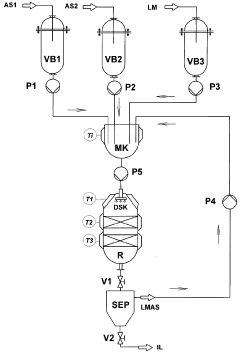
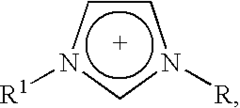
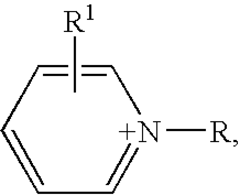
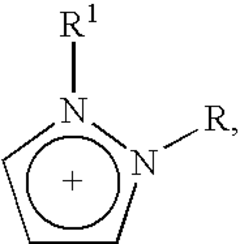
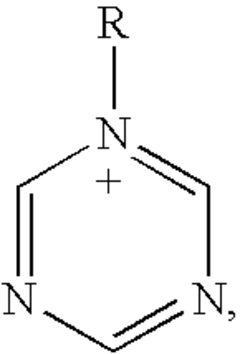
Electrocatalysis in ionic liquid media: selectivity controls
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Liquid Electrocatalysis Background and Objectives
Electrocatalysis in ionic liquids (ILs) represents a significant evolution in the field of electrochemistry, emerging from traditional aqueous and organic solvent systems to offer unprecedented control over reaction selectivity. The historical development of this technology can be traced back to the early 2000s when researchers began exploring ILs as alternative media for electrochemical processes, driven by their unique physicochemical properties including negligible vapor pressure, wide electrochemical windows, and high thermal stability.
The evolution of IL-based electrocatalysis has been marked by several key milestones, including the discovery of enhanced reaction rates for certain electrochemical transformations, improved catalyst stability, and novel reaction pathways unavailable in conventional media. Particularly noteworthy is the progression from simple proof-of-concept demonstrations to sophisticated systems capable of controlling reaction selectivity at the molecular level through careful tuning of the IL environment.
Current technological trends in this field are moving toward designer ILs with task-specific functionalities, integration with advanced catalyst materials such as metal-organic frameworks and nanostructured metals, and the development of computational models to predict IL-catalyst interactions. The convergence of these trends is creating new possibilities for selective electrochemical transformations with applications ranging from energy conversion to fine chemical synthesis.
The primary objective of research in IL electrocatalysis is to establish fundamental structure-property relationships that govern selectivity in electrochemical reactions. This includes understanding how the unique solvation environment provided by ILs influences reaction pathways, developing predictive models for rational IL selection, and creating design principles for IL-catalyst systems tailored to specific transformations.
Additional technical goals include expanding the temperature and potential windows for electrochemical processes, reducing the viscosity limitations of IL systems without compromising their beneficial properties, and developing recyclable IL-catalyst combinations that maintain performance over multiple cycles. These objectives align with broader sustainability goals by potentially enabling more energy-efficient processes with reduced waste generation.
The long-term vision for this technology encompasses the development of electrocatalytic systems capable of unprecedented selectivity control for challenging transformations such as CO2 reduction, nitrogen fixation, and selective C-H functionalization. Success in these areas would represent a paradigm shift in how we approach chemical manufacturing, potentially offering more sustainable alternatives to traditional thermochemical processes while opening new synthetic pathways previously considered impractical or impossible.
The evolution of IL-based electrocatalysis has been marked by several key milestones, including the discovery of enhanced reaction rates for certain electrochemical transformations, improved catalyst stability, and novel reaction pathways unavailable in conventional media. Particularly noteworthy is the progression from simple proof-of-concept demonstrations to sophisticated systems capable of controlling reaction selectivity at the molecular level through careful tuning of the IL environment.
Current technological trends in this field are moving toward designer ILs with task-specific functionalities, integration with advanced catalyst materials such as metal-organic frameworks and nanostructured metals, and the development of computational models to predict IL-catalyst interactions. The convergence of these trends is creating new possibilities for selective electrochemical transformations with applications ranging from energy conversion to fine chemical synthesis.
The primary objective of research in IL electrocatalysis is to establish fundamental structure-property relationships that govern selectivity in electrochemical reactions. This includes understanding how the unique solvation environment provided by ILs influences reaction pathways, developing predictive models for rational IL selection, and creating design principles for IL-catalyst systems tailored to specific transformations.
