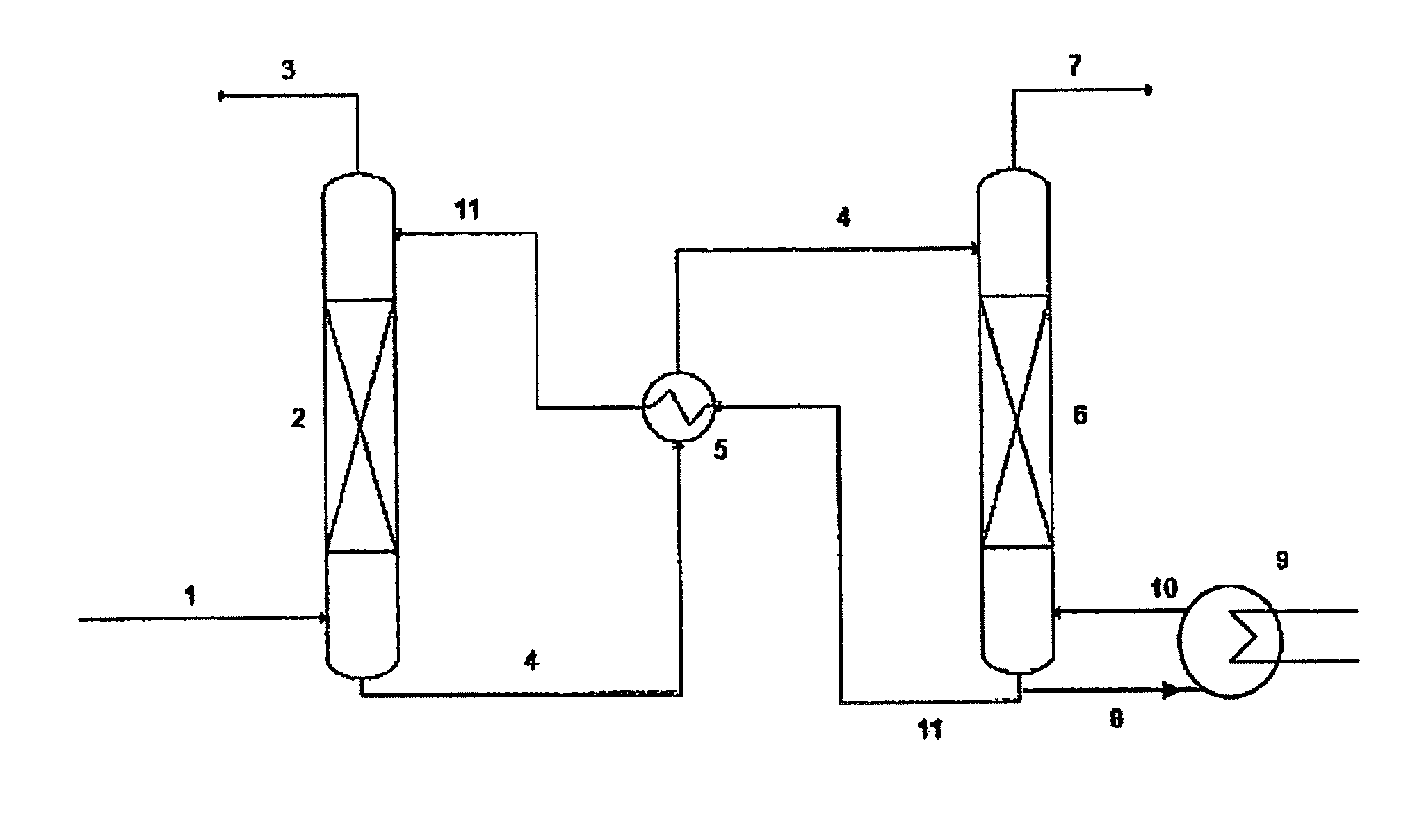
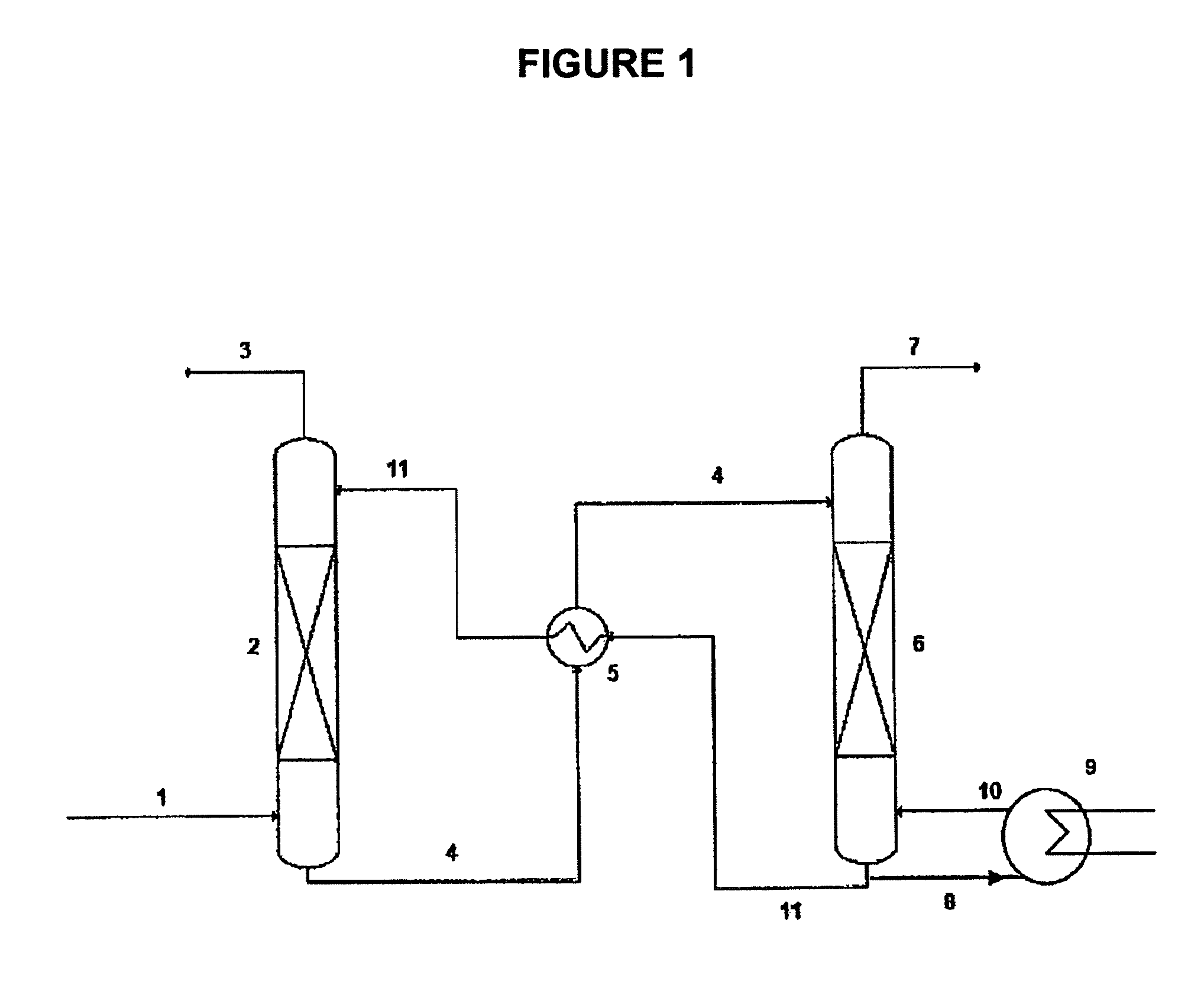
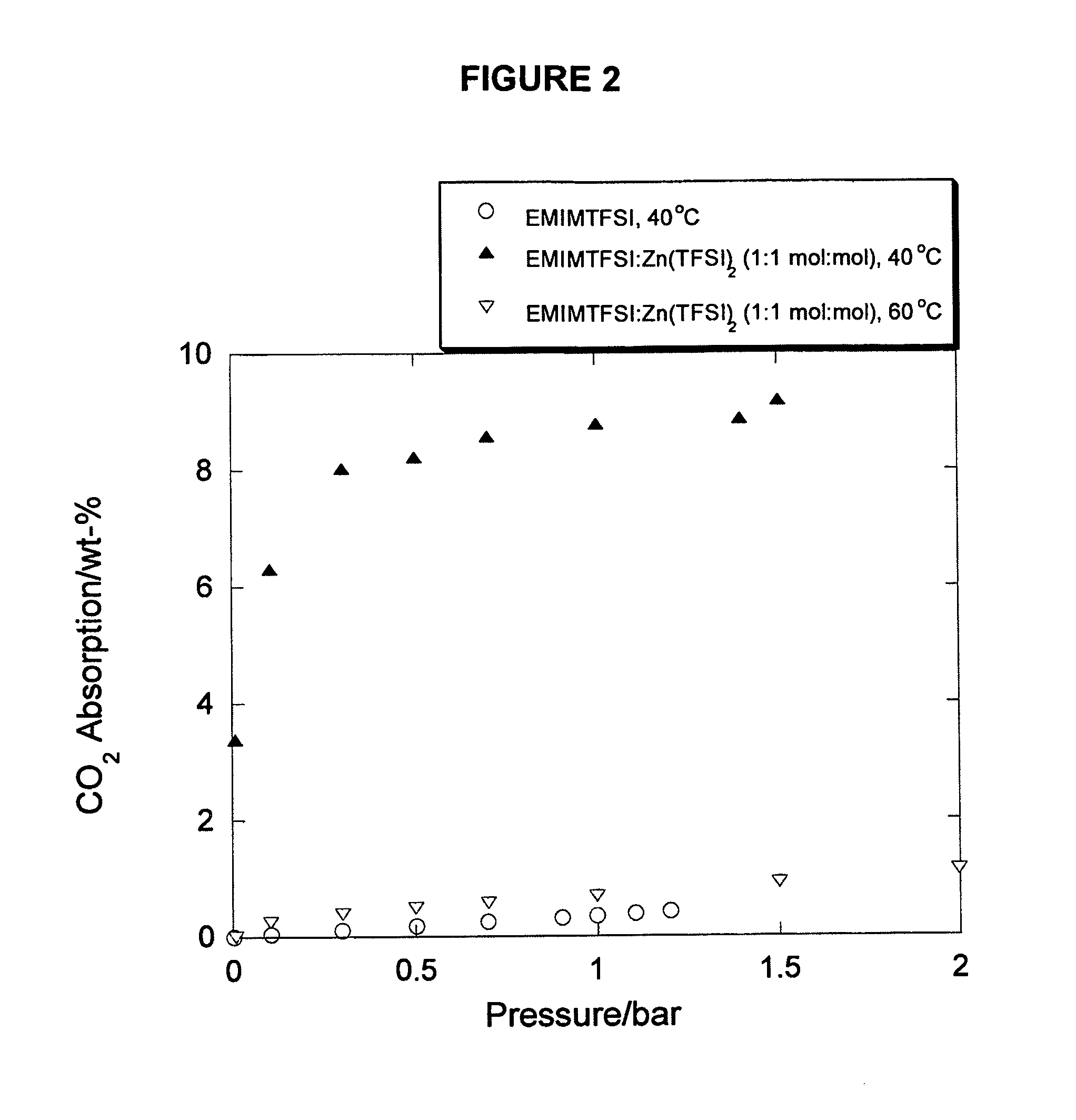
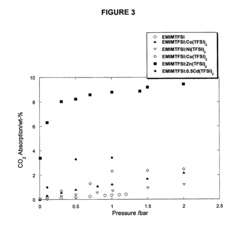
Task-specific ionic liquids in CO2 capture: absorption kinetics
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Liquids in CO2 Capture: Background and Objectives
Carbon dioxide (CO2) emissions have emerged as a critical environmental concern, contributing significantly to global climate change. The development of efficient CO2 capture technologies has become imperative in mitigating these effects. Among various approaches, ionic liquids (ILs) have gained substantial attention as promising sorbents for CO2 capture due to their unique physicochemical properties. The evolution of this technology can be traced back to the early 2000s when researchers first recognized the potential of ILs for gas absorption applications.
Task-specific ionic liquids (TSILs) represent a significant advancement in this field, designed with specific functional groups to enhance CO2 absorption capacity and selectivity. These designer solvents have evolved from conventional ILs through systematic molecular engineering to address the particular challenges of carbon capture. The technological trajectory has moved from simple imidazolium-based ILs to more complex structures incorporating amine functionalities, azolate anions, and other CO2-philic groups.
The primary technical objectives in this domain focus on overcoming the inherent limitations of traditional CO2 capture methods, particularly the high energy requirements for solvent regeneration and the degradation issues associated with conventional amine scrubbing. Specifically, researchers aim to develop TSILs with enhanced absorption kinetics while maintaining favorable thermodynamic properties, thereby reducing the energy penalty associated with the capture process.
