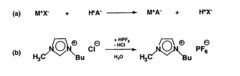
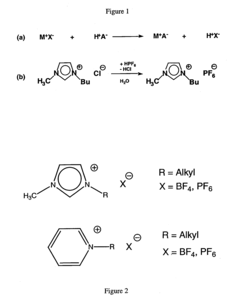
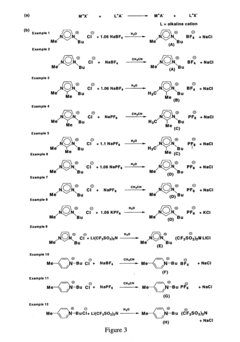
Analytical methods for ionic liquid purity and degradation
AUG 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Liquid Analysis Background and Objectives
Ionic liquids (ILs) have emerged as a revolutionary class of materials in chemical engineering and materials science over the past three decades. These non-molecular ionic compounds, liquid below 100°C, offer unique physicochemical properties including negligible vapor pressure, high thermal stability, and tunable solvation capabilities. The analysis of ionic liquid purity and degradation has become increasingly critical as their applications expand across industries including pharmaceuticals, electrochemistry, catalysis, and separation technologies.
The evolution of analytical methods for ionic liquids has paralleled their growing industrial relevance. Initial characterization techniques in the 1990s were primarily adapted from conventional solvent analysis, but these proved insufficient due to the unique properties of ILs. The field has since progressed toward specialized analytical approaches that address the complex nature of these materials, particularly their susceptibility to contamination and degradation under certain conditions.
Current technological trends indicate a shift toward multi-technique analytical platforms that can simultaneously evaluate multiple quality parameters of ionic liquids. This evolution is driven by the recognition that impurities at even trace levels can significantly impact IL performance in sensitive applications such as electrochemical devices and pharmaceutical processing.
The primary objective of developing advanced analytical methods for ionic liquid purity and degradation is to establish standardized protocols that ensure consistent quality across research and industrial applications. This includes the ability to detect and quantify common impurities such as water, halides, organic residues, and metal ions, as well as to characterize degradation products resulting from thermal, electrochemical, or chemical stress.
Another critical goal is to develop real-time monitoring capabilities that can track IL quality during operational use, particularly in continuous processes where degradation may occur progressively. This requires analytical techniques with sufficient sensitivity, selectivity, and temporal resolution to detect subtle changes in IL composition before they impact process performance.
From a technological perspective, the field aims to bridge the gap between laboratory analytical methods and practical industrial quality control. This necessitates the development of robust, user-friendly analytical tools that can be implemented in production environments without requiring extensive analytical expertise or complex sample preparation.
The advancement of computational models that can predict degradation pathways and impurity profiles represents another important objective, potentially enabling preemptive quality control strategies rather than reactive testing approaches. Such predictive capabilities would significantly enhance the efficiency of IL purification and stabilization efforts.
The evolution of analytical methods for ionic liquids has paralleled their growing industrial relevance. Initial characterization techniques in the 1990s were primarily adapted from conventional solvent analysis, but these proved insufficient due to the unique properties of ILs. The field has since progressed toward specialized analytical approaches that address the complex nature of these materials, particularly their susceptibility to contamination and degradation under certain conditions.
Current technological trends indicate a shift toward multi-technique analytical platforms that can simultaneously evaluate multiple quality parameters of ionic liquids. This evolution is driven by the recognition that impurities at even trace levels can significantly impact IL performance in sensitive applications such as electrochemical devices and pharmaceutical processing.
The primary objective of developing advanced analytical methods for ionic liquid purity and degradation is to establish standardized protocols that ensure consistent quality across research and industrial applications. This includes the ability to detect and quantify common impurities such as water, halides, organic residues, and metal ions, as well as to characterize degradation products resulting from thermal, electrochemical, or chemical stress.
Another critical goal is to develop real-time monitoring capabilities that can track IL quality during operational use, particularly in continuous processes where degradation may occur progressively. This requires analytical techniques with sufficient sensitivity, selectivity, and temporal resolution to detect subtle changes in IL composition before they impact process performance.
