Ionic liquid electrolytes for high-voltage batteries: design rules
AUG 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Liquid Electrolytes Background and Objectives
Ionic liquids (ILs) have emerged as promising electrolyte materials for next-generation high-voltage batteries, representing a significant evolution from conventional liquid electrolytes. The development of ionic liquid electrolytes can be traced back to the early 2000s when researchers began exploring alternatives to traditional carbonate-based electrolytes due to their limitations in thermal stability, flammability, and electrochemical windows. The unique properties of ionic liquids—including negligible vapor pressure, non-flammability, high thermal stability, and wide electrochemical windows—have positioned them as ideal candidates for addressing the challenges in high-voltage battery applications.
The technological evolution of ionic liquid electrolytes has progressed through several distinct phases. Initially, research focused on understanding the fundamental properties of various ionic liquid families, including imidazolium, pyrrolidinium, and quaternary ammonium-based ILs. Subsequently, efforts shifted toward optimizing ionic conductivity and electrochemical stability, which were initially limiting factors for practical applications. Recent advancements have concentrated on tailoring the molecular structure of ionic liquids to enhance compatibility with electrode materials and improve cycling performance at high voltages.
Current market trends indicate a growing demand for high-energy-density batteries capable of operating at voltages exceeding 4.5V, particularly in electric vehicles, grid storage, and portable electronics sectors. This demand is driving the need for electrolytes with enhanced oxidative stability and compatibility with high-voltage cathode materials such as LiNi0.5Mn1.5O4 and Li-rich layered oxides. Ionic liquid electrolytes represent a promising solution to meet these requirements, potentially enabling the development of batteries with energy densities 20-30% higher than current commercial systems.
The primary technical objectives for ionic liquid electrolytes in high-voltage batteries include: achieving ionic conductivities comparable to conventional electrolytes (>1 mS/cm at room temperature); expanding the electrochemical stability window to >5V vs. Li/Li+; enhancing interfacial compatibility with both cathode and anode materials; reducing viscosity while maintaining thermal stability; and developing cost-effective synthesis routes for large-scale production. Additionally, there is a focus on understanding structure-property relationships to establish clear design rules for molecular engineering of task-specific ionic liquids.
Looking forward, the field is moving toward multi-functional ionic liquid electrolytes that not only enable high-voltage operation but also contribute to improved safety, extended cycle life, and enhanced rate capability. The integration of computational modeling with experimental approaches is accelerating the discovery and optimization of novel ionic liquid structures tailored for specific battery chemistries and operating conditions.
The technological evolution of ionic liquid electrolytes has progressed through several distinct phases. Initially, research focused on understanding the fundamental properties of various ionic liquid families, including imidazolium, pyrrolidinium, and quaternary ammonium-based ILs. Subsequently, efforts shifted toward optimizing ionic conductivity and electrochemical stability, which were initially limiting factors for practical applications. Recent advancements have concentrated on tailoring the molecular structure of ionic liquids to enhance compatibility with electrode materials and improve cycling performance at high voltages.
Current market trends indicate a growing demand for high-energy-density batteries capable of operating at voltages exceeding 4.5V, particularly in electric vehicles, grid storage, and portable electronics sectors. This demand is driving the need for electrolytes with enhanced oxidative stability and compatibility with high-voltage cathode materials such as LiNi0.5Mn1.5O4 and Li-rich layered oxides. Ionic liquid electrolytes represent a promising solution to meet these requirements, potentially enabling the development of batteries with energy densities 20-30% higher than current commercial systems.
The primary technical objectives for ionic liquid electrolytes in high-voltage batteries include: achieving ionic conductivities comparable to conventional electrolytes (>1 mS/cm at room temperature); expanding the electrochemical stability window to >5V vs. Li/Li+; enhancing interfacial compatibility with both cathode and anode materials; reducing viscosity while maintaining thermal stability; and developing cost-effective synthesis routes for large-scale production. Additionally, there is a focus on understanding structure-property relationships to establish clear design rules for molecular engineering of task-specific ionic liquids.
Looking forward, the field is moving toward multi-functional ionic liquid electrolytes that not only enable high-voltage operation but also contribute to improved safety, extended cycle life, and enhanced rate capability. The integration of computational modeling with experimental approaches is accelerating the discovery and optimization of novel ionic liquid structures tailored for specific battery chemistries and operating conditions.