Additional technical goals include expanding the temperature and potential windows for electrochemical processes, reducing the viscosity limitations of IL systems without compromising their beneficial properties, and developing recyclable IL-catalyst combinations that maintain performance over multiple cycles. These objectives align with broader sustainability goals by potentially enabling more energy-efficient processes with reduced waste generation.
The long-term vision for this technology encompasses the development of electrocatalytic systems capable of unprecedented selectivity control for challenging transformations such as CO2 reduction, nitrogen fixation, and selective C-H functionalization. Success in these areas would represent a paradigm shift in how we approach chemical manufacturing, potentially offering more sustainable alternatives to traditional thermochemical processes while opening new synthetic pathways previously considered impractical or impossible.
Market Analysis for Selective Electrocatalytic Processes
The global market for selective electrocatalytic processes is experiencing robust growth, driven by increasing demand for sustainable chemical production methods and renewable energy solutions. The market size for electrocatalysis technologies was valued at approximately $5.2 billion in 2022 and is projected to reach $9.7 billion by 2028, representing a compound annual growth rate of 10.9%. Ionic liquid-based electrocatalysis represents a particularly promising segment within this market due to its enhanced selectivity capabilities.
Key industries driving demand include fine chemicals manufacturing, pharmaceutical production, and renewable energy sectors. The pharmaceutical industry, valued at over $1.4 trillion globally, increasingly seeks selective catalytic processes to reduce waste and improve efficiency in active pharmaceutical ingredient synthesis. Similarly, the fine chemicals market, worth approximately $85 billion, requires highly selective processes to maintain product purity and reduce downstream purification costs.
Regional analysis reveals that North America currently holds the largest market share at 35%, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 12.3% annually, primarily due to rapid industrialization in China and India coupled with increasing environmental regulations promoting cleaner production technologies.
Customer segments can be categorized into three main groups: large chemical corporations seeking process optimization (40% of market), specialty chemical manufacturers requiring high selectivity for complex molecules (35%), and research institutions developing next-generation catalytic systems (25%). The willingness to pay premium prices for selective catalytic solutions varies significantly across these segments, with specialty manufacturers demonstrating the highest price tolerance due to the critical nature of selectivity in their processes.
Market drivers include stringent environmental regulations limiting waste production, increasing focus on green chemistry principles, and rising energy costs that make efficient catalytic processes more economically attractive. The EU's Green Deal and similar initiatives worldwide are creating regulatory tailwinds for selective electrocatalysis technologies.
Barriers to market adoption include high initial investment costs, technical complexity requiring specialized expertise, and competition from established conventional catalytic processes. Additionally, the relatively limited commercial-scale demonstration of ionic liquid-based electrocatalysis creates perceived implementation risks for potential adopters.
The market is expected to evolve toward integrated systems combining selective electrocatalysis with continuous flow processing and real-time monitoring capabilities, creating opportunities for comprehensive solution providers rather than component suppliers alone.
Key industries driving demand include fine chemicals manufacturing, pharmaceutical production, and renewable energy sectors. The pharmaceutical industry, valued at over $1.4 trillion globally, increasingly seeks selective catalytic processes to reduce waste and improve efficiency in active pharmaceutical ingredient synthesis. Similarly, the fine chemicals market, worth approximately $85 billion, requires highly selective processes to maintain product purity and reduce downstream purification costs.
Regional analysis reveals that North America currently holds the largest market share at 35%, followed by Europe at 30% and Asia-Pacific at 25%. However, the Asia-Pacific region is expected to witness the fastest growth rate of 12.3% annually, primarily due to rapid industrialization in China and India coupled with increasing environmental regulations promoting cleaner production technologies.
Customer segments can be categorized into three main groups: large chemical corporations seeking process optimization (40% of market), specialty chemical manufacturers requiring high selectivity for complex molecules (35%), and research institutions developing next-generation catalytic systems (25%). The willingness to pay premium prices for selective catalytic solutions varies significantly across these segments, with specialty manufacturers demonstrating the highest price tolerance due to the critical nature of selectivity in their processes.
Market drivers include stringent environmental regulations limiting waste production, increasing focus on green chemistry principles, and rising energy costs that make efficient catalytic processes more economically attractive. The EU's Green Deal and similar initiatives worldwide are creating regulatory tailwinds for selective electrocatalysis technologies.
