanced characterization techniques to accelerate the discovery and optimization of next-generation electrolyte systems.
The development trajectory accelerated around 2015-2017 when researchers at Stanford University and Dalhousie University demonstrated improved cycling performance through novel electrolyte compositions incorporating fluorinated solvents and lithium salt additives. These early breakthroughs established the fundamental understanding that electrolyte engineering would be critical to the commercial viability of anode-free battery technology.
By 2018-2020, significant advancements emerged in high-concentration electrolytes (HCEs) and localized high-concentration electrolytes (LHCEs), which demonstrated superior interfacial stability and dendrite suppression capabilities. The incorporation of functional additives such as fluoroethylene carbonate (FEC) and vinylene carbonate (VC) further enhanced the formation of stable solid electrolyte interphases (SEI) on lithium metal surfaces.
Recent developments have focused on dual-salt electrolyte systems and the integration of ionic liquids to address the persistent challenges of coulombic efficiency and cycle life. Notably, research teams at institutions like the Pacific Northwest National Laboratory and the University of California have pioneered electrolyte formulations achieving over 400 cycles with minimal capacity degradation in anode-free configurations.
The primary technical objectives in this field now center on developing electrolyte formulations that can simultaneously address multiple challenges: preventing dendrite formation, enhancing coulombic efficiency beyond 99.9%, extending cycle life to >1000 cycles, maintaining performance at practical temperatures (-20°C to 60°C), and ensuring compatibility with high-voltage cathodes (>4.5V vs. Li/Li+).
Looking forward, the technology roadmap aims to achieve commercial-ready electrolyte formulations by 2025-2027 that enable anode-free batteries with energy densities exceeding 500 Wh/kg while maintaining safety and longevity comparable to conventional lithium-ion batteries. This ambitious goal requires interdisciplinary approaches combining computational modeling, high-throughput experimentation, and advanced characterization techniques to accelerate the discovery and optimization of next-generation electrolyte systems.
Market Analysis for Next-Generation Battery Technologies
The global battery market is experiencing a significant shift towards next-generation technologies, with anode-free batteries emerging as a promising contender in the energy storage landscape. Current market valuations place the advanced battery sector at approximately $95 billion, with projections indicating growth to $136 billion by 2026, representing a compound annual growth rate of 7.4%.
Anode-free battery technology specifically addresses critical market demands for higher energy density solutions, with potential energy density improvements of 60-80% compared to conventional lithium-ion batteries. This advancement directly responds to the electric vehicle industry's urgent need for extended range capabilities while reducing weight and manufacturing costs.
Consumer electronics represents another substantial market segment poised to benefit from electrolyte advancements in anode-free batteries. The sector's demand for longer-lasting, faster-charging, and thinner devices aligns perfectly with the value proposition of this technology. Market research indicates that manufacturers would pay a premium of 15-20% for battery solutions that deliver 30% longer runtime in the same form factor.
The stationary energy storage market presents perhaps the most substantial long-term opportunity. Grid-scale storage installations grew by 62% in 2022, with forecasts suggesting this acceleration will continue as renewable energy integration expands globally. Improved electrolyte formulations that enhance cycle life and safety characteristics position anode-free batteries as particularly attractive for this application.
Regional market analysis reveals varying adoption potentials. Asia-Pacific currently dominates battery manufacturing with 75% of global capacity, providing established infrastructure for new technology integration. North America and Europe are investing heavily in domestic battery supply chains, with combined government initiatives exceeding $20 billion specifically targeting next-generation battery technologies.
Market barriers include cost considerations, with current anode-free prototypes carrying a 30-40% premium over conventional lithium-ion cells. However, cost modeling suggests price parity could be achieved within 3-5 years as manufacturing scales and electrolyte formulations mature. Safety certification and standardization represent additional market entry challenges that will require 12-24 months to navigate.
