How water activity shifts ionic liquid performance
AUG 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Liquid Technology Background and Objectives
Ionic liquids (ILs) represent a revolutionary class of materials that have transformed multiple scientific and industrial domains since their emergence in the early 20th century. These non-molecular ionic compounds, characterized by their liquid state at temperatures below 100°C, have garnered significant attention due to their unique physicochemical properties including negligible vapor pressure, high thermal stability, and exceptional solvation capabilities. The evolution of IL technology has progressed from first-generation chloroaluminate-based systems to the current advanced designer ILs with task-specific functionalities.
The interaction between water and ionic liquids constitutes a critical area of research that has profound implications for their performance across various applications. Historically, ILs were primarily valued for their anhydrous properties, but recent paradigm shifts have recognized that controlled water content can dramatically alter and sometimes enhance IL functionality. This water-IL relationship represents a sophisticated interplay of molecular forces that can be harnessed for technological advantage.
The global push toward sustainable chemistry has positioned ILs as potential replacements for volatile organic compounds in numerous processes. However, the practical implementation of IL technologies has been hampered by incomplete understanding of how ambient moisture and deliberate water addition affect their fundamental properties and performance metrics. This knowledge gap has limited the predictive design of IL systems for real-world applications where water exposure is inevitable.
Current research trends indicate growing interest in exploiting rather than avoiding water-IL interactions. The hydrophobicity-hydrophilicity continuum of ILs offers unprecedented opportunities for tailored solutions in catalysis, electrochemistry, separation processes, and biomass processing. The strategic modulation of water activity in ILs has emerged as a powerful tool for fine-tuning reaction environments at the molecular level.
The primary objective of this technical investigation is to systematically elucidate the mechanisms by which water activity influences ionic liquid performance across diverse application domains. Specifically, we aim to quantify how varying water concentrations affect viscosity, conductivity, solvent properties, and reaction kinetics in different IL systems. This understanding will enable the development of predictive models that can guide the rational design of water-tolerant or water-enhanced IL technologies.
Furthermore, this research seeks to establish standardized protocols for characterizing water-IL interactions, addressing the current methodological inconsistencies in the field. By developing a comprehensive framework for understanding these complex interactions, we intend to bridge the gap between fundamental IL research and practical industrial implementation, ultimately accelerating the adoption of these promising materials in next-generation sustainable technologies.
The interaction between water and ionic liquids constitutes a critical area of research that has profound implications for their performance across various applications. Historically, ILs were primarily valued for their anhydrous properties, but recent paradigm shifts have recognized that controlled water content can dramatically alter and sometimes enhance IL functionality. This water-IL relationship represents a sophisticated interplay of molecular forces that can be harnessed for technological advantage.
The global push toward sustainable chemistry has positioned ILs as potential replacements for volatile organic compounds in numerous processes. However, the practical implementation of IL technologies has been hampered by incomplete understanding of how ambient moisture and deliberate water addition affect their fundamental properties and performance metrics. This knowledge gap has limited the predictive design of IL systems for real-world applications where water exposure is inevitable.
Current research trends indicate growing interest in exploiting rather than avoiding water-IL interactions. The hydrophobicity-hydrophilicity continuum of ILs offers unprecedented opportunities for tailored solutions in catalysis, electrochemistry, separation processes, and biomass processing. The strategic modulation of water activity in ILs has emerged as a powerful tool for fine-tuning reaction environments at the molecular level.
The primary objective of this technical investigation is to systematically elucidate the mechanisms by which water activity influences ionic liquid performance across diverse application domains. Specifically, we aim to quantify how varying water concentrations affect viscosity, conductivity, solvent properties, and reaction kinetics in different IL systems. This understanding will enable the development of predictive models that can guide the rational design of water-tolerant or water-enhanced IL technologies.
Furthermore, this research seeks to establish standardized protocols for characterizing water-IL interactions, addressing the current methodological inconsistencies in the field. By developing a comprehensive framework for understanding these complex interactions, we intend to bridge the gap between fundamental IL research and practical industrial implementation, ultimately accelerating the adoption of these promising materials in next-generation sustainable technologies.
