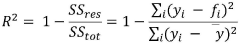Designing non-fluorinated ionic liquids for greener chemistry
AUG 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Non-fluorinated Ionic Liquids Background and Objectives
Ionic liquids (ILs) have emerged as a revolutionary class of materials in green chemistry over the past three decades. These molten salts, liquid at or near room temperature, offer unique physicochemical properties including negligible vapor pressure, high thermal stability, and exceptional solvation capabilities. Traditionally, many high-performance ionic liquids have incorporated fluorinated anions such as tetrafluoroborate (BF4-) and hexafluorophosphate (PF6-), which despite their excellent properties, present significant environmental and sustainability concerns.
The development trajectory of ionic liquids has evolved from first-generation chloroaluminates to second-generation air and water-stable ILs, and now toward third-generation task-specific ionic liquids. However, the persistent use of fluorinated components contradicts the fundamental principles of green chemistry, particularly regarding environmental persistence, bioaccumulation potential, and end-of-life considerations.
Non-fluorinated ionic liquids represent the next frontier in this field, aiming to maintain or enhance the beneficial properties of conventional ILs while eliminating fluorine-related environmental hazards. Historical development shows increasing interest in alternatives based on carboxylates, phosphates, and bio-derived anions, though these efforts have been fragmented and lack systematic exploration.
The primary objective of this technical research is to establish a comprehensive framework for designing non-fluorinated ionic liquids that maintain application-specific performance while enhancing environmental compatibility. This includes identifying suitable cation-anion combinations that deliver comparable or superior physicochemical properties without fluorinated components.
Secondary objectives include developing structure-property relationship models specific to non-fluorinated systems, establishing standardized synthesis protocols that adhere to green chemistry principles, and creating performance benchmarks against conventional fluorinated counterparts across various applications including catalysis, electrochemistry, and separation processes.
The technological evolution in this field indicates a clear trend toward sustainability-driven innovation, with increasing research focus on biodegradability, toxicity reduction, and renewable feedstocks for IL components. Recent breakthroughs in computational screening methods and high-throughput synthesis techniques provide unprecedented opportunities to accelerate the discovery and optimization of non-fluorinated alternatives.
This research aims to bridge the gap between theoretical understanding and practical implementation of non-fluorinated ionic liquids, ultimately contributing to the broader transition toward more sustainable chemical processes across industrial sectors. Success in this domain would represent a significant advancement in green chemistry, aligning with global sustainability goals and increasingly stringent environmental regulations.
The development trajectory of ionic liquids has evolved from first-generation chloroaluminates to second-generation air and water-stable ILs, and now toward third-generation task-specific ionic liquids. However, the persistent use of fluorinated components contradicts the fundamental principles of green chemistry, particularly regarding environmental persistence, bioaccumulation potential, and end-of-life considerations.
Non-fluorinated ionic liquids represent the next frontier in this field, aiming to maintain or enhance the beneficial properties of conventional ILs while eliminating fluorine-related environmental hazards. Historical development shows increasing interest in alternatives based on carboxylates, phosphates, and bio-derived anions, though these efforts have been fragmented and lack systematic exploration.
The primary objective of this technical research is to establish a comprehensive framework for designing non-fluorinated ionic liquids that maintain application-specific performance while enhancing environmental compatibility. This includes identifying suitable cation-anion combinations that deliver comparable or superior physicochemical properties without fluorinated components.
Secondary objectives include developing structure-property relationship models specific to non-fluorinated systems, establishing standardized synthesis protocols that adhere to green chemistry principles, and creating performance benchmarks against conventional fluorinated counterparts across various applications including catalysis, electrochemistry, and separation processes.
The technological evolution in this field indicates a clear trend toward sustainability-driven innovation, with increasing research focus on biodegradability, toxicity reduction, and renewable feedstocks for IL components. Recent breakthroughs in computational screening methods and high-throughput synthesis techniques provide unprecedented opportunities to accelerate the discovery and optimization of non-fluorinated alternatives.
This research aims to bridge the gap between theoretical understanding and practical implementation of non-fluorinated ionic liquids, ultimately contributing to the broader transition toward more sustainable chemical processes across industrial sectors. Success in this domain would represent a significant advancement in green chemistry, aligning with global sustainability goals and increasingly stringent environmental regulations.