Customer segment analysis indicates that premium electric vehicle manufacturers and aerospace applications will likely serve as early adopters, willing to absorb higher costs for the performance advantages. Mass market penetration will depend on achieving the projected cost reductions and demonstrating reliability at scale.
Anode-free battery technology specifically addresses critical market demands for higher energy density solutions, with potential energy density improvements of 60-80% compared to conventional lithium-ion batteries. This advancement directly responds to the electric vehicle industry's urgent need for extended range capabilities while reducing weight and manufacturing costs.
Consumer electronics represents another substantial market segment poised to benefit from electrolyte advancements in anode-free batteries. The sector's demand for longer-lasting, faster-charging, and thinner devices aligns perfectly with the value proposition of this technology. Market research indicates that manufacturers would pay a premium of 15-20% for battery solutions that deliver 30% longer runtime in the same form factor.
The stationary energy storage market presents perhaps the most substantial long-term opportunity. Grid-scale storage installations grew by 62% in 2022, with forecasts suggesting this acceleration will continue as renewable energy integration expands globally. Improved electrolyte formulations that enhance cycle life and safety characteristics position anode-free batteries as particularly attractive for this application.
Regional market analysis reveals varying adoption potentials. Asia-Pacific currently dominates battery manufacturing with 75% of global capacity, providing established infrastructure for new technology integration. North America and Europe are investing heavily in domestic battery supply chains, with combined government initiatives exceeding $20 billion specifically targeting next-generation battery technologies.
Market barriers include cost considerations, with current anode-free prototypes carrying a 30-40% premium over conventional lithium-ion cells. However, cost modeling suggests price parity could be achieved within 3-5 years as manufacturing scales and electrolyte formulations mature. Safety certification and standardization represent additional market entry challenges that will require 12-24 months to navigate.
Customer segment analysis indicates that premium electric vehicle manufacturers and aerospace applications will likely serve as early adopters, willing to absorb higher costs for the performance advantages. Mass market penetration will depend on achieving the projected cost reductions and demonstrating reliability at scale.
Current Electrolyte Formulation Challenges and Limitations
Despite the promising potential of anode-free batteries to achieve higher energy densities, current electrolyte formulations face significant challenges that hinder their commercial viability. The primary limitation stems from the inherent instability of lithium metal interfaces, which leads to uncontrolled dendrite growth during cycling. These dendrites can penetrate the separator, causing internal short circuits and catastrophic battery failure, presenting a major safety concern.
Conventional carbonate-based electrolytes that perform well in lithium-ion batteries demonstrate poor compatibility with anode-free configurations. They decompose rapidly at the lithium metal interface, forming unstable solid electrolyte interphase (SEI) layers that cannot effectively suppress dendrite formation. This results in low Coulombic efficiency, typically below 90% for initial cycles, whereas commercial viability requires values exceeding 99.9%.
Electrolyte formulations also struggle with limited lithium metal reversibility. During cycling, significant portions of lithium become electrochemically inactive due to side reactions with the electrolyte, leading to capacity fade and shortened cycle life. Most current formulations can only sustain 100-200 cycles before significant capacity degradation occurs, far below the 1,000+ cycles required for commercial applications.
The high reactivity of lithium metal with electrolyte components creates another major challenge. This reactivity not only consumes active lithium but also depletes electrolyte components, resulting in increased cell impedance and reduced rate capability over time. The continuous electrolyte decomposition leads to gas generation, causing cell swelling and further safety concerns.
Temperature sensitivity presents another significant limitation. Most advanced electrolyte formulations demonstrate acceptable performance only within narrow temperature ranges, typically 20-30°C. Performance deteriorates dramatically at lower temperatures due to increased electrolyte viscosity and reduced ionic conductivity, while elevated temperatures accelerate side reactions and SEI degradation.
From a manufacturing perspective, many promising electrolyte additives and formulations involve expensive or toxic components, limiting their scalability. Fluorinated compounds, which have shown promise in stabilizing lithium metal interfaces, often come with high costs and environmental concerns. Additionally, the sensitivity of these formulations to moisture and impurities necessitates stringent manufacturing conditions, further increasing production costs.