Barriers to market adoption include high initial investment costs, technical complexity requiring specialized expertise, and competition from established conventional catalytic processes. Additionally, the relatively limited commercial-scale demonstration of ionic liquid-based electrocatalysis creates perceived implementation risks for potential adopters.
The market is expected to evolve toward integrated systems combining selective electrocatalysis with continuous flow processing and real-time monitoring capabilities, creating opportunities for comprehensive solution providers rather than component suppliers alone.
Current Challenges in Ionic Liquid Electrocatalysis
Despite significant advancements in ionic liquid (IL) electrocatalysis, several critical challenges continue to impede the full realization of this technology's potential. One of the most pressing issues is the complex interplay between the IL structure and catalytic performance. The unique molecular architecture of ILs creates localized environments around catalyst surfaces that differ substantially from conventional aqueous or organic media, making predictive modeling exceptionally difficult.
The high viscosity of most ionic liquids presents a substantial mass transport limitation, reducing reaction rates and efficiency. This characteristic restricts the diffusion of reactants to catalytic sites and impedes product removal, ultimately limiting the practical application of IL-based electrocatalytic systems in industrial settings where throughput is paramount.
Temperature sensitivity poses another significant challenge. Many IL-based catalytic systems exhibit optimal performance within narrow temperature ranges, with dramatic changes in selectivity and activity occurring outside these windows. This temperature dependence complicates process control and scale-up efforts, particularly for exothermic reactions where heat management becomes critical.
Catalyst stability in IL environments remains problematic, with many conventional catalysts experiencing unexpected deactivation pathways unique to ionic liquid media. The strong coordination abilities of certain IL anions can lead to catalyst poisoning, while the potential for unexpected side reactions between catalyst materials and IL components can result in progressive performance degradation over time.
Water contamination represents a persistent challenge, as even trace amounts of moisture can significantly alter the electrochemical properties of ILs and impact catalytic selectivity. Maintaining anhydrous conditions at scale presents considerable engineering challenges, particularly in continuous processes where complete environmental isolation is impractical.
The electrochemical window limitations of certain ILs restrict the range of reactions that can be effectively catalyzed. While ILs generally offer wider electrochemical windows than aqueous systems, specific combinations of cations and anions may still undergo degradation at potentials required for certain target reactions, generating byproducts that interfere with catalytic processes.
Analytical challenges further complicate research progress, as traditional electrochemical characterization techniques often require modification for IL environments. The high viscosity, low conductivity, and unique solvation properties of ILs can distort electrochemical signals, making mechanistic studies and performance benchmarking difficult to standardize across different research groups.
The high viscosity of most ionic liquids presents a substantial mass transport limitation, reducing reaction rates and efficiency. This characteristic restricts the diffusion of reactants to catalytic sites and impedes product removal, ultimately limiting the practical application of IL-based electrocatalytic systems in industrial settings where throughput is paramount.
Temperature sensitivity poses another significant challenge. Many IL-based catalytic systems exhibit optimal performance within narrow temperature ranges, with dramatic changes in selectivity and activity occurring outside these windows. This temperature dependence complicates process control and scale-up efforts, particularly for exothermic reactions where heat management becomes critical.
Catalyst stability in IL environments remains problematic, with many conventional catalysts experiencing unexpected deactivation pathways unique to ionic liquid media. The strong coordination abilities of certain IL anions can lead to catalyst poisoning, while the potential for unexpected side reactions between catalyst materials and IL components can result in progressive performance degradation over time.
Water contamination represents a persistent challenge, as even trace amounts of moisture can significantly alter the electrochemical properties of ILs and impact catalytic selectivity. Maintaining anhydrous conditions at scale presents considerable engineering challenges, particularly in continuous processes where complete environmental isolation is impractical.
The electrochemical window limitations of certain ILs restrict the range of reactions that can be effectively catalyzed. While ILs generally offer wider electrochemical windows than aqueous systems, specific combinations of cations and anions may still undergo degradation at potentials required for certain target reactions, generating byproducts that interfere with catalytic processes.
Analytical challenges further complicate research progress, as traditional electrochemical characterization techniques often require modification for IL environments. The high viscosity, low conductivity, and unique solvation properties of ILs can distort electrochemical signals, making mechanistic studies and performance benchmarking difficult to standardize across different research groups.