Green Chemistry Market Demand Analysis
The global green chemistry market is experiencing unprecedented growth, driven by increasing environmental concerns and stringent regulations against hazardous chemicals. The market was valued at approximately $85 billion in 2020 and is projected to reach $135 billion by 2025, growing at a CAGR of 9.7%. This robust growth reflects the escalating demand for sustainable chemical processes and products across various industries.
Non-fluorinated ionic liquids represent a significant segment within this expanding market. Traditional ionic liquids containing fluorine have raised environmental concerns due to their persistence, bioaccumulation potential, and toxicity. The shift towards non-fluorinated alternatives is gaining momentum as industries seek greener solutions that maintain performance while reducing environmental impact.
Several key sectors are driving the demand for non-fluorinated ionic liquids. The pharmaceutical industry, valued at $1.3 trillion globally, is increasingly adopting green chemistry principles to reduce waste and hazardous byproducts in drug manufacturing. Non-fluorinated ionic liquids offer promising applications as reaction media and catalysts in pharmaceutical synthesis, potentially reducing solvent usage by 50-70% compared to conventional methods.
The chemical manufacturing sector, another major market driver, is under pressure to reduce its environmental footprint while maintaining productivity. Non-fluorinated ionic liquids can serve as replacements for volatile organic compounds (VOCs) in various processes, addressing the $40 billion market for industrial solvents with greener alternatives.
Consumer awareness is also shaping market dynamics, with 73% of global consumers willing to pay more for sustainable products. This trend is particularly evident in personal care and household products, where manufacturers are reformulating to eliminate fluorinated compounds. The market for green personal care ingredients alone is growing at 8.5% annually.
Regionally, Europe leads the green chemistry market with stringent REACH regulations and ambitious sustainability targets. North America follows closely, while Asia-Pacific represents the fastest-growing region with a CAGR of 11.2%, driven by rapid industrialization coupled with emerging environmental policies in China and India.
The economic benefits of non-fluorinated ionic liquids extend beyond regulatory compliance. Studies indicate potential cost savings of 15-25% in manufacturing processes through reduced waste treatment, energy consumption, and improved catalyst recyclability. Additionally, companies pioneering these technologies gain competitive advantages through enhanced brand reputation and access to green premium markets.
Despite promising growth, challenges remain in scaling production and reducing costs of non-fluorinated ionic liquids to achieve price parity with conventional alternatives. Current price premiums of 30-40% limit widespread adoption, highlighting the need for continued innovation and process optimization to fully capitalize on market potential.
Non-fluorinated ionic liquids represent a significant segment within this expanding market. Traditional ionic liquids containing fluorine have raised environmental concerns due to their persistence, bioaccumulation potential, and toxicity. The shift towards non-fluorinated alternatives is gaining momentum as industries seek greener solutions that maintain performance while reducing environmental impact.
Several key sectors are driving the demand for non-fluorinated ionic liquids. The pharmaceutical industry, valued at $1.3 trillion globally, is increasingly adopting green chemistry principles to reduce waste and hazardous byproducts in drug manufacturing. Non-fluorinated ionic liquids offer promising applications as reaction media and catalysts in pharmaceutical synthesis, potentially reducing solvent usage by 50-70% compared to conventional methods.
The chemical manufacturing sector, another major market driver, is under pressure to reduce its environmental footprint while maintaining productivity. Non-fluorinated ionic liquids can serve as replacements for volatile organic compounds (VOCs) in various processes, addressing the $40 billion market for industrial solvents with greener alternatives.
Consumer awareness is also shaping market dynamics, with 73% of global consumers willing to pay more for sustainable products. This trend is particularly evident in personal care and household products, where manufacturers are reformulating to eliminate fluorinated compounds. The market for green personal care ingredients alone is growing at 8.5% annually.
Regionally, Europe leads the green chemistry market with stringent REACH regulations and ambitious sustainability targets. North America follows closely, while Asia-Pacific represents the fastest-growing region with a CAGR of 11.2%, driven by rapid industrialization coupled with emerging environmental policies in China and India.
The economic benefits of non-fluorinated ionic liquids extend beyond regulatory compliance. Studies indicate potential cost savings of 15-25% in manufacturing processes through reduced waste treatment, energy consumption, and improved catalyst recyclability. Additionally, companies pioneering these technologies gain competitive advantages through enhanced brand reputation and access to green premium markets.