The lack of standardized testing protocols for evaluating electrolyte performance in anode-free configurations also impedes progress. Different research groups employ varying cell designs, testing conditions, and evaluation metrics, making direct comparisons between different electrolyte formulations challenging and slowing the identification of truly promising candidates.
Conventional carbonate-based electrolytes that perform well in lithium-ion batteries demonstrate poor compatibility with anode-free configurations. They decompose rapidly at the lithium metal interface, forming unstable solid electrolyte interphase (SEI) layers that cannot effectively suppress dendrite formation. This results in low Coulombic efficiency, typically below 90% for initial cycles, whereas commercial viability requires values exceeding 99.9%.
Electrolyte formulations also struggle with limited lithium metal reversibility. During cycling, significant portions of lithium become electrochemically inactive due to side reactions with the electrolyte, leading to capacity fade and shortened cycle life. Most current formulations can only sustain 100-200 cycles before significant capacity degradation occurs, far below the 1,000+ cycles required for commercial applications.
The high reactivity of lithium metal with electrolyte components creates another major challenge. This reactivity not only consumes active lithium but also depletes electrolyte components, resulting in increased cell impedance and reduced rate capability over time. The continuous electrolyte decomposition leads to gas generation, causing cell swelling and further safety concerns.
Temperature sensitivity presents another significant limitation. Most advanced electrolyte formulations demonstrate acceptable performance only within narrow temperature ranges, typically 20-30°C. Performance deteriorates dramatically at lower temperatures due to increased electrolyte viscosity and reduced ionic conductivity, while elevated temperatures accelerate side reactions and SEI degradation.
From a manufacturing perspective, many promising electrolyte additives and formulations involve expensive or toxic components, limiting their scalability. Fluorinated compounds, which have shown promise in stabilizing lithium metal interfaces, often come with high costs and environmental concerns. Additionally, the sensitivity of these formulations to moisture and impurities necessitates stringent manufacturing conditions, further increasing production costs.
The lack of standardized testing protocols for evaluating electrolyte performance in anode-free configurations also impedes progress. Different research groups employ varying cell designs, testing conditions, and evaluation metrics, making direct comparisons between different electrolyte formulations challenging and slowing the identification of truly promising candidates.
Leading Companies and Research Institutions in Advanced Electrolytes
The anode-free battery electrolyte formulation market is in an early growth phase, characterized by intensive R&D activities and emerging commercial applications. The global market size is projected to expand significantly as this technology promises higher energy densities and improved safety profiles compared to conventional lithium-ion batteries. Leading players include established battery manufacturers like LG Energy Solution and Samsung SDI, who are leveraging their extensive production capabilities, alongside specialized innovators such as Sila Nanotechnologies and BroadBit Batteries focusing on breakthrough formulations. Research institutions including Argonne National Laboratory, Max Planck Society, and CNRS are driving fundamental advancements, while chemical companies like Solvay and Capchem are developing specialized electrolyte components. The technology remains in transition from laboratory to commercial scale, with key challenges in stability and manufacturing scalability still being addressed.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced electrolyte formulations specifically designed for anode-free lithium metal batteries. Their approach focuses on high-concentration electrolytes (HCEs) and localized high-concentration electrolytes (LHCEs) that form stable solid electrolyte interphases (SEI) on the lithium metal surface. The company has pioneered dual-salt systems combining LiPF6 with LiFSI in carbonate-ester mixed solvents, which significantly improves the cycling stability of anode-free cells. Their proprietary electrolyte additives, including fluoroethylene carbonate (FEC) and vinylene carbonate (VC), help mitigate dendrite formation and enhance coulombic efficiency above 99.5% in anode-free configurations. LG has also developed novel flame-retardant additives that improve the safety profile without compromising electrochemical performance.