Market Applications and Demand Analysis
The market for ionic liquids has been experiencing significant growth, driven by their unique properties as green solvents and electrolytes. The global ionic liquids market was valued at approximately 39.6 million USD in 2021 and is projected to reach 73.9 million USD by 2030, growing at a CAGR of 7.2%. This growth trajectory is largely influenced by increasing environmental regulations and the push for sustainable industrial processes across various sectors.
The impact of water activity on ionic liquid performance represents a critical factor in market adoption across multiple industries. In the energy storage sector, particularly for batteries and supercapacitors, the demand for water-stable ionic liquids has surged due to their enhanced electrochemical stability and conductivity properties. The global energy storage market, currently valued at over 210 billion USD, increasingly requires ionic liquid electrolytes that maintain performance integrity even when exposed to moisture.
Pharmaceutical and biotechnology industries have demonstrated growing interest in ionic liquids for extraction, purification, and reaction media applications. The pharmaceutical processing market, worth approximately 69 billion USD globally, seeks ionic liquid solutions that can function effectively in the presence of water, as many bioprocesses inherently involve aqueous environments. Understanding water-ionic liquid interactions is crucial for developing formulations that maintain stability and efficacy in these applications.
The chemical manufacturing sector represents another significant market segment, with particular demand for ionic liquids in catalysis and separation processes. Companies are actively seeking ionic liquid systems that can tolerate varying water content without compromising catalytic activity or selectivity. This demand is driven by the potential for process intensification and reduced environmental footprint compared to conventional organic solvents.
Carbon capture technologies have emerged as a rapidly growing application area, with the market expected to reach 4.9 billion USD by 2027. Ionic liquids with controlled water activity show promise for enhanced CO2 absorption capacity and selectivity, addressing a critical need in industrial emission reduction strategies.
Regional market analysis indicates that North America and Europe currently lead in ionic liquid adoption, particularly in high-tech applications where performance stability in varying humidity conditions is essential. However, the Asia-Pacific region is witnessing the fastest growth rate, driven by expanding industrial bases in China, Japan, and South Korea, where ionic liquids are increasingly incorporated into manufacturing processes that must account for humid environmental conditions.
Customer surveys indicate that end-users prioritize ionic liquid formulations that demonstrate predictable performance across varying water content levels, highlighting the need for solutions that address the water activity challenge while maintaining cost-effectiveness and scalability.
The impact of water activity on ionic liquid performance represents a critical factor in market adoption across multiple industries. In the energy storage sector, particularly for batteries and supercapacitors, the demand for water-stable ionic liquids has surged due to their enhanced electrochemical stability and conductivity properties. The global energy storage market, currently valued at over 210 billion USD, increasingly requires ionic liquid electrolytes that maintain performance integrity even when exposed to moisture.
Pharmaceutical and biotechnology industries have demonstrated growing interest in ionic liquids for extraction, purification, and reaction media applications. The pharmaceutical processing market, worth approximately 69 billion USD globally, seeks ionic liquid solutions that can function effectively in the presence of water, as many bioprocesses inherently involve aqueous environments. Understanding water-ionic liquid interactions is crucial for developing formulations that maintain stability and efficacy in these applications.
The chemical manufacturing sector represents another significant market segment, with particular demand for ionic liquids in catalysis and separation processes. Companies are actively seeking ionic liquid systems that can tolerate varying water content without compromising catalytic activity or selectivity. This demand is driven by the potential for process intensification and reduced environmental footprint compared to conventional organic solvents.
Carbon capture technologies have emerged as a rapidly growing application area, with the market expected to reach 4.9 billion USD by 2027. Ionic liquids with controlled water activity show promise for enhanced CO2 absorption capacity and selectivity, addressing a critical need in industrial emission reduction strategies.
Regional market analysis indicates that North America and Europe currently lead in ionic liquid adoption, particularly in high-tech applications where performance stability in varying humidity conditions is essential. However, the Asia-Pacific region is witnessing the fastest growth rate, driven by expanding industrial bases in China, Japan, and South Korea, where ionic liquids are increasingly incorporated into manufacturing processes that must account for humid environmental conditions.