Current research trends indicate a growing interest in understanding the fundamental mechanisms governing CO2 absorption in ionic environments. This includes investigating the role of anion-cation interactions, the impact of functional group positioning, and the influence of structural modifications on absorption rates. Additionally, there is an increasing focus on developing computational models to predict TSIL performance, enabling more efficient screening of potential candidates.
The absorption kinetics of CO2 in TSILs represents a critical parameter determining the practical applicability of these materials in industrial settings. Fast absorption rates are essential for continuous processes, while the relationship between structural features and kinetic behavior provides valuable insights for molecular design. Recent studies have demonstrated that certain structural motifs, such as aprotic heterocyclic anions and functionalized cations, can significantly enhance absorption rates.
Looking forward, the technical trajectory is expected to move toward multi-functional TSILs that simultaneously address multiple aspects of the capture process, including absorption capacity, selectivity, kinetics, and stability. The integration of TSILs with supporting materials to form composite sorbents also represents a promising direction for overcoming mass transfer limitations inherent to high-viscosity liquids.
Task-specific ionic liquids (TSILs) represent a significant advancement in this field, designed with specific functional groups to enhance CO2 absorption capacity and selectivity. These designer solvents have evolved from conventional ILs through systematic molecular engineering to address the particular challenges of carbon capture. The technological trajectory has moved from simple imidazolium-based ILs to more complex structures incorporating amine functionalities, azolate anions, and other CO2-philic groups.
The primary technical objectives in this domain focus on overcoming the inherent limitations of traditional CO2 capture methods, particularly the high energy requirements for solvent regeneration and the degradation issues associated with conventional amine scrubbing. Specifically, researchers aim to develop TSILs with enhanced absorption kinetics while maintaining favorable thermodynamic properties, thereby reducing the energy penalty associated with the capture process.
Current research trends indicate a growing interest in understanding the fundamental mechanisms governing CO2 absorption in ionic environments. This includes investigating the role of anion-cation interactions, the impact of functional group positioning, and the influence of structural modifications on absorption rates. Additionally, there is an increasing focus on developing computational models to predict TSIL performance, enabling more efficient screening of potential candidates.
The absorption kinetics of CO2 in TSILs represents a critical parameter determining the practical applicability of these materials in industrial settings. Fast absorption rates are essential for continuous processes, while the relationship between structural features and kinetic behavior provides valuable insights for molecular design. Recent studies have demonstrated that certain structural motifs, such as aprotic heterocyclic anions and functionalized cations, can significantly enhance absorption rates.