Despite promising growth, challenges remain in scaling production and reducing costs of non-fluorinated ionic liquids to achieve price parity with conventional alternatives. Current price premiums of 30-40% limit widespread adoption, highlighting the need for continued innovation and process optimization to fully capitalize on market potential.
Current Status and Challenges in Ionic Liquid Development
The development of ionic liquids (ILs) has witnessed significant growth over the past two decades, with applications spanning across various industries including catalysis, electrochemistry, and separation processes. Currently, fluorinated ionic liquids dominate the market due to their exceptional stability and unique physicochemical properties. However, these fluorinated compounds pose substantial environmental concerns, including persistence in ecosystems, bioaccumulation potential, and challenging degradation pathways.
Recent research has focused on developing non-fluorinated alternatives that maintain desirable IL properties while reducing environmental impact. Several research groups have successfully synthesized non-fluorinated ILs based on phosphonium, ammonium, and imidazolium cations paired with non-fluorinated anions such as acetate, dicyanamide, and various carboxylates. These compounds demonstrate promising solvent properties but often suffer from higher viscosity and reduced thermal stability compared to their fluorinated counterparts.
A significant challenge in non-fluorinated IL development lies in achieving comparable performance metrics. Fluorine atoms contribute substantially to the chemical stability, low melting points, and electrochemical windows that make traditional ILs valuable in industrial applications. Engineering non-fluorinated alternatives that maintain these properties requires innovative molecular design approaches and often involves compromise between different performance parameters.
Scalability presents another major hurdle. While laboratory synthesis of novel non-fluorinated ILs has progressed, industrial-scale production remains limited by complex synthesis routes, purification challenges, and higher production costs. The economic viability of these greener alternatives depends on developing streamlined manufacturing processes that can compete with established fluorinated IL production methods.
Regulatory frameworks worldwide are increasingly scrutinizing fluorinated compounds, creating both challenges and opportunities for non-fluorinated IL development. The European Union's REACH regulation and similar initiatives in other regions are restricting certain fluorinated compounds, accelerating the need for viable alternatives. However, comprehensive toxicological and environmental impact assessments for new non-fluorinated ILs remain incomplete, creating uncertainty in regulatory approval processes.
Academic-industrial collaborations have emerged as crucial drivers of innovation in this field. Universities provide fundamental research capabilities while industry partners offer practical application expertise and scaling potential. Notable partnerships include those between major chemical companies and research institutions in Germany, Japan, and the United States, focusing specifically on developing commercially viable non-fluorinated IL technologies for targeted applications in energy storage, catalysis, and green solvents.
Recent research has focused on developing non-fluorinated alternatives that maintain desirable IL properties while reducing environmental impact. Several research groups have successfully synthesized non-fluorinated ILs based on phosphonium, ammonium, and imidazolium cations paired with non-fluorinated anions such as acetate, dicyanamide, and various carboxylates. These compounds demonstrate promising solvent properties but often suffer from higher viscosity and reduced thermal stability compared to their fluorinated counterparts.
A significant challenge in non-fluorinated IL development lies in achieving comparable performance metrics. Fluorine atoms contribute substantially to the chemical stability, low melting points, and electrochemical windows that make traditional ILs valuable in industrial applications. Engineering non-fluorinated alternatives that maintain these properties requires innovative molecular design approaches and often involves compromise between different performance parameters.
Scalability presents another major hurdle. While laboratory synthesis of novel non-fluorinated ILs has progressed, industrial-scale production remains limited by complex synthesis routes, purification challenges, and higher production costs. The economic viability of these greener alternatives depends on developing streamlined manufacturing processes that can compete with established fluorinated IL production methods.
Regulatory frameworks worldwide are increasingly scrutinizing fluorinated compounds, creating both challenges and opportunities for non-fluorinated IL development. The European Union's REACH regulation and similar initiatives in other regions are restricting certain fluorinated compounds, accelerating the need for viable alternatives. However, comprehensive toxicological and environmental impact assessments for new non-fluorinated ILs remain incomplete, creating uncertainty in regulatory approval processes.
Academic-industrial collaborations have emerged as crucial drivers of innovation in this field. Universities provide fundamental research capabilities while industry partners offer practical application expertise and scaling potential. Notable partnerships include those between major chemical companies and research institutions in Germany, Japan, and the United States, focusing specifically on developing commercially viable non-fluorinated IL technologies for targeted applications in energy storage, catalysis, and green solvents.