Customer surveys indicate that end-users prioritize ionic liquid formulations that demonstrate predictable performance across varying water content levels, highlighting the need for solutions that address the water activity challenge while maintaining cost-effectiveness and scalability.
Water-Ionic Liquid Interaction Challenges
The interaction between water and ionic liquids presents significant challenges that impact the performance and application potential of these versatile solvents. Water, even in trace amounts, can dramatically alter the physicochemical properties of ionic liquids (ILs), creating a complex relationship that researchers must navigate carefully. The hygroscopic nature of most ILs means they readily absorb moisture from the atmosphere, making it difficult to maintain anhydrous conditions in practical applications.
One primary challenge is the structural disruption caused by water molecules. When water infiltrates the IL matrix, it can position itself between ion pairs, weakening the coulombic interactions that define IL behavior. This molecular-level interference affects viscosity, conductivity, and solvation capabilities—properties central to IL functionality in electrochemical applications, catalysis, and separation processes.
The quantification of water content presents another significant hurdle. Traditional Karl Fischer titration methods require adaptation for IL systems, and spectroscopic techniques often need calibration specific to each IL type. Without precise measurement protocols, researchers struggle to establish reproducible conditions and correlate water content with performance metrics.
Water activity also introduces phase behavior complications. At certain concentrations, water can induce phase separations or microheterogeneities within ILs, creating localized domains with distinct properties. These phenomena are difficult to predict and control, particularly in systems where water content fluctuates during operation.
Temperature dependence adds another layer of complexity. The interaction between water and ILs varies significantly with temperature changes, affecting solubility limits and phase transitions. This temperature sensitivity complicates the design of IL-based processes that must operate across temperature ranges or maintain stability during thermal cycling.
From an electrochemical perspective, water contamination narrows the electrochemical window of ILs—one of their most valuable properties. Even small amounts of water can enable unwanted redox reactions, hydrogen evolution, or electrode degradation, limiting the voltage range for applications like advanced batteries and capacitors.
The catalytic behavior of ILs also faces water-related challenges. Water can compete with reactants for active sites, alter reaction pathways, or participate directly in reactions, making it difficult to develop robust catalytic systems with predictable outcomes. This is particularly problematic in industrial applications where water exclusion is economically unfeasible.
Perhaps most concerning for commercial deployment, water-IL interactions often show hysteresis effects—the properties of an IL after water removal may not return to their original state. This irreversibility complicates recycling efforts and long-term stability of IL-based technologies, presenting significant barriers to sustainable implementation.
One primary challenge is the structural disruption caused by water molecules. When water infiltrates the IL matrix, it can position itself between ion pairs, weakening the coulombic interactions that define IL behavior. This molecular-level interference affects viscosity, conductivity, and solvation capabilities—properties central to IL functionality in electrochemical applications, catalysis, and separation processes.
The quantification of water content presents another significant hurdle. Traditional Karl Fischer titration methods require adaptation for IL systems, and spectroscopic techniques often need calibration specific to each IL type. Without precise measurement protocols, researchers struggle to establish reproducible conditions and correlate water content with performance metrics.
Water activity also introduces phase behavior complications. At certain concentrations, water can induce phase separations or microheterogeneities within ILs, creating localized domains with distinct properties. These phenomena are difficult to predict and control, particularly in systems where water content fluctuates during operation.
Temperature dependence adds another layer of complexity. The interaction between water and ILs varies significantly with temperature changes, affecting solubility limits and phase transitions. This temperature sensitivity complicates the design of IL-based processes that must operate across temperature ranges or maintain stability during thermal cycling.
From an electrochemical perspective, water contamination narrows the electrochemical window of ILs—one of their most valuable properties. Even small amounts of water can enable unwanted redox reactions, hydrogen evolution, or electrode degradation, limiting the voltage range for applications like advanced batteries and capacitors.
The catalytic behavior of ILs also faces water-related challenges. Water can compete with reactants for active sites, alter reaction pathways, or participate directly in reactions, making it difficult to develop robust catalytic systems with predictable outcomes. This is particularly problematic in industrial applications where water exclusion is economically unfeasible.