From a technological perspective, the field aims to bridge the gap between laboratory analytical methods and practical industrial quality control. This necessitates the development of robust, user-friendly analytical tools that can be implemented in production environments without requiring extensive analytical expertise or complex sample preparation.
The advancement of computational models that can predict degradation pathways and impurity profiles represents another important objective, potentially enabling preemptive quality control strategies rather than reactive testing approaches. Such predictive capabilities would significantly enhance the efficiency of IL purification and stabilization efforts.
Market Demand for High-Purity Ionic Liquid Analysis
The global market for ionic liquids has been experiencing significant growth, with particular emphasis on high-purity ionic liquid analysis methods. This demand is primarily driven by the expanding applications of ionic liquids across various industries including pharmaceuticals, petrochemicals, renewable energy, and electronics. The unique properties of ionic liquids—such as negligible vapor pressure, high thermal stability, and excellent solvation capabilities—make them valuable alternatives to conventional organic solvents in numerous industrial processes.
Market research indicates that the pharmaceutical sector represents one of the largest consumers of high-purity ionic liquids, where they serve as reaction media, catalysts, and extraction solvents. In this sector, the demand for analytical methods to assess ionic liquid purity and monitor degradation is particularly acute due to stringent regulatory requirements and the critical nature of pharmaceutical manufacturing processes.
The renewable energy sector has emerged as another significant market driver, with ionic liquids being increasingly utilized in energy storage systems, particularly in next-generation batteries and supercapacitors. The performance and longevity of these energy storage devices are directly influenced by ionic liquid purity, creating substantial demand for advanced analytical techniques that can detect impurities at trace levels and monitor degradation products in real-time.
Chemical manufacturing companies have also demonstrated growing interest in high-purity ionic liquid analysis, as these compounds find applications in catalysis, separation processes, and as green solvents. The efficiency and selectivity of these processes depend heavily on ionic liquid purity, further fueling market demand for sophisticated analytical methods.
Regional market analysis reveals that North America and Europe currently lead in terms of demand for high-purity ionic liquid analytical methods, primarily due to their established chemical and pharmaceutical industries and stringent environmental regulations. However, the Asia-Pacific region is witnessing the fastest growth rate, driven by rapid industrialization, increasing R&D investments, and the expanding chemical manufacturing sector in countries like China, Japan, and South Korea.
End-users are increasingly demanding analytical methods that offer higher sensitivity, greater accuracy, and faster analysis times. There is particular interest in techniques that can detect degradation products at early stages, thereby preventing process inefficiencies and product quality issues. Additionally, there is growing demand for portable and online monitoring systems that enable real-time analysis of ionic liquid purity in industrial settings.
Market forecasts suggest that the demand for analytical methods for ionic liquid purity and degradation will continue to grow at a compound annual rate exceeding the overall chemical analysis market, reflecting the increasing industrial adoption of ionic liquids and the critical importance of their purity in various applications.
Market research indicates that the pharmaceutical sector represents one of the largest consumers of high-purity ionic liquids, where they serve as reaction media, catalysts, and extraction solvents. In this sector, the demand for analytical methods to assess ionic liquid purity and monitor degradation is particularly acute due to stringent regulatory requirements and the critical nature of pharmaceutical manufacturing processes.
The renewable energy sector has emerged as another significant market driver, with ionic liquids being increasingly utilized in energy storage systems, particularly in next-generation batteries and supercapacitors. The performance and longevity of these energy storage devices are directly influenced by ionic liquid purity, creating substantial demand for advanced analytical techniques that can detect impurities at trace levels and monitor degradation products in real-time.
Chemical manufacturing companies have also demonstrated growing interest in high-purity ionic liquid analysis, as these compounds find applications in catalysis, separation processes, and as green solvents. The efficiency and selectivity of these processes depend heavily on ionic liquid purity, further fueling market demand for sophisticated analytical methods.
Regional market analysis reveals that North America and Europe currently lead in terms of demand for high-purity ionic liquid analytical methods, primarily due to their established chemical and pharmaceutical industries and stringent environmental regulations. However, the Asia-Pacific region is witnessing the fastest growth rate, driven by rapid industrialization, increasing R&D investments, and the expanding chemical manufacturing sector in countries like China, Japan, and South Korea.