Market Analysis for High-Voltage Battery Applications
The high-voltage battery market is experiencing unprecedented growth driven by the global shift towards electrification across multiple sectors. The electric vehicle (EV) segment represents the largest application area, with projections indicating a compound annual growth rate of 25% through 2030. This expansion is fueled by stringent environmental regulations, government incentives, and increasing consumer acceptance of electric mobility solutions. Beyond automotive applications, high-voltage batteries are gaining significant traction in grid-scale energy storage systems, which are essential for integrating renewable energy sources into existing power infrastructures.
Consumer electronics manufacturers are increasingly adopting high-voltage battery technologies to meet demands for longer device operation and faster charging capabilities. This trend is particularly evident in premium smartphones, laptops, and wearable devices where energy density and performance are critical differentiators. The industrial sector also presents substantial growth opportunities, with applications ranging from automated guided vehicles to backup power systems requiring high energy density solutions.
Geographically, Asia-Pacific dominates the high-voltage battery market, with China, Japan, and South Korea leading in both production capacity and technological innovation. Europe follows as the second-largest market, driven by aggressive carbon reduction targets and substantial investments in electric mobility infrastructure. North America represents a rapidly growing market segment, particularly as domestic manufacturing capacity expands in response to supply chain security concerns.
The demand for ionic liquid electrolytes specifically is being shaped by performance requirements that conventional electrolytes cannot satisfy. End-users across sectors are prioritizing batteries with enhanced safety profiles, extended cycle life, and improved thermal stability—all attributes that ionic liquid electrolytes can potentially deliver. Market research indicates that manufacturers are willing to absorb premium costs for electrolyte solutions that enable operation above 4.5V, as this voltage threshold represents a significant performance breakthrough.
Regulatory factors are increasingly influencing market dynamics, with safety standards becoming more stringent following high-profile battery failure incidents. This regulatory environment favors ionic liquid electrolytes due to their non-flammable nature and reduced environmental impact compared to conventional organic electrolytes. Additionally, sustainability considerations are becoming important market drivers, with manufacturers seeking electrolyte technologies that align with circular economy principles and reduced carbon footprints.
The competitive landscape for high-voltage battery applications is intensifying, with major battery manufacturers forming strategic partnerships with electrolyte specialists to secure technological advantages. This collaboration trend indicates growing recognition of electrolyte formulation as a critical differentiator in next-generation battery performance rather than merely a component selection decision.
Consumer electronics manufacturers are increasingly adopting high-voltage battery technologies to meet demands for longer device operation and faster charging capabilities. This trend is particularly evident in premium smartphones, laptops, and wearable devices where energy density and performance are critical differentiators. The industrial sector also presents substantial growth opportunities, with applications ranging from automated guided vehicles to backup power systems requiring high energy density solutions.
Geographically, Asia-Pacific dominates the high-voltage battery market, with China, Japan, and South Korea leading in both production capacity and technological innovation. Europe follows as the second-largest market, driven by aggressive carbon reduction targets and substantial investments in electric mobility infrastructure. North America represents a rapidly growing market segment, particularly as domestic manufacturing capacity expands in response to supply chain security concerns.
The demand for ionic liquid electrolytes specifically is being shaped by performance requirements that conventional electrolytes cannot satisfy. End-users across sectors are prioritizing batteries with enhanced safety profiles, extended cycle life, and improved thermal stability—all attributes that ionic liquid electrolytes can potentially deliver. Market research indicates that manufacturers are willing to absorb premium costs for electrolyte solutions that enable operation above 4.5V, as this voltage threshold represents a significant performance breakthrough.
Regulatory factors are increasingly influencing market dynamics, with safety standards becoming more stringent following high-profile battery failure incidents. This regulatory environment favors ionic liquid electrolytes due to their non-flammable nature and reduced environmental impact compared to conventional organic electrolytes. Additionally, sustainability considerations are becoming important market drivers, with manufacturers seeking electrolyte technologies that align with circular economy principles and reduced carbon footprints.
The competitive landscape for high-voltage battery applications is intensifying, with major battery manufacturers forming strategic partnerships with electrolyte specialists to secure technological advantages. This collaboration trend indicates growing recognition of electrolyte formulation as a critical differentiator in next-generation battery performance rather than merely a component selection decision.