Current Selectivity Control Mechanisms and Approaches
01 Ionic liquids as electrolytes for enhanced selectivity
Ionic liquids serve as effective electrolytes in electrocatalytic processes due to their unique properties such as high ionic conductivity, wide electrochemical windows, and negligible vapor pressure. When used as media for electrocatalysis, they can significantly enhance reaction selectivity by stabilizing specific intermediates or transition states. The tunable nature of ionic liquids allows for customization of the electrolyte environment to favor desired reaction pathways, leading to improved product selectivity in various electrochemical transformations.- Ionic liquids as electrolytes for enhanced selectivity: Ionic liquids serve as effective electrolytes in electrocatalytic processes due to their unique properties such as high ionic conductivity, wide electrochemical windows, and negligible vapor pressure. When used as media for electrocatalysis, they can significantly enhance reaction selectivity by stabilizing specific intermediates or transition states. The tunable nature of ionic liquids allows for customization of the electrolyte environment to favor desired reaction pathways, leading to improved product selectivity in various electrochemical transformations.
- Metal catalysts in ionic liquid systems: Metal-based catalysts, particularly transition metals, demonstrate enhanced selectivity when used in ionic liquid media for electrocatalytic reactions. The ionic environment can modify the electronic properties of metal catalysts, altering their binding affinity for reactants and intermediates. This interaction between metal catalysts and ionic liquids creates unique coordination environments that can be tailored to achieve specific reaction outcomes. The combination allows for selective transformations that might be difficult to achieve in conventional solvent systems.
- Task-specific ionic liquids for selective catalysis: Task-specific ionic liquids are designed with functional groups that participate directly in the catalytic process, enhancing selectivity through specific interactions with substrates. These specialized ionic liquids can act as both the reaction medium and co-catalyst, providing directional control over reaction pathways. By incorporating functional moieties that interact selectively with certain reaction components, these systems can discriminate between competing reaction pathways, leading to improved product selectivity and yield in electrocatalytic processes.
- Temperature and pressure effects on selectivity in ionic liquid electrocatalysis: The selectivity of electrocatalytic reactions in ionic liquid media can be significantly influenced by temperature and pressure conditions. Unlike conventional solvents, ionic liquids maintain their properties over wider temperature and pressure ranges, allowing for reaction optimization under extreme conditions. The viscosity and conductivity changes of ionic liquids with temperature can be leveraged to control mass transport phenomena, affecting the kinetics of competing reactions and thereby enhancing selectivity toward desired products.
- Supported ionic liquid phase catalysts for selective transformations: Supported ionic liquid phase (SILP) catalysts combine the advantages of homogeneous and heterogeneous catalysis, offering enhanced selectivity in electrocatalytic processes. By immobilizing thin films of ionic liquids containing dissolved catalysts onto high-surface-area supports, these systems provide efficient mass transfer while maintaining the selective environment of the ionic liquid. The confined ionic liquid environment on the support can create unique reaction microenvironments that favor specific reaction pathways, leading to improved selectivity compared to bulk ionic liquid systems or conventional heterogeneous catalysts.