End-users are increasingly demanding analytical methods that offer higher sensitivity, greater accuracy, and faster analysis times. There is particular interest in techniques that can detect degradation products at early stages, thereby preventing process inefficiencies and product quality issues. Additionally, there is growing demand for portable and online monitoring systems that enable real-time analysis of ionic liquid purity in industrial settings.
Market forecasts suggest that the demand for analytical methods for ionic liquid purity and degradation will continue to grow at a compound annual rate exceeding the overall chemical analysis market, reflecting the increasing industrial adoption of ionic liquids and the critical importance of their purity in various applications.
Current Challenges in Ionic Liquid Purity Assessment
Despite significant advancements in ionic liquid (IL) technology, the assessment of IL purity and degradation remains a formidable challenge for researchers and industry practitioners. The complex chemical nature of ILs, characterized by their ionic composition and diverse structural variations, presents unique analytical difficulties not encountered with conventional molecular solvents. Current analytical methods often struggle to detect trace impurities that can significantly impact IL performance in critical applications.
A primary challenge lies in the detection limits of conventional analytical techniques when applied to ILs. Many standard methods fail to identify impurities below 1-2% concentration, yet even trace contaminants at parts-per-million levels can dramatically alter IL properties and performance. This sensitivity gap represents a significant barrier to quality control in high-precision applications such as electrochemical devices and catalytic processes.
The diversity of potential impurities further complicates analysis efforts. ILs may contain unreacted starting materials, halide residues, water, organic solvents, and degradation products—each requiring different analytical approaches for accurate quantification. Current methods often necessitate multiple analytical techniques to achieve comprehensive purity assessment, increasing analysis time and cost while introducing potential for inconsistent results across different methodologies.
Water contamination presents a particularly persistent challenge, as ILs are often hygroscopic. Even trace moisture can significantly alter physicochemical properties and catalytic performance. Despite advances in Karl Fischer titration and spectroscopic methods, accurate water quantification in diverse IL systems remains problematic, especially when water interacts strongly with the IL structure.
Degradation product identification represents another critical gap in current analytical capabilities. ILs undergo various degradation pathways depending on their chemical structure and exposure conditions. The resulting degradation products are often structurally similar to the parent IL, making separation and identification exceptionally difficult. Current chromatographic methods frequently struggle with resolution between these closely related compounds.
Standardization across the field presents an overarching challenge. The absence of universally accepted purity assessment protocols and reference materials hampers comparative studies and quality assurance efforts. Different research groups and manufacturers employ varied analytical approaches, making cross-comparison of results problematic and hindering the establishment of reliable purity specifications for commercial applications.
Real-time monitoring capabilities remain underdeveloped, limiting process control in industrial applications. Most current analytical methods require sample extraction and preparation, preventing continuous monitoring of IL purity during application. This gap is particularly problematic for electrochemical applications where degradation may occur progressively during operation.
A primary challenge lies in the detection limits of conventional analytical techniques when applied to ILs. Many standard methods fail to identify impurities below 1-2% concentration, yet even trace contaminants at parts-per-million levels can dramatically alter IL properties and performance. This sensitivity gap represents a significant barrier to quality control in high-precision applications such as electrochemical devices and catalytic processes.
The diversity of potential impurities further complicates analysis efforts. ILs may contain unreacted starting materials, halide residues, water, organic solvents, and degradation products—each requiring different analytical approaches for accurate quantification. Current methods often necessitate multiple analytical techniques to achieve comprehensive purity assessment, increasing analysis time and cost while introducing potential for inconsistent results across different methodologies.
Water contamination presents a particularly persistent challenge, as ILs are often hygroscopic. Even trace moisture can significantly alter physicochemical properties and catalytic performance. Despite advances in Karl Fischer titration and spectroscopic methods, accurate water quantification in diverse IL systems remains problematic, especially when water interacts strongly with the IL structure.
Degradation product identification represents another critical gap in current analytical capabilities. ILs undergo various degradation pathways depending on their chemical structure and exposure conditions. The resulting degradation products are often structurally similar to the parent IL, making separation and identification exceptionally difficult. Current chromatographic methods frequently struggle with resolution between these closely related compounds.