Current Status and Technical Barriers in Ionic Liquid Electrolytes
Ionic liquid electrolytes (ILEs) have emerged as promising alternatives to conventional organic electrolytes for high-voltage batteries due to their unique physicochemical properties. Currently, the global research landscape shows significant advancements in ILE development across North America, Europe, and East Asia, with Japan, South Korea, and China leading in patent applications and commercial implementations.
The current state of ILE technology demonstrates several advantages over traditional electrolytes, including negligible vapor pressure, non-flammability, wide electrochemical stability windows (up to 5-6V vs. Li/Li+), and excellent thermal stability. These properties directly address the safety concerns associated with conventional carbonate-based electrolytes. Recent breakthroughs have achieved ionic conductivities approaching 10^-3 S/cm at room temperature, making ILEs increasingly viable for practical applications.
Despite these advances, several critical technical barriers impede widespread adoption of ILEs in high-voltage battery systems. The most significant challenge remains their relatively high viscosity (typically 10-100 times higher than conventional electrolytes), which limits ion transport and results in lower rate capabilities. This viscosity issue becomes particularly problematic at lower operating temperatures, where some ILEs exhibit solidification or dramatic conductivity decreases.
Another major obstacle is the interfacial instability between ILEs and electrode materials, particularly at high voltages. While ILEs demonstrate wide electrochemical windows in isolation, their interaction with electrode surfaces often triggers decomposition reactions, leading to capacity fading and impedance growth. The formation of stable solid electrolyte interphase (SEI) layers remains inconsistent and highly dependent on specific ionic liquid compositions.
Cost factors present additional barriers, as most high-performance ionic liquids require complex synthesis routes and expensive precursors. Current production costs exceed conventional electrolytes by 5-10 times, limiting commercial viability despite their performance advantages.
Compatibility issues with conventional battery components also pose challenges. Many ILEs demonstrate corrosive behavior toward current collectors, separators, and packaging materials, necessitating redesign of multiple battery components. Additionally, the hygroscopic nature of many ionic liquids requires stringent moisture control during manufacturing and assembly processes.
Standardization remains underdeveloped, with diverse testing protocols and performance metrics making direct comparisons between different ILE systems difficult. This fragmentation in evaluation methodologies has slowed consensus-building around optimal design principles and hampered technology transfer from laboratory to industrial settings.
The current state of ILE technology demonstrates several advantages over traditional electrolytes, including negligible vapor pressure, non-flammability, wide electrochemical stability windows (up to 5-6V vs. Li/Li+), and excellent thermal stability. These properties directly address the safety concerns associated with conventional carbonate-based electrolytes. Recent breakthroughs have achieved ionic conductivities approaching 10^-3 S/cm at room temperature, making ILEs increasingly viable for practical applications.
Despite these advances, several critical technical barriers impede widespread adoption of ILEs in high-voltage battery systems. The most significant challenge remains their relatively high viscosity (typically 10-100 times higher than conventional electrolytes), which limits ion transport and results in lower rate capabilities. This viscosity issue becomes particularly problematic at lower operating temperatures, where some ILEs exhibit solidification or dramatic conductivity decreases.
Another major obstacle is the interfacial instability between ILEs and electrode materials, particularly at high voltages. While ILEs demonstrate wide electrochemical windows in isolation, their interaction with electrode surfaces often triggers decomposition reactions, leading to capacity fading and impedance growth. The formation of stable solid electrolyte interphase (SEI) layers remains inconsistent and highly dependent on specific ionic liquid compositions.
Cost factors present additional barriers, as most high-performance ionic liquids require complex synthesis routes and expensive precursors. Current production costs exceed conventional electrolytes by 5-10 times, limiting commercial viability despite their performance advantages.
Compatibility issues with conventional battery components also pose challenges. Many ILEs demonstrate corrosive behavior toward current collectors, separators, and packaging materials, necessitating redesign of multiple battery components. Additionally, the hygroscopic nature of many ionic liquids requires stringent moisture control during manufacturing and assembly processes.
Standardization remains underdeveloped, with diverse testing protocols and performance metrics making direct comparisons between different ILE systems difficult. This fragmentation in evaluation methodologies has slowed consensus-building around optimal design principles and hampered technology transfer from laboratory to industrial settings.