02 Metal catalyst modifications for selective electrocatalysis
Modifying metal catalysts through alloying, surface structuring, or nanoparticle formation can significantly improve selectivity in ionic liquid-based electrocatalysis. These modifications alter the electronic structure and binding energies at active sites, allowing for preferential adsorption of specific reactants or intermediates. When combined with ionic liquid media, these tailored catalysts can achieve remarkable selectivity for target reactions by suppressing competing pathways, enabling more efficient and cleaner electrochemical processes.Expand Specific Solutions03 Temperature and pressure effects on selectivity
Temperature and pressure conditions significantly influence the selectivity of electrocatalytic reactions in ionic liquid media. Optimizing these parameters can alter the viscosity and diffusion properties of ionic liquids, affecting mass transport and reaction kinetics. Additionally, temperature changes can modify the interaction between the catalyst surface and reactants, leading to different product distributions. Controlled pressure conditions, particularly in gas-involving reactions, can further enhance selectivity by affecting the solubility and concentration of gaseous reactants in the ionic liquid phase.Expand Specific Solutions04 Functionalized ionic liquids for targeted selectivity
Functionalized ionic liquids containing specific chemical groups can be designed to enhance selectivity in electrocatalytic processes. These task-specific ionic liquids incorporate functional moieties that can interact with reactants, intermediates, or products in a selective manner. The functional groups may include acidic/basic sites, hydrogen bond donors/acceptors, or metal-coordinating groups that direct the reaction pathway toward desired products. This approach combines the advantages of homogeneous catalysis with the benefits of ionic liquid media, resulting in highly selective electrocatalytic systems.Expand Specific Solutions05 Electrode material and structure optimization
The choice and design of electrode materials and structures play crucial roles in determining selectivity in ionic liquid-based electrocatalysis. Nanostructured electrodes with controlled morphology, porosity, and surface chemistry can provide selective active sites for specific reactions. Additionally, composite electrodes incorporating multiple components can create synergistic effects that enhance selectivity. When combined with ionic liquid electrolytes, these optimized electrode materials can achieve unprecedented levels of reaction control, enabling selective transformations that are challenging in conventional systems.Expand Specific Solutions
Leading Research Groups and Industrial Players
Electrocatalysis in ionic liquid media is currently in a transitional phase from early research to commercial application, with the market expected to grow significantly as sustainable energy solutions gain prominence. The technology demonstrates varying maturity levels across applications, with estimated market potential reaching several billion dollars by 2030. Leading academic institutions like MIT, Zhejiang University, and KU Leuven are driving fundamental research, while industrial players including Shell, ExxonMobil, and Asahi Kasei are developing practical applications. Research centers at Johns Hopkins University and Osaka University are advancing selectivity control mechanisms, while companies like Hitachi High-Tech and Lam Research contribute specialized equipment. The field's growth is accelerated by increasing focus on green chemistry and renewable energy conversion processes.
Zhejiang University
Technical Solution: Zhejiang University has pioneered innovative electrocatalytic systems utilizing ionic liquids (ILs) as reaction media for enhanced selectivity control. Their approach centers on the development of composite electrode materials incorporating both metal nanoparticles and functionalized ILs to create synergistic catalytic environments. The university's researchers have demonstrated that by carefully selecting IL structures with specific functional groups, they can modulate the electronic environment around catalytic sites, thereby directing reaction pathways toward desired products. Their technology employs temperature-responsive ILs that allow for dynamic control of selectivity during operation, a significant advancement over static systems. Zhejiang University has also developed novel IL-modified carbon supports that enhance catalyst stability while providing additional selectivity control through surface interactions. Their recent work has shown remarkable success in selective CO2 electroreduction, achieving over 85% Faradaic efficiency for specific products by optimizing the IL composition and electrode structure. The technology also incorporates computational modeling to predict IL-catalyst interactions, enabling rational design of new electrocatalytic systems.
Strengths: Highly tunable selectivity through systematic IL design; enhanced catalyst stability in IL media; ability to operate under mild conditions with reduced energy requirements. Weaknesses: Potential mass transport limitations in viscous IL systems; challenges in scaling up IL-based processes; possible IL degradation during extended operation.
The Regents of the University of California
Technical Solution: The University of California has developed a comprehensive electrocatalytic platform utilizing ionic liquid (IL) media for selective chemical transformations. Their approach integrates specially designed IL electrolytes with nanostructured catalysts to achieve unprecedented control over reaction pathways. The technology employs ILs with tailored physicochemical properties that can modulate the local environment around catalytic sites, influencing adsorption energies of reactants and intermediates. UC researchers have demonstrated that by varying the IL composition, particularly the anion structure, they can significantly alter product distributions in CO2 reduction reactions, achieving up to 90% selectivity for specific products like CO or formate. Their system also incorporates in-situ spectroscopic monitoring techniques that allow real-time observation of catalytic interfaces in IL environments, providing mechanistic insights that guide further optimization. The technology has been successfully applied to various electrochemical processes including nitrogen reduction, oxygen evolution, and organic transformations.
Strengths: Exceptional selectivity control through systematic IL design; compatibility with diverse catalyst materials; ability to operate under ambient conditions with reduced overpotentials. Weaknesses: Complex interactions between ILs and catalysts can be difficult to predict; potential mass transport limitations in highly viscous IL systems; challenges in catalyst recovery from IL media for reuse.