Standardization across the field presents an overarching challenge. The absence of universally accepted purity assessment protocols and reference materials hampers comparative studies and quality assurance efforts. Different research groups and manufacturers employ varied analytical approaches, making cross-comparison of results problematic and hindering the establishment of reliable purity specifications for commercial applications.
Real-time monitoring capabilities remain underdeveloped, limiting process control in industrial applications. Most current analytical methods require sample extraction and preparation, preventing continuous monitoring of IL purity during application. This gap is particularly problematic for electrochemical applications where degradation may occur progressively during operation.
Established Analytical Methods for Ionic Liquid Characterization
01 Purification methods for ionic liquids
Various purification methods can be employed to enhance the purity of ionic liquids, which is crucial for their performance in different applications. These methods include vacuum distillation, column chromatography, and extraction techniques. Purification processes help remove impurities such as halides, water, and organic residues that can affect the physical properties and chemical behavior of ionic liquids. High-purity ionic liquids exhibit better stability, conductivity, and catalytic activity.- Purification methods for ionic liquids: Various purification methods can be employed to enhance the purity of ionic liquids, which is crucial for their performance in different applications. These methods include vacuum distillation, column chromatography, extraction techniques, and membrane filtration. Purification processes help remove impurities such as halides, water, organic residues, and unreacted starting materials that can affect the physical properties and chemical behavior of ionic liquids.
- Degradation mechanisms and stability assessment: Ionic liquids can undergo various degradation processes including thermal decomposition, hydrolysis, oxidation, and photodegradation. Understanding these mechanisms is essential for predicting the stability and lifetime of ionic liquids in different applications. Stability assessment techniques include thermogravimetric analysis, spectroscopic methods, chromatography, and accelerated aging tests to evaluate the long-term performance and degradation products of ionic liquids under various environmental conditions.
- Impurity detection and characterization techniques: Advanced analytical techniques are employed to detect and characterize impurities in ionic liquids. These include nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry, high-performance liquid chromatography (HPLC), gas chromatography (GC), and electrochemical methods. These techniques help in identifying trace impurities, quantifying their concentrations, and understanding their impact on the properties and performance of ionic liquids in various applications.
- Stabilization strategies and additives: Various stabilization strategies and additives can be employed to enhance the stability and prevent degradation of ionic liquids. These include the addition of antioxidants, UV stabilizers, water scavengers, and specific functional groups that can improve thermal and chemical stability. Proper selection of cation and anion structures can also significantly influence the stability profile of ionic liquids, making them more resistant to degradation under challenging conditions.
- Recycling and regeneration of degraded ionic liquids: Methods for recycling and regenerating degraded ionic liquids are important for sustainable use and cost reduction. These include chemical treatments to remove degradation products, physical separation techniques, electrochemical regeneration, and thermal treatments. Recycling processes aim to restore the original properties and purity of ionic liquids after use or partial degradation, extending their lifecycle and reducing environmental impact in industrial applications.
02 Degradation mechanisms of ionic liquids
Ionic liquids can degrade through various mechanisms including thermal decomposition, hydrolysis, oxidation, and photodegradation. Understanding these degradation pathways is essential for predicting the stability and lifetime of ionic liquids in different applications. Factors such as temperature, moisture, oxygen exposure, and UV radiation can accelerate the degradation process. The degradation products may include smaller ionic species, neutral molecules, and gases that can affect the performance and safety of the systems containing ionic liquids.Expand Specific Solutions03 Analytical techniques for purity assessment
Several analytical techniques can be used to assess the purity of ionic liquids and monitor their degradation. These include nuclear magnetic resonance (NMR) spectroscopy, mass spectrometry, chromatography, thermal analysis, and electrochemical methods. These techniques provide information about the chemical composition, impurity content, and structural changes in ionic liquids. Regular monitoring of ionic liquid purity is important for quality control and ensuring consistent performance in applications such as catalysis, electrochemistry, and separations.Expand Specific Solutions04 Stabilization strategies for ionic liquids