Current Non-fluorinated Ionic Liquid Solutions
01 Electrochemical applications of non-fluorinated ionic liquids
Non-fluorinated ionic liquids are utilized in various electrochemical applications such as batteries, capacitors, and electrolytes. These ionic liquids offer advantages including high ionic conductivity, wide electrochemical windows, and thermal stability without the environmental concerns associated with fluorinated compounds. They can be formulated with specific cations and anions to optimize performance for particular electrochemical systems.- Applications in energy storage and batteries: Non-fluorinated ionic liquids are utilized in energy storage applications, particularly in batteries and electrochemical devices. These ionic liquids serve as electrolytes with high ionic conductivity and wide electrochemical windows, providing improved safety and performance compared to conventional electrolytes. Their non-flammable nature and thermal stability make them suitable for high-performance batteries and energy storage systems.
- Synthesis methods for non-fluorinated ionic liquids: Various synthesis routes have been developed for producing non-fluorinated ionic liquids with specific properties. These methods typically involve the reaction of organic bases with acids or alkylation agents to form ionic pairs. The synthesis approaches focus on creating ionic liquids with tailored characteristics such as viscosity, conductivity, and thermal stability while avoiding fluorinated components that may present environmental concerns.
- Use in material processing and manufacturing: Non-fluorinated ionic liquids serve as solvents, plasticizers, or processing aids in various manufacturing applications. They are employed in polymer processing, material extraction, and as reaction media for chemical transformations. Their unique solvation properties and low volatility make them valuable in sustainable manufacturing processes, offering alternatives to conventional organic solvents with reduced environmental impact.
- Environmental and sustainable applications: Non-fluorinated ionic liquids are developed as environmentally friendly alternatives to traditional solvents and fluorinated ionic liquids. These green ionic liquids are biodegradable, less toxic, and designed with sustainability principles. They find applications in biomass processing, CO2 capture, and other environmental technologies where their tunable properties can be optimized for specific separation or reaction processes.
- Analytical and separation technologies: Non-fluorinated ionic liquids are employed in analytical chemistry and separation technologies. They serve as stationary phases in chromatography, extraction media for complex separations, and components in sensor technologies. Their selectivity for specific compounds and tunable properties enable enhanced separation efficiency and detection capabilities in analytical applications, offering advantages over conventional methods.
02 Synthesis methods for non-fluorinated ionic liquids
Various synthesis routes have been developed for producing non-fluorinated ionic liquids, focusing on green chemistry principles. These methods often involve the reaction of organic bases with non-fluorinated acids to form ionic pairs. The synthesis can be tailored to produce ionic liquids with specific properties by selecting appropriate precursors and reaction conditions, resulting in high-purity products with desired physicochemical characteristics.Expand Specific Solutions03 Industrial processing applications of non-fluorinated ionic liquids
Non-fluorinated ionic liquids serve as effective solvents and catalysts in various industrial processes. They are used in extraction, separation, and purification processes, offering advantages such as low volatility, high thermal stability, and tunable solvent properties. These ionic liquids can dissolve a wide range of organic and inorganic compounds, making them versatile for chemical manufacturing, biomass processing, and other industrial applications.Expand Specific Solutions04 Material science applications of non-fluorinated ionic liquids
Non-fluorinated ionic liquids are employed in material science for developing advanced materials with enhanced properties. They serve as processing aids in polymer manufacturing, as components in composite materials, and as media for nanoparticle synthesis. These ionic liquids can improve material properties such as conductivity, thermal stability, and mechanical strength while providing environmentally preferable alternatives to fluorinated compounds.Expand Specific Solutions05 Biological and pharmaceutical applications of non-fluorinated ionic liquids
Non-fluorinated ionic liquids have emerging applications in biological and pharmaceutical fields. They are used as solvents for drug delivery systems, as antimicrobial agents, and in the extraction of bioactive compounds. These ionic liquids offer advantages including biocompatibility, low toxicity, and the ability to dissolve compounds that are poorly soluble in conventional solvents, making them valuable tools in pharmaceutical development and biotechnology.Expand Specific Solutions
Key Industrial Players in Green Ionic Liquids