Looking forward, the technical trajectory is expected to move toward multi-functional TSILs that simultaneously address multiple aspects of the capture process, including absorption capacity, selectivity, kinetics, and stability. The integration of TSILs with supporting materials to form composite sorbents also represents a promising direction for overcoming mass transfer limitations inherent to high-viscosity liquids.
Market Analysis for CO2 Capture Technologies
The global CO2 capture market is experiencing significant growth, driven by increasing environmental regulations and corporate sustainability commitments. As of 2023, the market was valued at approximately $2.6 billion, with projections indicating a compound annual growth rate (CAGR) of 19.2% through 2030, potentially reaching $7.8 billion. This growth trajectory is supported by governmental carbon reduction targets established under the Paris Agreement and subsequent climate accords.
Task-specific ionic liquids (TSILs) represent an emerging segment within the broader CO2 capture technology landscape. While traditional capture methods like amine scrubbing currently dominate with roughly 70% market share, TSILs are gaining attention due to their potential for improved absorption kinetics and reduced energy requirements. Market analysis indicates that innovative absorption technologies, including TSILs, could capture up to 15% of the total market by 2028 if current technical challenges are overcome.
Regional analysis reveals varying adoption patterns, with North America and Europe leading in research and implementation of advanced CO2 capture technologies. The Asia-Pacific region, particularly China and India, represents the fastest-growing market segment with 24% annual growth, driven by rapid industrialization coupled with new environmental regulations. These regions are increasingly investing in pilot projects utilizing task-specific ionic liquids for industrial applications.
Industry segmentation shows power generation remains the largest end-user sector for CO2 capture technologies (42% of market share), followed by oil and gas (27%), cement production (14%), and chemical manufacturing (11%). Task-specific ionic liquids show particular promise in the chemical manufacturing sector due to their compatibility with existing processes and potential for integration into production streams.
Economic analysis reveals that while initial implementation costs for TSIL-based capture systems remain 30-40% higher than conventional methods, their operational expenditure advantages could yield break-even points within 4-6 years of deployment. This economic equation is improving as manufacturing scales increase and more efficient synthesis methods for task-specific ionic liquids are developed.
Customer demand is increasingly focused on technologies that not only capture CO2 effectively but do so with minimal energy penalties. TSILs with enhanced absorption kinetics address this market need directly, potentially reducing the energy penalty by 15-25% compared to conventional amine scrubbing. This performance characteristic positions them favorably in market segments where energy costs represent a significant operational expense.
Task-specific ionic liquids (TSILs) represent an emerging segment within the broader CO2 capture technology landscape. While traditional capture methods like amine scrubbing currently dominate with roughly 70% market share, TSILs are gaining attention due to their potential for improved absorption kinetics and reduced energy requirements. Market analysis indicates that innovative absorption technologies, including TSILs, could capture up to 15% of the total market by 2028 if current technical challenges are overcome.
Regional analysis reveals varying adoption patterns, with North America and Europe leading in research and implementation of advanced CO2 capture technologies. The Asia-Pacific region, particularly China and India, represents the fastest-growing market segment with 24% annual growth, driven by rapid industrialization coupled with new environmental regulations. These regions are increasingly investing in pilot projects utilizing task-specific ionic liquids for industrial applications.
Industry segmentation shows power generation remains the largest end-user sector for CO2 capture technologies (42% of market share), followed by oil and gas (27%), cement production (14%), and chemical manufacturing (11%). Task-specific ionic liquids show particular promise in the chemical manufacturing sector due to their compatibility with existing processes and potential for integration into production streams.
Economic analysis reveals that while initial implementation costs for TSIL-based capture systems remain 30-40% higher than conventional methods, their operational expenditure advantages could yield break-even points within 4-6 years of deployment. This economic equation is improving as manufacturing scales increase and more efficient synthesis methods for task-specific ionic liquids are developed.
Customer demand is increasingly focused on technologies that not only capture CO2 effectively but do so with minimal energy penalties. TSILs with enhanced absorption kinetics address this market need directly, potentially reducing the energy penalty by 15-25% compared to conventional amine scrubbing. This performance characteristic positions them favorably in market segments where energy costs represent a significant operational expense.
Current Challenges in Task-specific Ionic Liquids Development
Despite the promising potential of task-specific ionic liquids (TSILs) for CO2 capture, several significant challenges impede their widespread industrial implementation. The primary obstacle remains the high viscosity of TSILs, which substantially reduces mass transfer rates and absorption kinetics. This viscosity issue becomes particularly problematic at higher CO2 loadings, creating a self-limiting effect where absorption efficiency decreases as more CO2 is captured. The viscosity challenge is further exacerbated at lower operating temperatures, limiting the operational flexibility of TSIL-based capture systems.
Cost factors present another major hurdle, as most TSILs require complex synthesis procedures involving expensive precursors and multi-step reactions. The current production methods remain predominantly laboratory-scale, lacking the economies of scale necessary for industrial viability. Estimates suggest that TSIL costs need to decrease by at least an order of magnitude to compete with conventional amine-based capture technologies.
