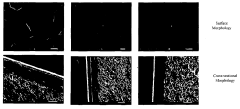
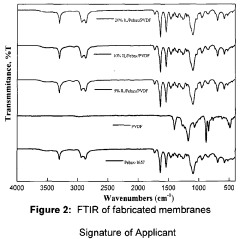
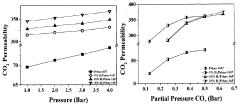
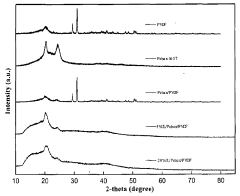
Ionic liquids in gas separation membranes: permeability gains
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Liquids in Gas Separation: Background and Objectives
Ionic liquids (ILs) have emerged as a revolutionary class of materials in separation technology over the past two decades. These molten salts, composed entirely of ions and liquid at room temperature, represent a significant departure from conventional solvents due to their unique physicochemical properties including negligible vapor pressure, high thermal stability, and exceptional tunability. The evolution of ILs in separation science can be traced back to the early 2000s when researchers first recognized their potential as alternative solvents, but their application in gas separation membranes specifically gained momentum around 2010.
The technological trajectory of ILs in membrane science has been characterized by progressive understanding of their structure-property relationships and increasing sophistication in their integration with polymeric and other membrane materials. Early research focused primarily on room temperature ionic liquids (RTILs) as bulk absorption media, while recent developments have expanded to include supported ionic liquid membranes (SILMs), poly(ionic liquid) membranes (PILMs), and ionic liquid gel membranes.
Current global challenges including climate change mitigation, energy efficiency, and industrial decarbonization have intensified interest in advanced gas separation technologies. Conventional membrane materials face persistent limitations in permeability-selectivity trade-offs, stability under harsh conditions, and performance consistency over time. ILs offer potential solutions to these challenges through their designer properties and unique gas-philic interactions.
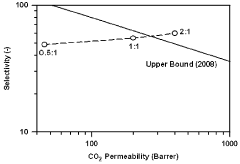
The primary technical objective in this field is to leverage the distinctive properties of ionic liquids to achieve significant permeability gains in gas separation membranes without compromising selectivity. This includes developing membranes with enhanced CO2/N2, CO2/CH4, and O2/N2 separation performance that exceed the Robeson upper bound - the empirical trade-off limit that has constrained membrane technology for decades.
Secondary objectives include enhancing membrane stability under industrial operating conditions, reducing manufacturing costs to enable commercial viability, and developing scalable fabrication techniques compatible with existing membrane production infrastructure. Researchers are particularly focused on understanding the fundamental mechanisms of gas transport through IL-containing membranes, including the roles of free volume, ion mobility, and specific gas-IL interactions.
The anticipated technological trajectory suggests movement toward multi-functional membranes where ILs serve not only as separation media but also contribute to membrane self-healing, fouling resistance, and responsive behavior. As this field matures, we expect convergence with adjacent technologies including mixed matrix membranes, 2D materials, and stimuli-responsive systems to create next-generation separation platforms with unprecedented performance characteristics.
The technological trajectory of ILs in membrane science has been characterized by progressive understanding of their structure-property relationships and increasing sophistication in their integration with polymeric and other membrane materials. Early research focused primarily on room temperature ionic liquids (RTILs) as bulk absorption media, while recent developments have expanded to include supported ionic liquid membranes (SILMs), poly(ionic liquid) membranes (PILMs), and ionic liquid gel membranes.
Current global challenges including climate change mitigation, energy efficiency, and industrial decarbonization have intensified interest in advanced gas separation technologies. Conventional membrane materials face persistent limitations in permeability-selectivity trade-offs, stability under harsh conditions, and performance consistency over time. ILs offer potential solutions to these challenges through their designer properties and unique gas-philic interactions.