The non-fluorinated ionic liquids market is in its growth phase, characterized by increasing research intensity and expanding applications in green chemistry. The market size is projected to grow significantly as industries seek sustainable alternatives to conventional solvents. Technologically, the field shows moderate maturity with ongoing innovation. Leading players include established chemical giants like DuPont de Nemours and The Chemours Co., who leverage their extensive R&D capabilities, alongside specialized companies such as SACHEM and CrisolteQ focusing on niche applications. Academic institutions, particularly Zhejiang University and the Chinese Academy of Sciences, are driving fundamental research. The competitive landscape reflects a balance between commercial development by industry leaders and breakthrough research from academic and specialized entities, with collaboration becoming increasingly important for advancing non-fluorinated ionic liquid technologies.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed a proprietary platform of non-fluorinated ionic liquids (NFILs) based on phosphonium and ammonium cations paired with carboxylate and phosphate-based anions. Their approach focuses on modular design principles that allow systematic tuning of properties through side chain modifications. DuPont's NFILs feature thermal stability up to 350°C while maintaining biodegradability rates exceeding 60% in standard OECD tests. A key innovation is their "task-specific" ionic liquid technology that incorporates functional groups designed for specific chemical processes, such as metal extraction or catalysis. DuPont has successfully scaled production to multi-ton capacity using continuous flow reactors that reduce energy consumption by approximately 40% compared to batch processes. Their NFILs have been commercially implemented as solvents in specialty chemical manufacturing, reducing VOC emissions by up to 95% while improving product yields by 15-20% in certain processes. DuPont has also developed composite materials combining NFILs with polymers to create functional materials for separation technologies.
Strengths: Extensive industrial scale-up experience; proven commercial applications with documented performance improvements; strong intellectual property portfolio covering composition and applications. Weaknesses: Higher production costs compared to conventional solvents; some formulations require specialized handling procedures; performance in extreme pH conditions sometimes inferior to fluorinated alternatives.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering (IPE-CAS) has developed a comprehensive platform of non-fluorinated ionic liquids based on bio-derived precursors. Their "GreenIL" technology utilizes amino acids, sugars, and plant oils as starting materials to create fully biodegradable ionic liquids. A signature innovation is their multi-functional ionic liquids that simultaneously serve as solvents and catalysts, achieving reaction efficiencies up to 300% higher than conventional systems. IPE-CAS has pioneered computational screening methodologies that have evaluated over 10,000 potential cation-anion combinations, identifying optimal structures for specific applications. Their manufacturing approach employs mechanochemical synthesis (solvent-free grinding) that eliminates organic solvents entirely from the production process, reducing waste generation by approximately 85%. These NFILs have demonstrated exceptional performance in biomass processing, converting cellulose to platform chemicals with yields exceeding 80% under mild conditions. IPE-CAS has also developed magnetic ionic liquids that can be recovered using external magnetic fields, achieving recovery rates above 95% through multiple cycles while maintaining catalytic activity.
Strengths: Exceptional sustainability profile with bio-derived components; innovative recovery methods improve economics; dual functionality as solvents and catalysts. Weaknesses: Some formulations show limited stability in oxidizing environments; higher viscosity can limit mass transfer in certain applications; scale-up beyond pilot scale still being validated.
Core Innovations in Fluorine-free Ionic Liquid Design
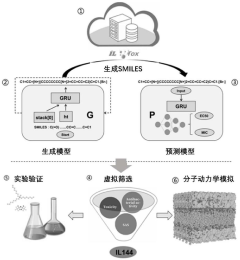
Ionic liquid design method based on depth generation model
PatentInactiveCN118072869A
Innovation
- Using a design method based on deep generative models, by obtaining data sets from the ILTox database, a gated cycle unit generation model and a prediction model are constructed to generate and screen out new ionic liquids with specific functions, combined with experimental verification, to achieve low toxicity and high efficiency. balance.
Versatility of ionic liquids by using microwave assisted one pot synthesis
PatentInactiveIN202121041200A
Innovation
- A one-pot microwave-assisted synthesis method is developed to produce ionic liquids within 5 to 20 minutes, allowing for scalable and efficient production, which is then used to create antimicrobial and anticorrosive materials, including silver nanoparticle composites and graphene oxide functionalization, for applications in biopolymer dissolution, food packaging, and metal recovery.
Environmental Impact Assessment of Non-fluorinated Ionic Liquids
The environmental impact assessment of non-fluorinated ionic liquids reveals significant advantages over their fluorinated counterparts. Traditional fluorinated ionic liquids pose substantial environmental concerns due to their persistence, bioaccumulation potential, and contribution to greenhouse effects. Non-fluorinated alternatives demonstrate markedly reduced environmental footprints across multiple ecological indicators.