Strengths: Superior cycling stability with high coulombic efficiency; established manufacturing infrastructure for rapid commercialization; comprehensive intellectual property portfolio in electrolyte formulations. Weaknesses: Higher production costs compared to conventional electrolytes; some formulations show performance degradation at extreme temperatures; limited compatibility with certain cathode chemistries.
Samsung SDI Co., Ltd.
Technical Solution: Samsung SDI has developed proprietary "High-Efficiency Lithium Interface Stabilizer" (HELIS) electrolyte technology specifically for anode-free battery applications. Their formulation incorporates fluorinated ether-based solvents combined with lithium salts containing both TFSI and FSI anions, creating a robust passivation layer on the in-situ formed lithium metal. The company's electrolyte system features carefully balanced concentrations of LiTFSI and LiFSI (typically 2-3M) in a mixture of fluorinated ethers and conventional carbonates. Samsung has also integrated nano-sized ceramic particles as suspension additives that help regulate lithium deposition morphology. Their latest generation incorporates ionic liquid components that further suppress dendrite formation while enhancing thermal stability. The HELIS technology has demonstrated over 400 cycles in prototype anode-free cells with minimal capacity degradation.
Strengths: Excellent dendrite suppression capabilities; superior thermal stability compared to conventional electrolytes; compatible with high-voltage cathodes. Weaknesses: Complex manufacturing process increases production costs; some components have limited availability in the supply chain; performance in low-temperature environments needs improvement.
Key Patents and Scientific Breakthroughs in Electrolyte Chemistry
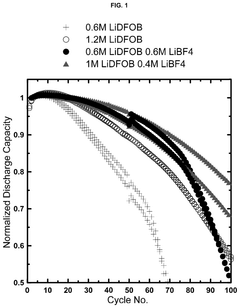
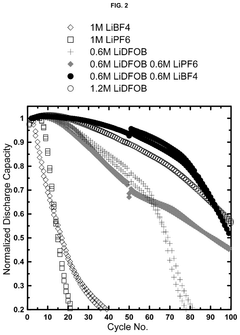
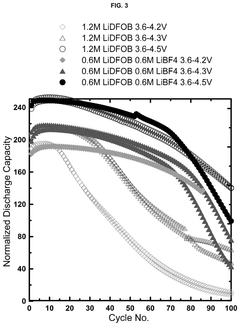
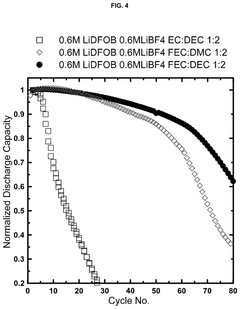
Electrolytes with lithium difluoro(oxalato)borate and lithium tetrafluoroborate salts for lithium metal and anode-free cells
PatentActiveUS12388115B2
Innovation
- A dual salt electrolyte composition comprising lithium difluoro(oxalato)borate (LiDFOB) and lithium tetrafluoroborate (LiBF4) combined with a solvent such as fluorethylene carbonate, applied under uniaxial stack pressure, enhances capacity retention in anode-free lithium batteries.
Electrolyte for anode-free lithium metal battery and anode-free lithium metal battery comprising same
PatentWO2024205200A1
Innovation
- An electrolyte containing a metal salt with a standard reduction potential lower than -1.68V, capable of forming an alloy with lithium and belonging to period 4 or higher of the periodic table, is introduced to suppress side reactions and reduce internal resistance by forming a lithium alloy with the metal cations, which precipitate on the negative electrode current collector.
Safety and Stability Considerations for Anode-Free Electrolytes
Safety considerations for anode-free battery electrolytes represent a critical challenge in their commercial viability. The absence of a pre-deposited anode creates unique safety risks, particularly during the initial lithium plating process where uneven deposition can lead to dendrite formation. These dendrites may penetrate the separator, causing internal short circuits and potentially catastrophic thermal runaway events. Current electrolyte formulations must balance ionic conductivity with stability against lithium metal's high reactivity.