Current Design Strategies for High-Voltage Ionic Liquid Electrolytes
01 Cation and anion selection for ionic liquid electrolytes
The selection of appropriate cations and anions is crucial for designing effective ionic liquid electrolytes. Cations such as imidazolium, pyrrolidinium, and quaternary ammonium, combined with anions like TFSI, BF4, and PF6, can be tailored to achieve specific electrochemical properties. The structure and size of these ions affect conductivity, viscosity, and electrochemical stability window, which are essential parameters for battery performance.- Cation and anion selection for ionic liquid electrolytes: The selection of appropriate cations and anions is crucial for designing effective ionic liquid electrolytes. Cations such as imidazolium, pyrrolidinium, and quaternary ammonium, combined with anions like TFSI, BF4, and PF6 can be tailored to achieve specific electrochemical properties. The structure of these ions affects conductivity, viscosity, and electrochemical stability window, which are essential parameters for battery performance.
- Additives and modifiers for enhanced performance: Various additives can be incorporated into ionic liquid electrolytes to enhance their performance characteristics. These include lithium salts to improve lithium-ion transport, flame retardants to enhance safety, and functional polymers to modify viscosity and mechanical properties. Certain additives can also form protective solid electrolyte interphase (SEI) layers on electrodes, improving cycling stability and battery lifespan.
- Temperature and pressure considerations in design: Ionic liquid electrolytes must be designed with consideration for operating temperature ranges and pressure conditions. Optimizing the composition to maintain performance across wide temperature ranges is essential for applications in extreme environments. Some ionic liquids exhibit excellent thermal stability up to 300°C and negligible vapor pressure, making them suitable for high-temperature applications where conventional electrolytes would degrade or evaporate.
- Electrode compatibility and interface engineering: Designing ionic liquid electrolytes requires careful consideration of their compatibility with electrode materials. The electrolyte-electrode interface plays a crucial role in determining battery performance and safety. Engineering this interface through surface modifications, protective coatings, or tailored electrolyte compositions can minimize unwanted side reactions, reduce impedance, and improve cycling stability.
- Manufacturing processes and scalability considerations: The design rules for ionic liquid electrolytes must account for manufacturing processes and scalability. Considerations include purification methods to remove impurities that can affect electrochemical performance, mixing protocols to ensure homogeneity, and cost-effective production techniques. Advanced manufacturing approaches such as continuous flow processes and quality control measures are essential for transitioning from laboratory-scale to commercial production.
02 Additives and modifiers for enhanced performance
Various additives and modifiers can be incorporated into ionic liquid electrolytes to enhance their performance characteristics. These include lithium salts to improve lithium-ion transport, flame retardants to enhance safety, and functional polymers to optimize mechanical properties. Specific additives can also address issues such as electrode-electrolyte interface stability and cycling efficiency in battery applications.Expand Specific Solutions03 Temperature stability and operating range optimization
Designing ionic liquid electrolytes with wide temperature operating ranges is essential for various applications. This involves selecting ion combinations that maintain low viscosity at low temperatures while preserving thermal stability at high temperatures. Eutectic mixtures of different ionic liquids can be employed to extend the liquid range and improve conductivity across broader temperature windows.Expand Specific Solutions04 Electrode compatibility and interface engineering
Successful ionic liquid electrolyte design requires consideration of electrode compatibility and interface phenomena. This includes developing formulations that form stable solid electrolyte interphases (SEI) on anodes and cathodes, minimizing parasitic reactions, and ensuring good wettability with electrode materials. Tailoring the ionic liquid structure to specific electrode chemistries can significantly improve cycling stability and battery lifespan.Expand Specific Solutions05 Manufacturing processes and scalability considerations
The design rules for ionic liquid electrolytes must account for manufacturing processes and scalability. This includes developing synthesis routes that minimize impurities, which can significantly affect electrochemical performance. Considerations for large-scale production, such as cost-effective precursors, simplified purification methods, and environmentally friendly processes, are essential for commercial viability of ionic liquid electrolytes in energy storage applications.Expand Specific Solutions
Leading Companies and Research Institutions in Ionic Liquid Development
The ionic liquid electrolytes for high-voltage batteries market is currently in a growth phase, with increasing demand driven by the need for safer, higher-energy-density battery solutions. The global market size is expanding rapidly, projected to reach significant value as electric vehicle adoption accelerates. Technologically, the field shows moderate maturity with ongoing innovation challenges. Leading players include established corporations like Toshiba, Hyundai Motor, and Bridgestone alongside specialized entities such as Sionic Energy and Wildcat Discovery Technologies focusing on breakthrough formulations. Academic-industrial partnerships are prominent, with institutions like Georgia Tech Research Corp, Shanghai Jiao Tong University, and Northwestern University collaborating with industry to overcome performance limitations in cycle life, ionic conductivity, and manufacturing costs that currently prevent widespread commercialization.