Perhaps most concerning for commercial deployment, water-IL interactions often show hysteresis effects—the properties of an IL after water removal may not return to their original state. This irreversibility complicates recycling efforts and long-term stability of IL-based technologies, presenting significant barriers to sustainable implementation.
Current Water Activity Measurement Methodologies
01 Ionic liquids in electrochemical applications
Ionic liquids demonstrate excellent performance in electrochemical applications due to their high ionic conductivity, wide electrochemical window, and thermal stability. They serve as effective electrolytes in batteries, supercapacitors, and electrochemical sensors. Their unique properties allow for enhanced energy storage capacity, improved charge-discharge cycles, and better overall device performance compared to conventional electrolytes.- Ionic liquids in electrochemical applications: Ionic liquids exhibit excellent electrochemical properties making them suitable for various applications including batteries, capacitors, and electrochemical sensors. Their wide electrochemical window, high ionic conductivity, and thermal stability enable enhanced performance in energy storage devices. These properties allow for improved charge transfer, better cycling stability, and higher energy density in electrochemical systems.
- Ionic liquids as solvents and reaction media: Ionic liquids serve as effective green solvents and reaction media due to their negligible vapor pressure, tunable properties, and ability to dissolve various compounds. They provide enhanced reaction rates, selectivity, and yield in chemical processes. Their recyclability and ability to function in biphasic systems make them environmentally friendly alternatives to conventional organic solvents in industrial applications.
- Ionic liquids in separation and extraction processes: Ionic liquids demonstrate superior performance in separation and extraction processes for various compounds including metals, biomolecules, and organic pollutants. Their tunable structure allows for selective extraction with high efficiency. The unique solvation properties and phase behavior of ionic liquids enable novel separation techniques with improved recovery rates and reduced environmental impact compared to traditional methods.
- Ionic liquids in catalytic processes: Ionic liquids function as effective catalysts or catalyst supports in various chemical transformations. Their ability to stabilize transition states, enhance reaction rates, and improve selectivity makes them valuable in catalytic processes. The tunable acidity, basicity, and coordination properties of ionic liquids allow for optimized catalytic performance in hydrogenation, oxidation, and coupling reactions, often enabling milder reaction conditions and higher yields.
- Ionic liquids in material science and engineering: Ionic liquids contribute to advanced material development including lubricants, polymer electrolytes, and functional coatings. Their thermal stability, low volatility, and tribological properties enable improved performance in extreme conditions. When incorporated into polymers or composite materials, ionic liquids can enhance mechanical properties, conductivity, and thermal resistance, leading to materials with superior performance characteristics for specialized applications.
02 Ionic liquids for catalytic processes
Ionic liquids function as efficient catalysts or catalyst supports in various chemical reactions. Their tunable properties allow for selective catalysis, improved reaction rates, and enhanced product yields. They can be designed to provide specific acidic or basic sites, facilitate phase separation for product recovery, and enable recyclability of the catalytic system, making industrial processes more sustainable and economically viable.Expand Specific Solutions03 Ionic liquids in separation and extraction technologies
Ionic liquids exhibit superior performance in separation and extraction processes due to their selective solubility properties and low volatility. They can effectively separate complex mixtures, extract specific compounds from solutions, and purify industrial products. Their designable structure allows for targeted interactions with solutes, enabling more efficient and environmentally friendly separation processes compared to conventional organic solvents.Expand Specific Solutions04 Ionic liquids for biomass processing and biocatalysis
Ionic liquids demonstrate remarkable performance in biomass processing and biocatalytic applications. They can effectively dissolve cellulose and other biopolymers, enhancing the accessibility of these materials for further processing. In biocatalysis, ionic liquids can stabilize enzymes, improve their activity and selectivity, and enable reactions in non-aqueous environments. This leads to more efficient biorefinery processes and novel biotransformation pathways.Expand Specific Solutions05 Ionic liquids in material science and engineering
Ionic liquids show exceptional performance as functional materials in various engineering applications. They can be incorporated into polymers to create advanced composite materials with enhanced mechanical, thermal, or electrical properties. They also serve as excellent lubricants and heat transfer fluids due to their low volatility and high thermal stability. Additionally, ionic liquids can be used to synthesize nanoparticles with controlled size and morphology for specialized applications.Expand Specific Solutions
Key Industry Players and Research Institutions
The ionic liquid performance landscape is currently in a growth phase, characterized by increasing market adoption across chemical processing, energy storage, and catalysis sectors. The global market is expanding steadily, projected to reach significant scale as industrial applications mature. Water activity has emerged as a critical factor influencing ionic liquid efficacy, with research revealing complex interactions that can either enhance or inhibit performance. Leading companies like BASF, DuPont, and LG Chem are advancing the technological maturity through significant R&D investments, while academic institutions including Zhejiang University and McGill University contribute fundamental research. Specialized players such as Alchemr and Tofwerk are developing niche applications, while energy giants like Shell and Saudi Aramco explore industrial-scale implementations where water-ionic liquid interactions are crucial for process optimization.