Thermal stability remains a significant concern, as anode-free systems typically operate with higher energy density and reduced thermal management capabilities. Electrolytes must maintain performance across wider temperature ranges while preventing exothermic decomposition reactions. Recent advancements have focused on incorporating flame-retardant additives such as phosphates and fluorinated compounds, which can significantly reduce flammability without severely compromising electrochemical performance.
Voltage stability presents another crucial safety parameter, with electrolytes needing to withstand high voltage conditions during charging. Conventional carbonate-based electrolytes often decompose at voltages required for high-energy cathodes, creating gases that increase internal pressure and compromise cell integrity. Novel formulations incorporating sulfones and nitriles have demonstrated improved oxidative stability, extending the upper voltage limit while maintaining compatibility with lithium metal.
Long-term cycling stability directly impacts safety profiles, as electrolyte degradation products can accelerate dendrite growth and increase internal resistance. The continuous formation and breakdown of the solid electrolyte interphase (SEI) consumes active lithium and electrolyte components, potentially leading to dry-out conditions that compromise safety. Localized lithium depletion can create hotspots during charging, increasing thermal runaway risks.
Environmental and manufacturing safety also warrant consideration, as many advanced electrolyte components involve toxic or environmentally persistent materials. Fluorinated compounds, while effective for performance, present environmental concerns and workplace hazards during production and disposal. Recent research has explored more benign alternatives such as natural-derived solvents and water-processable salt systems that maintain performance while reducing environmental impact.
Standardized safety testing protocols specifically designed for anode-free systems remain underdeveloped, creating challenges in consistent safety evaluation. Current tests often fail to capture the unique failure modes of these systems, particularly during early cycling when the anode is being established. The development of specialized testing methodologies that simulate real-world conditions and accelerated aging will be essential for accurate safety assessment and regulatory approval of next-generation anode-free batteries.
Thermal stability remains a significant concern, as anode-free systems typically operate with higher energy density and reduced thermal management capabilities. Electrolytes must maintain performance across wider temperature ranges while preventing exothermic decomposition reactions. Recent advancements have focused on incorporating flame-retardant additives such as phosphates and fluorinated compounds, which can significantly reduce flammability without severely compromising electrochemical performance.
Voltage stability presents another crucial safety parameter, with electrolytes needing to withstand high voltage conditions during charging. Conventional carbonate-based electrolytes often decompose at voltages required for high-energy cathodes, creating gases that increase internal pressure and compromise cell integrity. Novel formulations incorporating sulfones and nitriles have demonstrated improved oxidative stability, extending the upper voltage limit while maintaining compatibility with lithium metal.
Long-term cycling stability directly impacts safety profiles, as electrolyte degradation products can accelerate dendrite growth and increase internal resistance. The continuous formation and breakdown of the solid electrolyte interphase (SEI) consumes active lithium and electrolyte components, potentially leading to dry-out conditions that compromise safety. Localized lithium depletion can create hotspots during charging, increasing thermal runaway risks.
Environmental and manufacturing safety also warrant consideration, as many advanced electrolyte components involve toxic or environmentally persistent materials. Fluorinated compounds, while effective for performance, present environmental concerns and workplace hazards during production and disposal. Recent research has explored more benign alternatives such as natural-derived solvents and water-processable salt systems that maintain performance while reducing environmental impact.
Standardized safety testing protocols specifically designed for anode-free systems remain underdeveloped, creating challenges in consistent safety evaluation. Current tests often fail to capture the unique failure modes of these systems, particularly during early cycling when the anode is being established. The development of specialized testing methodologies that simulate real-world conditions and accelerated aging will be essential for accurate safety assessment and regulatory approval of next-generation anode-free batteries.