Stability concerns also plague TSIL development, with many promising candidates exhibiting degradation under real-world operating conditions. Thermal degradation at regeneration temperatures, oxidative degradation in the presence of flue gas impurities, and hydrolytic instability in moisture-rich environments all compromise long-term performance. This degradation not only reduces capture efficiency over time but also potentially generates toxic byproducts requiring additional treatment.
The absorption-desorption energy balance presents another significant challenge. While TSILs generally offer lower heat of absorption compared to conventional amines (beneficial for reducing regeneration energy), this often correlates with slower absorption kinetics. Engineering this trade-off remains difficult, as modifications that enhance absorption rates frequently increase regeneration energy requirements, negating one of the key advantages of TSIL technology.
Scalability issues further complicate industrial adoption. Current laboratory demonstrations typically operate at milliliter scales, while industrial applications would require cubic meter volumes. The behavior of TSILs in large-scale contactors, including mass transfer characteristics, fluid dynamics, and long-term stability under continuous operation, remains largely unexplored. Additionally, materials compatibility concerns exist, as some TSILs exhibit corrosive properties toward common industrial construction materials.
Environmental and toxicological uncertainties constitute another barrier. The environmental fate, biodegradability, and potential ecological impacts of TSILs and their degradation products remain poorly characterized. Regulatory frameworks for large-scale deployment of these novel materials are still evolving, creating uncertainty for potential industrial adopters.
Cost factors present another major hurdle, as most TSILs require complex synthesis procedures involving expensive precursors and multi-step reactions. The current production methods remain predominantly laboratory-scale, lacking the economies of scale necessary for industrial viability. Estimates suggest that TSIL costs need to decrease by at least an order of magnitude to compete with conventional amine-based capture technologies.
Stability concerns also plague TSIL development, with many promising candidates exhibiting degradation under real-world operating conditions. Thermal degradation at regeneration temperatures, oxidative degradation in the presence of flue gas impurities, and hydrolytic instability in moisture-rich environments all compromise long-term performance. This degradation not only reduces capture efficiency over time but also potentially generates toxic byproducts requiring additional treatment.
The absorption-desorption energy balance presents another significant challenge. While TSILs generally offer lower heat of absorption compared to conventional amines (beneficial for reducing regeneration energy), this often correlates with slower absorption kinetics. Engineering this trade-off remains difficult, as modifications that enhance absorption rates frequently increase regeneration energy requirements, negating one of the key advantages of TSIL technology.
Scalability issues further complicate industrial adoption. Current laboratory demonstrations typically operate at milliliter scales, while industrial applications would require cubic meter volumes. The behavior of TSILs in large-scale contactors, including mass transfer characteristics, fluid dynamics, and long-term stability under continuous operation, remains largely unexplored. Additionally, materials compatibility concerns exist, as some TSILs exhibit corrosive properties toward common industrial construction materials.
Environmental and toxicological uncertainties constitute another barrier. The environmental fate, biodegradability, and potential ecological impacts of TSILs and their degradation products remain poorly characterized. Regulatory frameworks for large-scale deployment of these novel materials are still evolving, creating uncertainty for potential industrial adopters.
State-of-the-art Absorption Kinetics Solutions
01 Ionic liquids for gas absorption and separation
Task-specific ionic liquids can be designed for selective gas absorption and separation processes. These ionic liquids have tailored structures that enhance their affinity for specific gases, improving absorption capacity and kinetics. The absorption kinetics can be optimized by modifying the cation and anion structures, as well as by incorporating functional groups that interact favorably with target gas molecules. These materials offer advantages over conventional solvents including higher selectivity, stability, and reduced volatility.- Ionic liquids for gas absorption and separation: Task-specific ionic liquids can be designed for selective gas absorption and separation processes. These ionic liquids exhibit unique absorption kinetics that allow for efficient capture of specific gases such as CO2, SO2, and other greenhouse gases. The absorption mechanism typically involves chemical interactions between the functional groups of the ionic liquid and the target gas molecules, resulting in high selectivity and capacity. The kinetics of absorption can be tuned by modifying the structure of the ionic liquid, particularly the anion and cation components.
- Heat transfer applications of ionic liquids: Ionic liquids demonstrate favorable absorption kinetics for heat transfer applications. Their unique thermal properties, including high thermal stability and tunable heat capacity, make them suitable for absorption refrigeration systems and thermal energy storage. The absorption kinetics of these systems are influenced by the viscosity and thermal conductivity of the ionic liquids, which can be modified through structural design. These materials offer advantages over conventional heat transfer fluids, including lower vapor pressure and wider operating temperature ranges.
- Measurement and characterization of ionic liquid absorption kinetics: Various analytical techniques and methodologies have been developed to measure and characterize the absorption kinetics of task-specific ionic liquids. These include spectroscopic methods, gravimetric analysis, and specialized apparatus designed to monitor absorption rates in real-time. Understanding the kinetic parameters such as diffusion coefficients, activation energy, and rate constants is crucial for optimizing ionic liquid performance in various applications. Mathematical models have been developed to describe the absorption behavior, taking into account factors such as mass transfer limitations and chemical reaction rates.