Various strategies can be employed to enhance the stability and prevent degradation of ionic liquids. These include the addition of stabilizers or antioxidants, structural modification of the ionic liquid components, and controlling storage and handling conditions. Proper selection of cation and anion combinations can also improve thermal and chemical stability. Stabilization approaches help extend the service life of ionic liquids in demanding applications such as high-temperature processes, electrochemical devices, and industrial separations.Expand Specific Solutions05 Recycling and regeneration of degraded ionic liquids
Methods for recycling and regenerating degraded ionic liquids are important for sustainable use and cost reduction. These methods include chemical treatments to remove degradation products, physical separation techniques, and reactivation processes. Recycling approaches can restore the original properties of the ionic liquids and extend their useful life. The ability to regenerate ionic liquids is particularly valuable in industrial applications where large volumes are used, such as in extraction processes, catalytic reactions, and electrochemical systems.Expand Specific Solutions
Key Industry Players in Ionic Liquid Analysis
The ionic liquid purity and degradation analysis market is in a growth phase, with increasing demand driven by expanding applications in various industries. The market is characterized by a mix of established chemical companies like BASF, Merck Patent GmbH, and ExxonMobil Technology & Engineering Co., alongside specialized analytical service providers. Technical maturity varies across analytical methods, with academic institutions (Chinese Academy of Sciences, Nanjing University, Aalto University) driving fundamental research while industrial players focus on application-specific solutions. Companies like Kurita Water Industries and Evonik Operations are developing proprietary analytical techniques for monitoring ionic liquid degradation in industrial processes. The competitive landscape is evolving as analytical technologies advance from traditional chromatographic methods toward more sophisticated spectroscopic and electrochemical approaches, with increasing emphasis on real-time monitoring capabilities.
BASF Corp.
Technical Solution: BASF has developed comprehensive analytical protocols for ionic liquid purity assessment combining multiple techniques including NMR spectroscopy, mass spectrometry, and chromatographic methods. Their approach utilizes 1H and 13C NMR for structural confirmation and quantification of impurities down to 0.1% levels. For degradation analysis, BASF employs accelerated aging studies under controlled temperature and humidity conditions, coupled with HPLC-MS to identify degradation products. Their proprietary QuEChERS (Quick, Easy, Cheap, Effective, Rugged, and Safe) adaptation specifically optimizes sample preparation for ionic liquids, reducing matrix effects and improving detection limits to sub-ppm levels. BASF has also pioneered thermal stability assessment using TGA-MS (Thermogravimetric Analysis coupled with Mass Spectrometry) to characterize decomposition pathways and establish shelf-life predictions for industrial applications.
Strengths: Comprehensive multi-technique approach provides redundant verification; industrial-scale validation ensures reliability in manufacturing settings; proprietary sample preparation methods enhance sensitivity. Weaknesses: Methods require expensive analytical instrumentation; some techniques have limited throughput for high-volume quality control applications.
Merck Patent GmbH
Technical Solution: Merck has established a comprehensive analytical framework for ionic liquid purity determination and degradation monitoring. Their approach integrates high-resolution mass spectrometry (HRMS) with ion mobility separation to achieve detailed characterization of ionic liquid compositions. For purity assessment, Merck employs a combination of reversed-phase UHPLC with charged aerosol detection (CAD) and evaporative light scattering detection (ELSD), enabling quantification of non-chromophoric impurities without the need for individual reference standards. Their degradation analysis methodology incorporates accelerated stability testing under controlled conditions with periodic sampling and analysis by LC-MS/MS to identify degradation products and establish kinetic models. Merck has developed specialized Karl Fischer titration protocols optimized for hygroscopic ionic liquids to accurately determine water content, a critical parameter affecting purity and stability. Additionally, they utilize multinuclear NMR techniques (1H, 13C, 19F, 31P as applicable) for structural verification and impurity profiling.
Strengths: Comprehensive characterization without requiring reference standards for all impurities; high-throughput screening capabilities suitable for quality control; specialized water determination methods for hygroscopic samples. Weaknesses: Some advanced instrumentation requirements limit accessibility; method development needed for each new ionic liquid class; challenging quantification of structurally similar impurities.
Critical Technologies for Detecting Ionic Liquid Impurities
Method for preparing high-purity ionic liquids
PatentInactiveUS20040074842A1
Innovation
- A method involving a monophasic or biphasic system where an ionic liquid precursor is mixed with a stoichiometric amount of an inorganic salt and an inert liquid, followed by filtration and purification using filter aids like aluminum oxide or activated carbon, to produce high-purity ionic liquids free from acid or base traces.