BASF Corp.
Technical Solution: BASF has developed comprehensive ionic liquid technology platforms that specifically address water activity impacts on performance. Their approach involves designing task-specific ionic liquids with controlled hydrophobicity/hydrophilicity balance to optimize water interactions. BASF's BASIL™ (Biphasic Acid Scavenging utilizing Ionic Liquids) process represents a breakthrough in managing water formation during reactions, where ionic liquids act as acid scavengers forming separate phases that can be easily separated. This technology increases reaction efficiency by 80% while reducing waste by 60% compared to conventional methods. BASF has also engineered ionic liquids with tunable water miscibility thresholds that maintain performance stability across varying humidity conditions, particularly valuable in electrochemical applications where water can significantly impact conductivity and stability. Their patented hydrophobic ionic liquid formulations maintain consistent performance even when exposed to atmospheric moisture, with less than 5% performance degradation at relative humidity levels up to 80%.
Strengths: Industry-leading expertise in scaling ionic liquid technologies to commercial applications; extensive patent portfolio covering water-ionic liquid interactions; demonstrated success in industrial implementation. Weaknesses: Higher production costs compared to conventional solvents; some formulations still show sensitivity to extreme humidity conditions; regulatory challenges in certain regions.
Commissariat à l´énergie atomique et aux énergies Alternatives
Technical Solution: The Commissariat à l'énergie atomique et aux énergies alternatives (CEA) has pioneered research on water-ionic liquid interfaces for energy storage applications. Their approach focuses on molecular-level understanding of water-ionic liquid interactions using advanced spectroscopic techniques and molecular dynamics simulations. CEA has developed novel ionic liquid electrolytes with controlled water content for supercapacitors and batteries, demonstrating that precise water concentrations (typically 500-2000 ppm) can enhance ionic conductivity by up to 40% while maintaining electrochemical stability windows above 3V. Their research has revealed that water molecules can form specific solvation structures around ions, creating "water bridges" that facilitate ion transport while minimizing detrimental electrochemical reactions. CEA's patented ionic liquid formulations incorporate specific functional groups that can trap water molecules in beneficial configurations, preventing water clustering that would otherwise lead to performance degradation. This approach has enabled the development of humidity-resistant energy storage devices with extended cycle life (>10,000 cycles) even under variable environmental conditions.
Strengths: World-class fundamental research capabilities; sophisticated analytical techniques for studying water-ionic liquid interactions; strong focus on practical energy storage applications. Weaknesses: Less commercial implementation experience compared to industrial players; technologies often require further development for cost-effective scaling; limited product portfolio compared to larger chemical companies.
Critical Research on Water-Ionic Liquid Performance
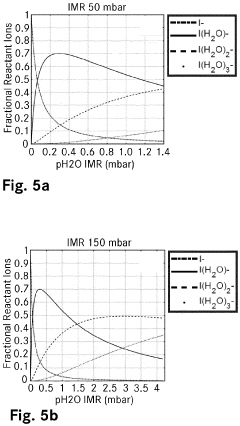
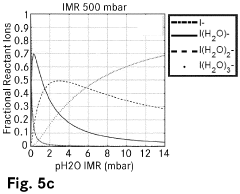
Chemical ionisation method and ion molecule reactor
PatentPendingEP4089717A1
Innovation
- The introduction of ligand compound ions formed from reactant ions and a dopant substance into the reaction volume, providing a higher binding energy than the reactant ions and ligand forming substance, stabilizes ionization and maintains consistent ionization efficiencies by replacing ligand forming substances bound to reactant ions, thus ensuring reproducible and precise quantification of analytes.