Sionic Energy, Inc.
Technical Solution: Sionic Energy has developed a proprietary ionic liquid electrolyte system specifically designed for high-voltage lithium-ion batteries. Their approach focuses on using novel ionic liquid compositions with wide electrochemical stability windows exceeding 5V, enabling operation with high-voltage cathode materials. The company's technology incorporates fluorinated anions and asymmetric cations that minimize electrolyte decomposition at high voltages while maintaining good ionic conductivity. Sionic's electrolytes feature additives that form stable solid-electrolyte interphase (SEI) layers on electrode surfaces, preventing continuous electrolyte degradation. Their formulations also demonstrate enhanced thermal stability up to 300°C, significantly reducing thermal runaway risks compared to conventional carbonate-based electrolytes that typically decompose around 150°C.
Strengths: Superior voltage stability window allowing compatibility with high-voltage cathode materials; exceptional thermal safety profile reducing battery fire risks; improved cycle life at elevated voltages. Weaknesses: Higher viscosity than conventional electrolytes potentially limiting power performance at low temperatures; higher production costs compared to traditional electrolytes; may require specialized manufacturing processes for integration.
Toshiba Corp.
Technical Solution: Toshiba has pioneered a hybrid ionic liquid electrolyte system for high-voltage battery applications that combines conventional organic solvents with carefully selected ionic liquids. Their approach utilizes phosphonium and pyrrolidinium-based ionic liquids mixed with fluorinated organic solvents to achieve electrochemical stability above 4.5V vs. Li/Li+. Toshiba's technology incorporates lithium salts with weakly coordinating anions such as LiTFSI and LiFSI to enhance ionic conductivity while maintaining oxidative stability. A key innovation in their formulation is the use of multi-functional additives that simultaneously protect both cathode and anode interfaces, creating robust passivation layers that prevent continuous electrolyte oxidation. Their electrolytes have demonstrated stable cycling with high-voltage NMC and LNMO cathodes, showing capacity retention above 80% after 1000 cycles at elevated voltages.
Strengths: Balanced approach combining ionic liquid benefits with conventional electrolyte performance; demonstrated compatibility with existing manufacturing processes; good low-temperature performance compared to pure ionic liquid systems. Weaknesses: Still contains some flammable organic components reducing safety benefits compared to pure ionic liquid systems; higher cost than conventional electrolytes; potential long-term stability issues at extreme voltages above 4.8V.
Key Patents and Scientific Breakthroughs in Ionic Liquid Formulations
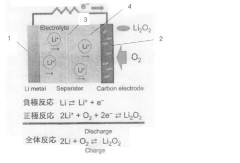
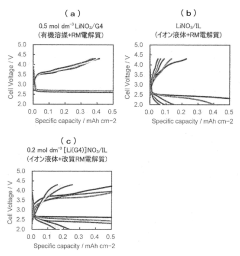
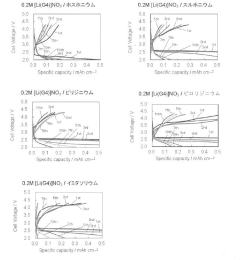
Ionic liquid electrolyte for lithium-air secondary battery and lithium-air secondary battery with redox mediator capability
PatentActiveJP2022002206A
Innovation
- The use of an ionic liquid electrolyte with a modified redox mediator complexed with a Li salt, such as LiNO3, LiBr, or LiI, dissolved in an ionic liquid, along with specific ligands like glymes, sulfoxides, and nitriles, enhances RM solubility and stability, reducing charging overvoltage and improving energy efficiency.