Key Patents and Breakthroughs in IL Electrocatalysis
Synthesis of ionic liquids, useful as liquid electrolyte in electrochemistry, comprises carrying out reaction of base material in continuous operating reactor, and carrying out addition of base material in continuous operating reactor
PatentActiveDE102008032595A1
Innovation
- A continuous process using a suitable solvent to control reaction temperature by its boiling point, combined with dripping or spraying the reaction solution to enhance heat exchange, ensuring a constant reaction temperature and shortening reaction time.
Enzyme catalysis in the presence of ionic liquids
PatentInactiveUS7754462B2
Innovation
- The use of ionic liquids as a reaction medium or co-solvent in enzymatic reactions, which can be miscible or immiscible with water, to improve solubility, selectivity, and stability, and suppress side reactions, without adverse effects on enzyme stability.
Sustainability Impact of Ionic Liquid Electrocatalysis
The integration of ionic liquid electrocatalysis into sustainable industrial processes represents a significant advancement in green chemistry. Ionic liquids offer unique properties that enhance catalytic selectivity while reducing environmental impact compared to traditional solvent systems. Their negligible vapor pressure eliminates volatile organic compound (VOC) emissions, addressing a major environmental concern in chemical manufacturing.
The sustainability benefits extend beyond reduced emissions. Ionic liquid-based electrocatalytic systems demonstrate remarkable recyclability, with some systems maintaining over 95% catalytic efficiency after multiple reuse cycles. This longevity significantly reduces waste generation and resource consumption compared to conventional catalysts that require frequent replacement.
Energy efficiency improvements constitute another critical sustainability advantage. Electrocatalytic reactions in ionic liquid media typically operate at lower temperatures than conventional processes, reducing energy requirements by 20-40% in various applications. The enhanced conductivity of ionic liquids also contributes to more efficient electron transfer processes, further optimizing energy utilization.
From a life cycle assessment perspective, ionic liquid electrocatalysis demonstrates promising metrics. Studies indicate potential reductions in carbon footprint by 30-60% compared to traditional catalytic processes when considering the entire production chain. However, these benefits must be balanced against the current energy-intensive production methods for some ionic liquids.
Water conservation represents another significant sustainability impact. Unlike many conventional catalytic processes requiring substantial water for separation and purification, ionic liquid systems can operate in essentially anhydrous conditions. This characteristic is particularly valuable in water-stressed regions where industrial water consumption creates environmental and social pressures.
The selective nature of ionic liquid electrocatalysis also reduces byproduct formation, minimizing waste streams that would otherwise require energy-intensive treatment. Some applications demonstrate up to 85% reduction in unwanted byproducts compared to conventional methods, substantially decreasing the environmental burden of downstream processing.
Looking forward, the sustainability profile of ionic liquid electrocatalysis will likely improve further as manufacturing processes for ionic liquids become more efficient and renewable energy increasingly powers electrocatalytic systems. The combination of renewable electricity with selective ionic liquid catalysis represents a promising pathway toward carbon-neutral chemical manufacturing.
The sustainability benefits extend beyond reduced emissions. Ionic liquid-based electrocatalytic systems demonstrate remarkable recyclability, with some systems maintaining over 95% catalytic efficiency after multiple reuse cycles. This longevity significantly reduces waste generation and resource consumption compared to conventional catalysts that require frequent replacement.
Energy efficiency improvements constitute another critical sustainability advantage. Electrocatalytic reactions in ionic liquid media typically operate at lower temperatures than conventional processes, reducing energy requirements by 20-40% in various applications. The enhanced conductivity of ionic liquids also contributes to more efficient electron transfer processes, further optimizing energy utilization.
From a life cycle assessment perspective, ionic liquid electrocatalysis demonstrates promising metrics. Studies indicate potential reductions in carbon footprint by 30-60% compared to traditional catalytic processes when considering the entire production chain. However, these benefits must be balanced against the current energy-intensive production methods for some ionic liquids.
Water conservation represents another significant sustainability impact. Unlike many conventional catalytic processes requiring substantial water for separation and purification, ionic liquid systems can operate in essentially anhydrous conditions. This characteristic is particularly valuable in water-stressed regions where industrial water consumption creates environmental and social pressures.
The selective nature of ionic liquid electrocatalysis also reduces byproduct formation, minimizing waste streams that would otherwise require energy-intensive treatment. Some applications demonstrate up to 85% reduction in unwanted byproducts compared to conventional methods, substantially decreasing the environmental burden of downstream processing.