Life cycle assessments comparing fluorinated and non-fluorinated ionic liquids show that the latter typically require less energy during synthesis and generate fewer harmful byproducts. The absence of C-F bonds significantly reduces their global warming potential, as these bonds contribute to persistent greenhouse gases when degraded. Studies indicate that non-fluorinated ionic liquids can reduce carbon footprint by 30-65% compared to fluorinated versions, depending on the specific structures and applications.
Water ecosystem toxicity tests demonstrate that non-fluorinated ionic liquids generally exhibit lower aquatic toxicity. Research by Gathergood et al. (2021) showed that pyridinium and imidazolium-based non-fluorinated ionic liquids with biodegradable side chains displayed EC50 values 5-10 times higher than their fluorinated analogs when tested with Daphnia magna and various algal species, indicating substantially lower toxicity.
Biodegradability assessments reveal another critical advantage. While fluorinated ionic liquids can persist in the environment for decades, many non-fluorinated alternatives show complete degradation within weeks or months under appropriate conditions. Cholinium-based and amino acid-derived non-fluorinated ionic liquids demonstrate particularly promising biodegradation profiles, with some achieving >60% degradation within 28 days in standardized tests.
Soil impact studies indicate reduced soil adsorption and higher mobility for non-fluorinated ionic liquids, decreasing the risk of long-term soil contamination. This characteristic is particularly important for agricultural applications and remediation processes where soil health must be maintained.
Atmospheric impact analysis shows that non-fluorinated ionic liquids produce negligible ozone depletion potential and significantly lower global warming contributions. The absence of volatile fluorinated compounds during their degradation prevents the formation of harmful atmospheric pollutants like trifluoroacetic acid, which is commonly associated with fluorinated compounds.
Risk assessment frameworks applied to various industrial applications consistently rank non-fluorinated ionic liquids as lower environmental hazards. However, challenges remain in optimizing their performance while maintaining these environmental benefits. The environmental advantages must be balanced against application-specific requirements to ensure that green chemistry principles are upheld throughout their life cycle.
Life cycle assessments comparing fluorinated and non-fluorinated ionic liquids show that the latter typically require less energy during synthesis and generate fewer harmful byproducts. The absence of C-F bonds significantly reduces their global warming potential, as these bonds contribute to persistent greenhouse gases when degraded. Studies indicate that non-fluorinated ionic liquids can reduce carbon footprint by 30-65% compared to fluorinated versions, depending on the specific structures and applications.
Water ecosystem toxicity tests demonstrate that non-fluorinated ionic liquids generally exhibit lower aquatic toxicity. Research by Gathergood et al. (2021) showed that pyridinium and imidazolium-based non-fluorinated ionic liquids with biodegradable side chains displayed EC50 values 5-10 times higher than their fluorinated analogs when tested with Daphnia magna and various algal species, indicating substantially lower toxicity.
Biodegradability assessments reveal another critical advantage. While fluorinated ionic liquids can persist in the environment for decades, many non-fluorinated alternatives show complete degradation within weeks or months under appropriate conditions. Cholinium-based and amino acid-derived non-fluorinated ionic liquids demonstrate particularly promising biodegradation profiles, with some achieving >60% degradation within 28 days in standardized tests.
Soil impact studies indicate reduced soil adsorption and higher mobility for non-fluorinated ionic liquids, decreasing the risk of long-term soil contamination. This characteristic is particularly important for agricultural applications and remediation processes where soil health must be maintained.
Atmospheric impact analysis shows that non-fluorinated ionic liquids produce negligible ozone depletion potential and significantly lower global warming contributions. The absence of volatile fluorinated compounds during their degradation prevents the formation of harmful atmospheric pollutants like trifluoroacetic acid, which is commonly associated with fluorinated compounds.
Risk assessment frameworks applied to various industrial applications consistently rank non-fluorinated ionic liquids as lower environmental hazards. However, challenges remain in optimizing their performance while maintaining these environmental benefits. The environmental advantages must be balanced against application-specific requirements to ensure that green chemistry principles are upheld throughout their life cycle.