Environmental Impact and Sustainability of Advanced Electrolyte Materials
The environmental impact of electrolyte formulations for anode-free batteries represents a critical consideration in the broader context of sustainable energy storage solutions. Traditional lithium-ion battery electrolytes often contain fluorinated compounds and organic solvents that pose significant environmental and safety concerns throughout their lifecycle. Advanced electrolyte formulations for anode-free batteries present an opportunity to address these challenges while enhancing performance characteristics.
Current electrolyte systems frequently utilize fluorinated salts like LiPF6, which can generate toxic hydrogen fluoride upon exposure to moisture. Additionally, conventional carbonate-based solvents are highly flammable and derived from non-renewable petroleum sources. The environmental footprint of these materials extends from resource extraction through manufacturing to end-of-life disposal, contributing to resource depletion, pollution, and waste management challenges.
Emerging research in anode-free battery electrolytes demonstrates promising directions for environmental improvement. Water-based electrolytes represent a significant advancement, eliminating volatile organic compounds and reducing fire hazards. Similarly, ionic liquid electrolytes offer non-flammability and exceptional stability while being potentially recyclable, though their synthesis currently involves complex processes with environmental implications.
Life cycle assessment (LCA) studies indicate that advanced electrolyte formulations can reduce the carbon footprint of battery production by 15-30% compared to conventional systems. This reduction stems from lower energy requirements during manufacturing and decreased hazardous waste generation. Furthermore, improved electrolyte stability extends battery lifespan, reducing the frequency of replacement and associated environmental impacts.
The recyclability of advanced electrolytes presents both opportunities and challenges. Novel separation techniques enable the recovery of electrolyte components with up to 85% efficiency in laboratory settings. However, industrial-scale implementation remains limited by economic and technical barriers. Biodegradable electrolyte components derived from renewable resources are emerging as promising alternatives, though their performance characteristics require further optimization.
Regulatory frameworks increasingly emphasize environmental considerations in battery technology development. The European Union's Battery Directive and similar regulations worldwide are driving innovation toward greener electrolyte formulations with reduced toxicity and improved recyclability. These regulatory pressures, combined with consumer demand for sustainable technologies, accelerate the transition toward environmentally benign electrolyte systems for next-generation anode-free batteries.
Current electrolyte systems frequently utilize fluorinated salts like LiPF6, which can generate toxic hydrogen fluoride upon exposure to moisture. Additionally, conventional carbonate-based solvents are highly flammable and derived from non-renewable petroleum sources. The environmental footprint of these materials extends from resource extraction through manufacturing to end-of-life disposal, contributing to resource depletion, pollution, and waste management challenges.
Emerging research in anode-free battery electrolytes demonstrates promising directions for environmental improvement. Water-based electrolytes represent a significant advancement, eliminating volatile organic compounds and reducing fire hazards. Similarly, ionic liquid electrolytes offer non-flammability and exceptional stability while being potentially recyclable, though their synthesis currently involves complex processes with environmental implications.
Life cycle assessment (LCA) studies indicate that advanced electrolyte formulations can reduce the carbon footprint of battery production by 15-30% compared to conventional systems. This reduction stems from lower energy requirements during manufacturing and decreased hazardous waste generation. Furthermore, improved electrolyte stability extends battery lifespan, reducing the frequency of replacement and associated environmental impacts.
The recyclability of advanced electrolytes presents both opportunities and challenges. Novel separation techniques enable the recovery of electrolyte components with up to 85% efficiency in laboratory settings. However, industrial-scale implementation remains limited by economic and technical barriers. Biodegradable electrolyte components derived from renewable resources are emerging as promising alternatives, though their performance characteristics require further optimization.
Regulatory frameworks increasingly emphasize environmental considerations in battery technology development. The European Union's Battery Directive and similar regulations worldwide are driving innovation toward greener electrolyte formulations with reduced toxicity and improved recyclability. These regulatory pressures, combined with consumer demand for sustainable technologies, accelerate the transition toward environmentally benign electrolyte systems for next-generation anode-free batteries.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!