Purification of ionic liquids
PatentInactiveEP1824575A1
Innovation
- A method involving partial crystallization of ionic liquids from their melt followed by separation of crystals from the residual melt, utilizing techniques like seeding and dynamic or static melt crystallization, which allows for the removal of impurities and achieves high purity levels of 85% to 99.999% weight, while being thermally gentle and efficient.
Environmental Impact of Ionic Liquid Analysis Methods
The environmental implications of ionic liquid analysis methods have become increasingly significant as these chemicals gain wider industrial application. Traditional analytical techniques for determining ionic liquid purity and degradation often involve hazardous solvents and reagents, contributing to laboratory waste streams with potential environmental consequences. Chromatographic methods, particularly HPLC and GC, typically require substantial volumes of organic solvents that pose disposal challenges and contribute to volatile organic compound (VOC) emissions.
Spectroscopic techniques present a more environmentally favorable alternative, with NMR and FTIR requiring minimal sample preparation and generating negligible waste. However, the energy consumption of these instruments, particularly NMR spectrometers with their superconducting magnets requiring cryogenic cooling, represents a significant environmental footprint that is often overlooked in method selection.
Mass spectrometry-based techniques vary considerably in their environmental impact. While they offer exceptional sensitivity, many MS methods require sample preparation steps involving extraction solvents. The vacuum systems and high-energy ionization sources also contribute to the overall energy consumption profile of these analytical approaches.
Recent developments in green analytical chemistry have led to more environmentally conscious ionic liquid analysis methods. Miniaturization through microfluidic platforms has dramatically reduced solvent and sample volume requirements. Additionally, ambient ionization mass spectrometry techniques eliminate many sample preparation steps, reducing chemical waste generation while maintaining analytical performance.
Life cycle assessment (LCA) studies comparing various analytical methods for ionic liquid analysis reveal that the environmental impact extends beyond immediate laboratory waste. The production of specialized reagents, instrument manufacturing, and energy consumption during operation all contribute significantly to the overall environmental footprint. These assessments indicate that method selection should consider not only analytical performance but also comprehensive environmental impact metrics.
Regulatory frameworks increasingly require consideration of environmental aspects in analytical method development. The REACH regulation in Europe and similar initiatives globally have prompted analytical chemists to develop greener alternatives for ionic liquid analysis that minimize hazardous waste generation while maintaining necessary sensitivity and specificity for purity and degradation assessments.
The development of biodegradable reagents specifically designed for ionic liquid analysis represents an emerging frontier in reducing environmental impact. These specialized materials aim to replace persistent chemicals in sample preparation and analysis workflows, ensuring that the analytical process itself does not contribute to environmental contamination when monitoring these potentially persistent compounds.
Spectroscopic techniques present a more environmentally favorable alternative, with NMR and FTIR requiring minimal sample preparation and generating negligible waste. However, the energy consumption of these instruments, particularly NMR spectrometers with their superconducting magnets requiring cryogenic cooling, represents a significant environmental footprint that is often overlooked in method selection.
Mass spectrometry-based techniques vary considerably in their environmental impact. While they offer exceptional sensitivity, many MS methods require sample preparation steps involving extraction solvents. The vacuum systems and high-energy ionization sources also contribute to the overall energy consumption profile of these analytical approaches.
Recent developments in green analytical chemistry have led to more environmentally conscious ionic liquid analysis methods. Miniaturization through microfluidic platforms has dramatically reduced solvent and sample volume requirements. Additionally, ambient ionization mass spectrometry techniques eliminate many sample preparation steps, reducing chemical waste generation while maintaining analytical performance.
Life cycle assessment (LCA) studies comparing various analytical methods for ionic liquid analysis reveal that the environmental impact extends beyond immediate laboratory waste. The production of specialized reagents, instrument manufacturing, and energy consumption during operation all contribute significantly to the overall environmental footprint. These assessments indicate that method selection should consider not only analytical performance but also comprehensive environmental impact metrics.