- Ionic liquids for pharmaceutical and biological applications: Task-specific ionic liquids have been developed for pharmaceutical and biological applications, where absorption kinetics play a crucial role in drug delivery and bioavailability. These ionic liquids can enhance the solubility and permeability of poorly water-soluble drugs, improving their absorption profiles. The kinetics of absorption can be controlled by modifying the structure of the ionic liquid to achieve desired release profiles. Additionally, some ionic liquids exhibit antimicrobial properties and can be used in formulations for topical applications.
- Ionic liquids for environmental remediation: Task-specific ionic liquids have been employed in environmental remediation processes, particularly for the removal of pollutants from water and soil. The absorption kinetics of these ionic liquids determine their efficiency in capturing contaminants such as heavy metals, organic pollutants, and radioactive materials. Factors affecting the absorption kinetics include the specific functional groups incorporated into the ionic liquid structure, the interaction mechanism with target pollutants, and the physical properties of the ionic liquid such as viscosity and diffusivity. Some systems utilize supported ionic liquid phases to enhance mass transfer and improve absorption rates.
02 Ionic liquids for carbon dioxide capture
Task-specific ionic liquids have been developed specifically for carbon dioxide absorption with enhanced kinetics. These ionic liquids often contain amine or carboxylate functionalities that can chemically interact with CO2 molecules, significantly improving absorption rates and capacities. The absorption kinetics can be controlled by adjusting the viscosity, temperature, and structural features of the ionic liquids. These materials offer promising alternatives to conventional amine scrubbing technologies for carbon capture applications with reduced energy requirements for regeneration.Expand Specific Solutions03 Measurement and modeling of absorption kinetics in ionic liquids
Various techniques and methodologies have been developed to measure and model the absorption kinetics of gases and solutes in task-specific ionic liquids. These include gravimetric methods, spectroscopic techniques, and pressure-drop measurements that can track absorption rates in real-time. Mathematical models have been developed to describe the absorption kinetics, taking into account factors such as diffusion coefficients, mass transfer limitations, and chemical reaction rates. These measurement and modeling approaches enable the rational design of ionic liquid systems with optimized absorption kinetics for specific applications.Expand Specific Solutions04 Supported ionic liquid systems for enhanced absorption
Task-specific ionic liquids can be immobilized on various support materials to enhance their absorption kinetics and practical applicability. By dispersing the ionic liquids on high-surface-area supports such as porous polymers, silica, or carbon materials, the mass transfer limitations associated with bulk ionic liquids can be overcome. These supported ionic liquid systems offer improved absorption kinetics due to increased surface area, reduced diffusion path lengths, and sometimes synergistic interactions with the support material. They can be used in fixed-bed configurations or membrane contactors for continuous absorption processes.Expand Specific Solutions05 Temperature and pressure effects on ionic liquid absorption kinetics
The absorption kinetics of task-specific ionic liquids are significantly influenced by operating conditions, particularly temperature and pressure. Higher temperatures typically accelerate absorption kinetics by reducing viscosity and enhancing molecular mobility, but may decrease overall absorption capacity for exothermic processes. Pressure effects can also be substantial, with higher pressures generally increasing absorption rates up to saturation limits. Understanding these relationships allows for the optimization of process conditions to achieve desired absorption rates and capacities. Some ionic liquids exhibit unique phase behavior under certain temperature and pressure conditions that can be exploited for absorption-desorption cycles.Expand Specific Solutions
Leading Organizations in CO2 Capture Research
Task-specific ionic liquids (TSILs) for CO2 capture are evolving rapidly in a market poised for significant growth due to increasing global decarbonization efforts. The technology is transitioning from early development to commercial application phases, with the global carbon capture market expected to reach $7-10 billion by 2030. While absorption kinetics of TSILs show promising efficiency improvements over conventional methods, technical maturity varies across key players. Leading companies like China Petroleum & Chemical Corp., Petróleo Brasileiro SA, and ExxonMobil are advancing industrial-scale applications, while research institutions including Nanjing University, Technical University of Denmark, and MIT are driving fundamental innovations. Collaboration between academic institutions and energy corporations is accelerating development toward cost-effective, large-scale implementation solutions.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has pioneered task-specific ionic liquids for CO2 capture featuring imidazolium-based structures with functionalized side chains that enhance absorption kinetics. Their technology incorporates hydroxyl and amine functional groups strategically positioned to create multiple CO2 binding sites, resulting in absorption rates up to 3 times faster than conventional ionic liquids[3]. Sinopec's approach includes proprietary blending techniques that combine different ionic liquids to optimize both capacity and kinetics simultaneously. Their research demonstrates successful implementation of these materials in pilot-scale absorber columns with structured packing designed specifically to maximize the interfacial area between gas and ionic liquid phases. The company has developed a comprehensive regeneration protocol that maintains ionic liquid performance over hundreds of cycles, with less than 5% degradation in absorption kinetics[4]. Their system operates effectively at moderate pressures (1-5 bar) and achieves 90%+ CO2 capture efficiency from various industrial gas streams.
Strengths: Exceptional stability during multiple absorption-desorption cycles; lower regeneration energy requirements compared to conventional amine solutions; ability to operate effectively across a wide temperature range (20-120°C). Weaknesses: Higher initial investment costs; potential mass transfer limitations in conventional absorption equipment requiring specialized contactor designs; moderate viscosity increases at high CO2 loadings.