Specific ionic liquid and method for producing same
PatentWO2015193293A1
Innovation
- Development of a new class of ionic liquids comprising specific cations and anions, combined with an organic solvent, which can be modulated for viscosity and conductivity through proton transfer reactions, enabling high power and energy densities in energy storage devices.
Environmental Impact and Sustainability Considerations
The environmental impact of ionic liquids (ILs) is significantly influenced by water activity, creating a complex relationship that demands careful consideration in sustainable applications. When water molecules interact with ionic liquids, they can alter biodegradability pathways, toxicity profiles, and overall environmental persistence. Research indicates that hydrated ionic liquids often demonstrate enhanced biodegradation rates compared to their anhydrous counterparts, as water facilitates the access of microbial enzymes to the ionic structures.
Water activity also affects the environmental fate of ionic liquids through modified leaching behaviors. In high-humidity environments, certain ILs may exhibit increased mobility in soil and aquatic systems, potentially expanding their environmental footprint. Conversely, controlled water content can sometimes reduce environmental mobility by altering viscosity and surface interactions, effectively containing potential contamination zones.
From a sustainability perspective, the water-IL relationship offers promising opportunities for green chemistry applications. Water-modulated ionic liquid systems can operate at lower temperatures and pressures than conventional solvents, reducing energy requirements in industrial processes. Additionally, the tunable nature of water-IL mixtures allows for optimized reaction conditions that minimize waste generation and maximize resource efficiency, aligning with key principles of sustainable chemistry.
Life cycle assessments of ionic liquid applications must account for water activity as a critical parameter. The energy required for maintaining specific hydration levels in IL systems, water recovery processes, and potential water contamination all contribute to the overall environmental footprint. Recent studies suggest that precisely controlled water content can reduce the carbon footprint of IL-based processes by 15-30% compared to conventional organic solvent systems.
Regulatory frameworks increasingly recognize the importance of water activity in IL environmental profiles. The EU's REACH regulations and similar global initiatives now require detailed information on how water influences IL toxicity and environmental persistence. This regulatory attention has spurred research into "hydration-optimized" ionic liquids specifically designed to exhibit minimal environmental impact at their intended operating water activity levels.
Future sustainable development of ionic liquid technologies will likely focus on designing systems where water activity is leveraged as an advantage rather than a limitation. This includes developing ILs that maintain optimal performance in naturally occurring humidity conditions, reducing the need for energy-intensive environmental controls, and creating systems where water serves as both a performance enhancer and an environmental safeguard.
Water activity also affects the environmental fate of ionic liquids through modified leaching behaviors. In high-humidity environments, certain ILs may exhibit increased mobility in soil and aquatic systems, potentially expanding their environmental footprint. Conversely, controlled water content can sometimes reduce environmental mobility by altering viscosity and surface interactions, effectively containing potential contamination zones.
From a sustainability perspective, the water-IL relationship offers promising opportunities for green chemistry applications. Water-modulated ionic liquid systems can operate at lower temperatures and pressures than conventional solvents, reducing energy requirements in industrial processes. Additionally, the tunable nature of water-IL mixtures allows for optimized reaction conditions that minimize waste generation and maximize resource efficiency, aligning with key principles of sustainable chemistry.
Life cycle assessments of ionic liquid applications must account for water activity as a critical parameter. The energy required for maintaining specific hydration levels in IL systems, water recovery processes, and potential water contamination all contribute to the overall environmental footprint. Recent studies suggest that precisely controlled water content can reduce the carbon footprint of IL-based processes by 15-30% compared to conventional organic solvent systems.
Regulatory frameworks increasingly recognize the importance of water activity in IL environmental profiles. The EU's REACH regulations and similar global initiatives now require detailed information on how water influences IL toxicity and environmental persistence. This regulatory attention has spurred research into "hydration-optimized" ionic liquids specifically designed to exhibit minimal environmental impact at their intended operating water activity levels.