Safety and Stability Considerations for High-Voltage Applications
Safety considerations for ionic liquid electrolytes in high-voltage battery applications are paramount due to the increased energy density and operational risks. Traditional organic electrolytes face significant safety challenges at high voltages (>4.5V), including thermal runaway, gas evolution, and potential fire hazards. Ionic liquid electrolytes offer inherent advantages with their non-flammability, negligible vapor pressure, and high thermal stability, substantially reducing these risks.
The stability of ionic liquid electrolytes at high voltages depends critically on their electrochemical window, which must exceed the operational voltage of the battery system. Recent research demonstrates that certain ionic liquids, particularly those based on pyrrolidinium and piperidinium cations paired with bis(trifluoromethanesulfonyl)imide (TFSI) anions, can maintain stability up to 5.5V vs. Li/Li+, making them suitable candidates for high-voltage applications.
Chemical compatibility between ionic liquid electrolytes and electrode materials presents another crucial stability consideration. High-voltage cathode materials like LiNi0.5Mn1.5O4 and Li-rich layered oxides can catalyze electrolyte decomposition. Engineering ionic liquids with specific functional groups that form stable solid electrolyte interphase (SEI) layers can mitigate this issue, enhancing long-term cycling stability.
Temperature-dependent behavior significantly impacts safety profiles. While ionic liquids generally exhibit superior thermal stability compared to conventional electrolytes, their high viscosity at lower temperatures can impede ion transport. Designing ionic liquid electrolytes with lower melting points and incorporating appropriate additives can address this limitation, ensuring reliable performance across wider temperature ranges.
Aging mechanisms and degradation pathways require careful consideration for long-term stability. High-voltage operation accelerates decomposition reactions at electrode-electrolyte interfaces. Studies indicate that fluorinated anions in ionic liquids demonstrate enhanced resistance to oxidative decomposition, while the presence of impurities, particularly water, can dramatically reduce electrochemical stability windows and trigger unwanted side reactions.
Standardized safety protocols for ionic liquid electrolytes in high-voltage batteries remain underdeveloped. Establishing comprehensive testing methodologies that evaluate thermal stability, gas evolution under abuse conditions, and compatibility with current collector materials is essential for commercial viability. Recent advancements in in-situ characterization techniques provide valuable insights into degradation mechanisms, enabling more targeted design approaches for next-generation ionic liquid electrolytes with enhanced safety profiles.
The stability of ionic liquid electrolytes at high voltages depends critically on their electrochemical window, which must exceed the operational voltage of the battery system. Recent research demonstrates that certain ionic liquids, particularly those based on pyrrolidinium and piperidinium cations paired with bis(trifluoromethanesulfonyl)imide (TFSI) anions, can maintain stability up to 5.5V vs. Li/Li+, making them suitable candidates for high-voltage applications.
Chemical compatibility between ionic liquid electrolytes and electrode materials presents another crucial stability consideration. High-voltage cathode materials like LiNi0.5Mn1.5O4 and Li-rich layered oxides can catalyze electrolyte decomposition. Engineering ionic liquids with specific functional groups that form stable solid electrolyte interphase (SEI) layers can mitigate this issue, enhancing long-term cycling stability.
Temperature-dependent behavior significantly impacts safety profiles. While ionic liquids generally exhibit superior thermal stability compared to conventional electrolytes, their high viscosity at lower temperatures can impede ion transport. Designing ionic liquid electrolytes with lower melting points and incorporating appropriate additives can address this limitation, ensuring reliable performance across wider temperature ranges.
Aging mechanisms and degradation pathways require careful consideration for long-term stability. High-voltage operation accelerates decomposition reactions at electrode-electrolyte interfaces. Studies indicate that fluorinated anions in ionic liquids demonstrate enhanced resistance to oxidative decomposition, while the presence of impurities, particularly water, can dramatically reduce electrochemical stability windows and trigger unwanted side reactions.
Standardized safety protocols for ionic liquid electrolytes in high-voltage batteries remain underdeveloped. Establishing comprehensive testing methodologies that evaluate thermal stability, gas evolution under abuse conditions, and compatibility with current collector materials is essential for commercial viability. Recent advancements in in-situ characterization techniques provide valuable insights into degradation mechanisms, enabling more targeted design approaches for next-generation ionic liquid electrolytes with enhanced safety profiles.