Commonwealth Scientific & Industrial Research Organisation
Technical Solution: CSIRO has developed advanced task-specific ionic liquids (TSILs) for CO2 capture featuring novel molecular architectures that significantly enhance absorption kinetics while maintaining high capacity. Their proprietary technology incorporates strategically designed functional groups that create multiple binding sites for CO2, resulting in absorption rates up to 3-4 times faster than conventional ionic liquids[7]. CSIRO's approach includes innovative synthesis methods that reduce production costs by approximately 35% compared to traditional routes. Their research demonstrates successful implementation of these materials in both conventional absorption columns and advanced membrane contactors, with specialized designs that optimize the interfacial area between gas and liquid phases. The organization has developed comprehensive mathematical models that accurately predict absorption kinetics across various operating conditions, enabling precise process optimization. CSIRO's technology includes proprietary regeneration protocols that maintain TSIL performance over hundreds of cycles with minimal degradation in kinetic performance. Their systems have been successfully tested with actual industrial gas streams, demonstrating robust performance even in the presence of common contaminants like SOx and NOx[8].
Strengths: Exceptional stability in the presence of common flue gas contaminants; versatile application across different contactor technologies; comprehensive kinetic modeling enabling precise process optimization. Weaknesses: Some formulations require specialized handling due to moisture sensitivity; higher viscosity compared to conventional amine solutions necessitating modified equipment designs; moderate energy requirements for regeneration.
Key Patents and Research Breakthroughs
Ionic liquids
PatentInactiveUS20120186993A1
Innovation
- The use of an ionic liquid absorbent containing specific anions, metal species, and optionally organic cations, which are in a liquid state at operating conditions, allowing for chemical absorption of gases like CO2, H2S, SOx, NOx, and CO through interaction with the metal species, enhancing absorption capacity and stability.
Ionic liquids
PatentInactiveEP2459300A1
Innovation
- The use of ionic liquid absorbents containing specific anions and metal species, which interact with CO2 to enhance absorption capacity through a chemical absorption mechanism, and include organic cations and ligands to modify the electronic environment of the metal species, thereby stabilizing gas interactions during absorption and destabilizing them during desorption, reducing energy input for desorption.
Environmental Impact Assessment
The deployment of task-specific ionic liquids (TSILs) for CO2 capture presents significant environmental implications that warrant comprehensive assessment. These novel materials offer promising alternatives to conventional amine-based solvents, potentially reducing the ecological footprint of carbon capture processes. When evaluating TSILs from an environmental perspective, their complete lifecycle must be considered, from synthesis to disposal or regeneration.
Primary environmental benefits of TSILs include their negligible vapor pressure, which virtually eliminates volatile organic compound (VOC) emissions—a major environmental concern with traditional capture methods. Additionally, the high thermal stability of TSILs reduces degradation during regeneration cycles, minimizing waste generation and extending operational lifespans. Studies indicate that properly designed TSILs can reduce the energy penalty associated with CO2 capture by 15-30% compared to monoethanolamine (MEA) systems, translating to lower indirect emissions from power consumption.
However, several environmental challenges remain unresolved. The synthesis of TSILs often involves multiple reaction steps and purification processes that may utilize hazardous chemicals and generate substantial waste streams. Life Cycle Assessment (LCA) studies suggest that the environmental benefits of TSILs during operation must be weighed against potential impacts during production. Furthermore, the biodegradability of many TSILs remains questionable, raising concerns about their long-term environmental persistence if released.
Water consumption represents another critical environmental factor. While TSILs generally demonstrate lower water requirements than aqueous amine solutions, certain hydrophilic ionic liquids may introduce water contamination issues. Recent research indicates that functionalized TSILs with hydrophobic anions can significantly reduce water uptake, thereby minimizing treatment requirements and associated environmental impacts.
Toxicological profiles of TSILs vary considerably depending on their specific chemical structures. Ecotoxicity studies reveal that some TSILs exhibit aquatic toxicity, particularly those containing longer alkyl chains or certain functional groups. Emerging research focuses on developing environmentally benign TSILs derived from bio-based precursors, which demonstrate reduced ecotoxicity while maintaining CO2 absorption performance.
The scalability of TSIL production presents additional environmental considerations. Current synthesis methods often involve energy-intensive processes and specialized reagents, potentially limiting their net environmental benefit at industrial scales. Advances in green chemistry approaches, including solvent-free synthesis and continuous flow processing, show promise for reducing the environmental footprint of TSIL manufacturing while enabling economically viable large-scale production.
Primary environmental benefits of TSILs include their negligible vapor pressure, which virtually eliminates volatile organic compound (VOC) emissions—a major environmental concern with traditional capture methods. Additionally, the high thermal stability of TSILs reduces degradation during regeneration cycles, minimizing waste generation and extending operational lifespans. Studies indicate that properly designed TSILs can reduce the energy penalty associated with CO2 capture by 15-30% compared to monoethanolamine (MEA) systems, translating to lower indirect emissions from power consumption.
However, several environmental challenges remain unresolved. The synthesis of TSILs often involves multiple reaction steps and purification processes that may utilize hazardous chemicals and generate substantial waste streams. Life Cycle Assessment (LCA) studies suggest that the environmental benefits of TSILs during operation must be weighed against potential impacts during production. Furthermore, the biodegradability of many TSILs remains questionable, raising concerns about their long-term environmental persistence if released.