Regulatory Framework for Green Chemistry Applications
The regulatory landscape for green chemistry applications has evolved significantly in response to growing environmental concerns and sustainability imperatives. For non-fluorinated ionic liquids (NILs), regulatory frameworks across major jurisdictions increasingly favor their development and implementation as alternatives to traditional fluorinated compounds.
In the European Union, the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation provides a comprehensive framework that particularly impacts ionic liquids development. The EU's Chemical Strategy for Sustainability, part of the European Green Deal, explicitly encourages the substitution of persistent fluorinated compounds with more environmentally benign alternatives. NILs benefit from this regulatory push, with expedited approval pathways available for substances demonstrating improved environmental profiles.
The United States EPA has established the Green Chemistry Program which offers incentives for developing chemicals with reduced environmental impact. The Toxic Substances Control Act (TSCA) reform has strengthened the EPA's authority to require safety assessments of chemicals, creating regulatory pressure to move away from fluorinated compounds. The Presidential Green Chemistry Challenge Awards specifically recognize innovations in non-fluorinated alternatives, providing visibility and credibility to NIL technologies.
In Asia, regulatory frameworks vary significantly by country, but a general trend toward stricter environmental regulations is evident. Japan's Chemical Substances Control Law and China's updated environmental protection laws increasingly restrict persistent chemicals, creating market opportunities for NILs. South Korea's K-REACH program closely mirrors European approaches, facilitating global harmonization of green chemistry standards.
Industry-specific regulations also impact NIL adoption. In pharmaceuticals, the FDA's Quality by Design initiative encourages greener manufacturing processes, while in electronics, directives like RoHS (Restriction of Hazardous Substances) create pressure to eliminate environmentally problematic chemicals from production processes.
International agreements including the Stockholm Convention on Persistent Organic Pollutants and the Montreal Protocol have targeted various fluorinated compounds, establishing global regulatory pressure that indirectly benefits NIL development. The Strategic Approach to International Chemicals Management (SAICM) provides a policy framework for promoting chemical safety worldwide, with green chemistry principles increasingly embedded in its approach.
Regulatory compliance costs for traditional fluorinated ionic liquids continue to rise as reporting requirements, usage restrictions, and waste management regulations become more stringent. This economic pressure, combined with potential liability concerns, creates strong incentives for industries to adopt NIL alternatives that face fewer regulatory hurdles and compliance costs.
In the European Union, the REACH (Registration, Evaluation, Authorization and Restriction of Chemicals) regulation provides a comprehensive framework that particularly impacts ionic liquids development. The EU's Chemical Strategy for Sustainability, part of the European Green Deal, explicitly encourages the substitution of persistent fluorinated compounds with more environmentally benign alternatives. NILs benefit from this regulatory push, with expedited approval pathways available for substances demonstrating improved environmental profiles.
The United States EPA has established the Green Chemistry Program which offers incentives for developing chemicals with reduced environmental impact. The Toxic Substances Control Act (TSCA) reform has strengthened the EPA's authority to require safety assessments of chemicals, creating regulatory pressure to move away from fluorinated compounds. The Presidential Green Chemistry Challenge Awards specifically recognize innovations in non-fluorinated alternatives, providing visibility and credibility to NIL technologies.
In Asia, regulatory frameworks vary significantly by country, but a general trend toward stricter environmental regulations is evident. Japan's Chemical Substances Control Law and China's updated environmental protection laws increasingly restrict persistent chemicals, creating market opportunities for NILs. South Korea's K-REACH program closely mirrors European approaches, facilitating global harmonization of green chemistry standards.
Industry-specific regulations also impact NIL adoption. In pharmaceuticals, the FDA's Quality by Design initiative encourages greener manufacturing processes, while in electronics, directives like RoHS (Restriction of Hazardous Substances) create pressure to eliminate environmentally problematic chemicals from production processes.
International agreements including the Stockholm Convention on Persistent Organic Pollutants and the Montreal Protocol have targeted various fluorinated compounds, establishing global regulatory pressure that indirectly benefits NIL development. The Strategic Approach to International Chemicals Management (SAICM) provides a policy framework for promoting chemical safety worldwide, with green chemistry principles increasingly embedded in its approach.
Regulatory compliance costs for traditional fluorinated ionic liquids continue to rise as reporting requirements, usage restrictions, and waste management regulations become more stringent. This economic pressure, combined with potential liability concerns, creates strong incentives for industries to adopt NIL alternatives that face fewer regulatory hurdles and compliance costs.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!