Environmental Impact and Sustainability of Ionic Liquid Electrolytes
The environmental impact of ionic liquid electrolytes represents a critical consideration in their development for high-voltage battery applications. Unlike conventional organic electrolytes that pose significant flammability and toxicity concerns, ionic liquids offer inherently lower vapor pressure and reduced flammability, substantially decreasing the risk of thermal runaway and associated environmental hazards in battery systems.
From a life cycle perspective, ionic liquid electrolytes demonstrate promising sustainability advantages. Their exceptional thermal stability and wide electrochemical windows enable longer battery lifespans, potentially reducing the frequency of battery replacement and associated electronic waste. This longevity factor contributes significantly to resource conservation and waste reduction across the battery value chain.
The synthesis of ionic liquids, however, presents certain environmental challenges. Traditional preparation methods often involve energy-intensive processes and potentially hazardous reagents. Recent advances in green chemistry approaches have yielded more environmentally benign synthesis routes, including solvent-free reactions and bio-derived precursors, which substantially reduce the environmental footprint of ionic liquid production.
Biodegradability represents another crucial aspect of ionic liquid environmental impact. While conventional ionic liquids with fluorinated anions exhibit limited biodegradability, newer generations incorporating biodegradable components such as amino acid-derived cations and natural-product-based structures show improved environmental fate profiles. These biodegradable ionic liquids decompose more readily in natural environments, minimizing long-term ecological accumulation risks.
Recycling and recovery strategies for ionic liquid electrolytes remain underdeveloped compared to conventional battery components. Their non-volatile nature facilitates potential recovery through liquid-liquid extraction techniques, though commercial-scale processes require further optimization. Emerging technologies utilizing supercritical CO2 extraction show promise for efficient ionic liquid recovery with minimal environmental impact.
The carbon footprint associated with ionic liquid production currently exceeds that of conventional electrolytes due to complex synthesis requirements. However, life cycle assessments indicate that this initial environmental cost may be offset by extended battery lifetimes and improved safety profiles. Future development of renewable energy-powered synthesis methods could further enhance the sustainability proposition of ionic liquid electrolytes.
Water consumption and potential aquatic toxicity must also be considered in comprehensive environmental assessments. While ionic liquids generally exhibit lower water solubility than conventional organic electrolytes, their potential ecological impacts upon accidental release require careful evaluation through standardized ecotoxicological testing protocols.
From a life cycle perspective, ionic liquid electrolytes demonstrate promising sustainability advantages. Their exceptional thermal stability and wide electrochemical windows enable longer battery lifespans, potentially reducing the frequency of battery replacement and associated electronic waste. This longevity factor contributes significantly to resource conservation and waste reduction across the battery value chain.
The synthesis of ionic liquids, however, presents certain environmental challenges. Traditional preparation methods often involve energy-intensive processes and potentially hazardous reagents. Recent advances in green chemistry approaches have yielded more environmentally benign synthesis routes, including solvent-free reactions and bio-derived precursors, which substantially reduce the environmental footprint of ionic liquid production.
Biodegradability represents another crucial aspect of ionic liquid environmental impact. While conventional ionic liquids with fluorinated anions exhibit limited biodegradability, newer generations incorporating biodegradable components such as amino acid-derived cations and natural-product-based structures show improved environmental fate profiles. These biodegradable ionic liquids decompose more readily in natural environments, minimizing long-term ecological accumulation risks.
Recycling and recovery strategies for ionic liquid electrolytes remain underdeveloped compared to conventional battery components. Their non-volatile nature facilitates potential recovery through liquid-liquid extraction techniques, though commercial-scale processes require further optimization. Emerging technologies utilizing supercritical CO2 extraction show promise for efficient ionic liquid recovery with minimal environmental impact.
The carbon footprint associated with ionic liquid production currently exceeds that of conventional electrolytes due to complex synthesis requirements. However, life cycle assessments indicate that this initial environmental cost may be offset by extended battery lifetimes and improved safety profiles. Future development of renewable energy-powered synthesis methods could further enhance the sustainability proposition of ionic liquid electrolytes.
Water consumption and potential aquatic toxicity must also be considered in comprehensive environmental assessments. While ionic liquids generally exhibit lower water solubility than conventional organic electrolytes, their potential ecological impacts upon accidental release require careful evaluation through standardized ecotoxicological testing protocols.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!