Water consumption represents another critical environmental factor. While TSILs generally demonstrate lower water requirements than aqueous amine solutions, certain hydrophilic ionic liquids may introduce water contamination issues. Recent research indicates that functionalized TSILs with hydrophobic anions can significantly reduce water uptake, thereby minimizing treatment requirements and associated environmental impacts.
Toxicological profiles of TSILs vary considerably depending on their specific chemical structures. Ecotoxicity studies reveal that some TSILs exhibit aquatic toxicity, particularly those containing longer alkyl chains or certain functional groups. Emerging research focuses on developing environmentally benign TSILs derived from bio-based precursors, which demonstrate reduced ecotoxicity while maintaining CO2 absorption performance.
The scalability of TSIL production presents additional environmental considerations. Current synthesis methods often involve energy-intensive processes and specialized reagents, potentially limiting their net environmental benefit at industrial scales. Advances in green chemistry approaches, including solvent-free synthesis and continuous flow processing, show promise for reducing the environmental footprint of TSIL manufacturing while enabling economically viable large-scale production.
Scalability and Industrial Implementation Strategies
The scaling of task-specific ionic liquids (TSILs) for CO2 capture from laboratory to industrial scale presents significant challenges that must be addressed systematically. Current industrial implementation primarily exists as pilot projects, with full-scale commercial deployment still under development. The viscosity of TSILs remains a critical barrier, as it increases substantially upon CO2 absorption, leading to mass transfer limitations and reduced absorption kinetics in large-scale operations.
To overcome these challenges, several engineering approaches have emerged. Process intensification techniques, including the development of supported ionic liquid membranes and microchannel reactors, have shown promise in enhancing mass transfer rates while minimizing the required TSIL volume. These approaches effectively increase the surface area-to-volume ratio, partially mitigating the viscosity-related limitations that become more pronounced at industrial scales.
Economic feasibility represents another crucial consideration for industrial implementation. The synthesis costs of TSILs currently exceed those of conventional solvents by a significant margin, with specialized functional groups further increasing production expenses. A comprehensive techno-economic analysis conducted by the National Energy Technology Laboratory suggests that cost reduction pathways must focus on extending TSIL lifetime, improving regeneration efficiency, and developing more economical synthesis routes.
Manufacturing scalability requires standardized production protocols that maintain consistent TSIL quality across large production volumes. Recent advances in continuous flow chemistry have demonstrated potential for more efficient TSIL synthesis at scale, reducing batch-to-batch variations and lowering production costs. Several chemical manufacturers have begun establishing dedicated production lines for TSILs, though capacity remains limited compared to conventional industrial solvents.
Integration with existing carbon capture infrastructure presents both challenges and opportunities. Retrofitting conventional absorption columns requires modifications to accommodate the unique properties of TSILs, including specialized materials to prevent corrosion and redesigned internals to manage higher viscosity fluids. Hybrid systems combining TSILs with conventional solvents have emerged as a transitional approach, allowing gradual implementation while leveraging existing infrastructure.
Regulatory frameworks and standardization efforts are developing to support industrial adoption. Organizations including the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM) are working to establish standard testing protocols and performance metrics specifically for TSIL-based carbon capture systems, which will facilitate broader commercial acceptance and implementation.
To overcome these challenges, several engineering approaches have emerged. Process intensification techniques, including the development of supported ionic liquid membranes and microchannel reactors, have shown promise in enhancing mass transfer rates while minimizing the required TSIL volume. These approaches effectively increase the surface area-to-volume ratio, partially mitigating the viscosity-related limitations that become more pronounced at industrial scales.
Economic feasibility represents another crucial consideration for industrial implementation. The synthesis costs of TSILs currently exceed those of conventional solvents by a significant margin, with specialized functional groups further increasing production expenses. A comprehensive techno-economic analysis conducted by the National Energy Technology Laboratory suggests that cost reduction pathways must focus on extending TSIL lifetime, improving regeneration efficiency, and developing more economical synthesis routes.
Manufacturing scalability requires standardized production protocols that maintain consistent TSIL quality across large production volumes. Recent advances in continuous flow chemistry have demonstrated potential for more efficient TSIL synthesis at scale, reducing batch-to-batch variations and lowering production costs. Several chemical manufacturers have begun establishing dedicated production lines for TSILs, though capacity remains limited compared to conventional industrial solvents.
Integration with existing carbon capture infrastructure presents both challenges and opportunities. Retrofitting conventional absorption columns requires modifications to accommodate the unique properties of TSILs, including specialized materials to prevent corrosion and redesigned internals to manage higher viscosity fluids. Hybrid systems combining TSILs with conventional solvents have emerged as a transitional approach, allowing gradual implementation while leveraging existing infrastructure.
Regulatory frameworks and standardization efforts are developing to support industrial adoption. Organizations including the International Organization for Standardization (ISO) and the American Society for Testing and Materials (ASTM) are working to establish standard testing protocols and performance metrics specifically for TSIL-based carbon capture systems, which will facilitate broader commercial acceptance and implementation.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!