Future sustainable development of ionic liquid technologies will likely focus on designing systems where water activity is leveraged as an advantage rather than a limitation. This includes developing ILs that maintain optimal performance in naturally occurring humidity conditions, reducing the need for energy-intensive environmental controls, and creating systems where water serves as both a performance enhancer and an environmental safeguard.
Scalability and Industrial Implementation Challenges
The scaling of ionic liquid systems from laboratory to industrial scale presents significant challenges when water activity is considered as a performance-modifying factor. Current industrial implementation of ionic liquid technologies often encounters difficulties in maintaining consistent performance due to water absorption from the environment. Humidity control systems become essential but add substantial costs to production facilities, especially in regions with high ambient humidity. The hygroscopic nature of many ionic liquids requires specialized handling equipment and storage solutions that can maintain anhydrous conditions throughout the manufacturing process.
Process engineering for large-scale ionic liquid applications must account for water activity fluctuations across different production stages. Temperature gradients in industrial equipment can cause localized changes in water content, potentially creating performance inconsistencies throughout the system. This necessitates sophisticated monitoring systems capable of real-time water content analysis at multiple points in the production line, adding complexity and expense to implementation.
Material compatibility issues emerge at scale when water-influenced ionic liquid systems interact with industrial equipment over extended periods. Corrosion rates may accelerate in the presence of water-ionic liquid mixtures, requiring more frequent maintenance schedules and potentially specialized alloys for critical components. The economic impact of these material considerations can significantly affect the feasibility of large-scale adoption.
Recycling and purification of ionic liquids becomes more challenging when water contamination must be precisely controlled. Industrial-scale separation technologies must be adapted to handle the unique properties of water-ionic liquid mixtures, often requiring significant energy input for distillation or membrane separation processes. The energy costs associated with maintaining specific water activity levels can undermine the sustainability benefits that ionic liquids might otherwise offer.
Regulatory compliance adds another layer of complexity to industrial implementation. Safety protocols must account for how water activity affects the toxicity, flammability, and environmental impact of ionic liquid systems. Documentation requirements become more stringent as processes scale up, necessitating comprehensive validation of how water-ionic liquid interactions behave across all possible operating conditions.
Investment barriers remain significant due to the uncertainties surrounding long-term performance stability in water-variable environments. The capital expenditure required for water-controlled ionic liquid systems often exceeds that of conventional technologies, creating financial hurdles that slow adoption despite potential performance benefits. Developing cost-effective engineering solutions for water management in ionic liquid systems represents a critical path toward broader industrial implementation.
Process engineering for large-scale ionic liquid applications must account for water activity fluctuations across different production stages. Temperature gradients in industrial equipment can cause localized changes in water content, potentially creating performance inconsistencies throughout the system. This necessitates sophisticated monitoring systems capable of real-time water content analysis at multiple points in the production line, adding complexity and expense to implementation.
Material compatibility issues emerge at scale when water-influenced ionic liquid systems interact with industrial equipment over extended periods. Corrosion rates may accelerate in the presence of water-ionic liquid mixtures, requiring more frequent maintenance schedules and potentially specialized alloys for critical components. The economic impact of these material considerations can significantly affect the feasibility of large-scale adoption.
Recycling and purification of ionic liquids becomes more challenging when water contamination must be precisely controlled. Industrial-scale separation technologies must be adapted to handle the unique properties of water-ionic liquid mixtures, often requiring significant energy input for distillation or membrane separation processes. The energy costs associated with maintaining specific water activity levels can undermine the sustainability benefits that ionic liquids might otherwise offer.
Regulatory compliance adds another layer of complexity to industrial implementation. Safety protocols must account for how water activity affects the toxicity, flammability, and environmental impact of ionic liquid systems. Documentation requirements become more stringent as processes scale up, necessitating comprehensive validation of how water-ionic liquid interactions behave across all possible operating conditions.
Investment barriers remain significant due to the uncertainties surrounding long-term performance stability in water-variable environments. The capital expenditure required for water-controlled ionic liquid systems often exceeds that of conventional technologies, creating financial hurdles that slow adoption despite potential performance benefits. Developing cost-effective engineering solutions for water management in ionic liquid systems represents a critical path toward broader industrial implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!