Looking forward, the sustainability profile of ionic liquid electrocatalysis will likely improve further as manufacturing processes for ionic liquids become more efficient and renewable energy increasingly powers electrocatalytic systems. The combination of renewable electricity with selective ionic liquid catalysis represents a promising pathway toward carbon-neutral chemical manufacturing.
Scale-up Considerations for Industrial Implementation
The transition from laboratory-scale electrocatalytic processes in ionic liquid media to industrial implementation presents significant engineering challenges. Scaling up requires careful consideration of reactor design, as ionic liquids possess unique physical properties including high viscosity and density compared to conventional aqueous electrolytes. Industrial reactors must incorporate enhanced mass transport mechanisms to overcome diffusion limitations that become more pronounced at larger scales. Flow-cell configurations with optimized electrode geometries have demonstrated promising results in pilot studies, allowing for continuous operation while maintaining selectivity advantages.
Material selection becomes critical at industrial scale due to the corrosive nature of some ionic liquids and their interactions with electrode materials over extended operation periods. Stainless steel and specialized polymer components have shown adequate chemical resistance, though long-term stability testing remains essential for specific ionic liquid/catalyst combinations. Additionally, electrode materials must maintain their selectivity characteristics when manufactured at larger dimensions, often requiring modified fabrication techniques to preserve nanoscale features responsible for catalytic performance.
Energy efficiency considerations are paramount for commercial viability. The higher viscosity of ionic liquids typically results in increased ohmic resistance compared to aqueous systems, necessitating careful cell design to minimize energy losses. Temperature management systems must be integrated to maintain optimal operating conditions, as selectivity in ionic liquid media demonstrates significant temperature dependence. Recent advances in electrode architecture, including hierarchical porous structures, have shown promise in reducing energy requirements while preserving selectivity benefits.
Process integration presents another dimension of scale-up complexity. Ionic liquid recovery and recycling systems must be incorporated into industrial designs to offset the higher cost of these specialized electrolytes. Membrane separation technologies have emerged as effective solutions for continuous ionic liquid recovery with minimal degradation. Furthermore, product separation strategies must be optimized for the specific physicochemical properties of ionic liquids, which often differ substantially from conventional electrolyte systems.
Economic feasibility ultimately depends on balancing increased capital costs against improved selectivity benefits. Preliminary techno-economic analyses suggest that ionic liquid-based electrocatalytic processes become commercially competitive when selectivity improvements exceed 15-20% compared to conventional approaches, particularly for high-value chemical transformations. The development of lower-cost ionic liquid formulations specifically designed for industrial-scale electrocatalysis represents an active research direction with significant potential to improve economic viability.
Material selection becomes critical at industrial scale due to the corrosive nature of some ionic liquids and their interactions with electrode materials over extended operation periods. Stainless steel and specialized polymer components have shown adequate chemical resistance, though long-term stability testing remains essential for specific ionic liquid/catalyst combinations. Additionally, electrode materials must maintain their selectivity characteristics when manufactured at larger dimensions, often requiring modified fabrication techniques to preserve nanoscale features responsible for catalytic performance.
Energy efficiency considerations are paramount for commercial viability. The higher viscosity of ionic liquids typically results in increased ohmic resistance compared to aqueous systems, necessitating careful cell design to minimize energy losses. Temperature management systems must be integrated to maintain optimal operating conditions, as selectivity in ionic liquid media demonstrates significant temperature dependence. Recent advances in electrode architecture, including hierarchical porous structures, have shown promise in reducing energy requirements while preserving selectivity benefits.
Process integration presents another dimension of scale-up complexity. Ionic liquid recovery and recycling systems must be incorporated into industrial designs to offset the higher cost of these specialized electrolytes. Membrane separation technologies have emerged as effective solutions for continuous ionic liquid recovery with minimal degradation. Furthermore, product separation strategies must be optimized for the specific physicochemical properties of ionic liquids, which often differ substantially from conventional electrolyte systems.
Economic feasibility ultimately depends on balancing increased capital costs against improved selectivity benefits. Preliminary techno-economic analyses suggest that ionic liquid-based electrocatalytic processes become commercially competitive when selectivity improvements exceed 15-20% compared to conventional approaches, particularly for high-value chemical transformations. The development of lower-cost ionic liquid formulations specifically designed for industrial-scale electrocatalysis represents an active research direction with significant potential to improve economic viability.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!