Regulatory frameworks increasingly require consideration of environmental aspects in analytical method development. The REACH regulation in Europe and similar initiatives globally have prompted analytical chemists to develop greener alternatives for ionic liquid analysis that minimize hazardous waste generation while maintaining necessary sensitivity and specificity for purity and degradation assessments.
The development of biodegradable reagents specifically designed for ionic liquid analysis represents an emerging frontier in reducing environmental impact. These specialized materials aim to replace persistent chemicals in sample preparation and analysis workflows, ensuring that the analytical process itself does not contribute to environmental contamination when monitoring these potentially persistent compounds.
Standardization Efforts in Ionic Liquid Quality Control
The standardization of quality control methods for ionic liquids represents a critical frontier in advancing their industrial application. Currently, several international organizations are actively developing standardized protocols for assessing ionic liquid purity and monitoring degradation processes. The International Union of Pure and Applied Chemistry (IUPAC) has established a dedicated working group focused on creating unified nomenclature and analytical standards specifically for ionic liquids, addressing the inconsistencies that have historically hampered cross-laboratory comparisons.
ASTM International has recently published several standard test methods applicable to ionic liquid analysis, including protocols for water content determination, halide impurity quantification, and thermal stability assessment. These standards provide crucial benchmarks for manufacturers and end-users to evaluate product quality consistently. Similarly, the International Organization for Standardization (ISO) is developing comprehensive guidelines for ionic liquid characterization that incorporate multiple analytical techniques within a standardized framework.
Industry consortia have emerged as significant contributors to standardization efforts. The Ionic Liquids Consortium (ILC), comprising major chemical manufacturers and academic institutions, has established a round-robin testing program where identical ionic liquid samples are analyzed across multiple laboratories to validate analytical methods and establish reproducibility parameters. This collaborative approach has proven effective in identifying robust analytical procedures suitable for standardization.
Regional standardization bodies, particularly in regions with strong ionic liquid research presence such as the European Union, China, and the United States, have developed complementary guidelines tailored to specific industrial applications. The European Committee for Standardization (CEN) has focused on standards for ionic liquids in electrochemical applications, while Chinese authorities have prioritized standards for ionic liquids in separation processes.
Certification programs for ionic liquid purity are beginning to emerge, with several analytical service providers offering standardized testing packages based on these developing international standards. These certification services provide manufacturers with independent verification of product quality and help build market confidence in ionic liquid technologies.
The harmonization of these various standardization efforts remains an ongoing challenge. Current initiatives focus on establishing minimum reporting requirements for ionic liquid characterization data in scientific literature and technical documentation, ensuring that critical parameters such as water content, halide impurities, and spectroscopic profiles are consistently documented using standardized analytical methods.
ASTM International has recently published several standard test methods applicable to ionic liquid analysis, including protocols for water content determination, halide impurity quantification, and thermal stability assessment. These standards provide crucial benchmarks for manufacturers and end-users to evaluate product quality consistently. Similarly, the International Organization for Standardization (ISO) is developing comprehensive guidelines for ionic liquid characterization that incorporate multiple analytical techniques within a standardized framework.
Industry consortia have emerged as significant contributors to standardization efforts. The Ionic Liquids Consortium (ILC), comprising major chemical manufacturers and academic institutions, has established a round-robin testing program where identical ionic liquid samples are analyzed across multiple laboratories to validate analytical methods and establish reproducibility parameters. This collaborative approach has proven effective in identifying robust analytical procedures suitable for standardization.
Regional standardization bodies, particularly in regions with strong ionic liquid research presence such as the European Union, China, and the United States, have developed complementary guidelines tailored to specific industrial applications. The European Committee for Standardization (CEN) has focused on standards for ionic liquids in electrochemical applications, while Chinese authorities have prioritized standards for ionic liquids in separation processes.
Certification programs for ionic liquid purity are beginning to emerge, with several analytical service providers offering standardized testing packages based on these developing international standards. These certification services provide manufacturers with independent verification of product quality and help build market confidence in ionic liquid technologies.
The harmonization of these various standardization efforts remains an ongoing challenge. Current initiatives focus on establishing minimum reporting requirements for ionic liquid characterization data in scientific literature and technical documentation, ensuring that critical parameters such as water content, halide impurities, and spectroscopic profiles are consistently documented using standardized analytical methods.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!