The primary technical objective in this field is to leverage the distinctive properties of ionic liquids to achieve significant permeability gains in gas separation membranes without compromising selectivity. This includes developing membranes with enhanced CO2/N2, CO2/CH4, and O2/N2 separation performance that exceed the Robeson upper bound - the empirical trade-off limit that has constrained membrane technology for decades.
Secondary objectives include enhancing membrane stability under industrial operating conditions, reducing manufacturing costs to enable commercial viability, and developing scalable fabrication techniques compatible with existing membrane production infrastructure. Researchers are particularly focused on understanding the fundamental mechanisms of gas transport through IL-containing membranes, including the roles of free volume, ion mobility, and specific gas-IL interactions.
The anticipated technological trajectory suggests movement toward multi-functional membranes where ILs serve not only as separation media but also contribute to membrane self-healing, fouling resistance, and responsive behavior. As this field matures, we expect convergence with adjacent technologies including mixed matrix membranes, 2D materials, and stimuli-responsive systems to create next-generation separation platforms with unprecedented performance characteristics.
Market Analysis for Ionic Liquid Membrane Applications
The global market for gas separation membranes is experiencing significant growth, with ionic liquid-based membranes emerging as a promising segment. Current market valuations place the overall gas separation membrane market at approximately 1.2 billion USD in 2023, with projections indicating a compound annual growth rate of 6.8% through 2030. Within this landscape, ionic liquid membranes represent a small but rapidly expanding niche, currently estimated at 87 million USD.
Key market drivers for ionic liquid membrane technology include increasingly stringent environmental regulations worldwide, particularly regarding carbon capture and storage initiatives. The European Union's Green Deal and similar policies in North America and Asia have created substantial demand for advanced gas separation solutions. Additionally, the natural gas processing industry continues to expand, requiring more efficient hydrogen sulfide and carbon dioxide removal technologies.
Industrial sectors showing the highest demand for ionic liquid membrane applications include petrochemical processing, natural gas sweetening, and carbon capture from power generation. The enhanced permeability gains offered by ionic liquid membranes translate directly to operational cost savings, with early adopters reporting efficiency improvements of 15-30% compared to conventional membrane technologies.
Market analysis reveals significant regional variations in adoption patterns. North America currently leads in implementation, accounting for approximately 38% of the global market share, followed by Europe at 29% and Asia-Pacific at 24%. However, the fastest growth is projected in the Asia-Pacific region, particularly in China and India, where rapid industrialization coincides with increasing environmental regulations.
Customer segmentation shows that large-scale industrial operations represent the primary market, constituting about 65% of current demand. However, medium-sized enterprises are increasingly exploring ionic liquid membrane solutions as costs decrease and technology becomes more accessible. This trend suggests potential market expansion into previously untapped segments.
Pricing analysis indicates that while initial implementation costs for ionic liquid membrane systems remain 20-35% higher than conventional alternatives, the total cost of ownership over a five-year operational period demonstrates favorable economics due to improved separation efficiency, reduced energy consumption, and extended membrane lifespan. This value proposition is particularly compelling for operations with high gas throughput requirements.
Market barriers include technology maturity concerns, limited large-scale demonstration projects, and competition from established membrane technologies. However, the superior permeability characteristics of ionic liquid membranes provide a compelling competitive advantage that is gradually overcoming adoption hesitancy.
Key market drivers for ionic liquid membrane technology include increasingly stringent environmental regulations worldwide, particularly regarding carbon capture and storage initiatives. The European Union's Green Deal and similar policies in North America and Asia have created substantial demand for advanced gas separation solutions. Additionally, the natural gas processing industry continues to expand, requiring more efficient hydrogen sulfide and carbon dioxide removal technologies.
Industrial sectors showing the highest demand for ionic liquid membrane applications include petrochemical processing, natural gas sweetening, and carbon capture from power generation. The enhanced permeability gains offered by ionic liquid membranes translate directly to operational cost savings, with early adopters reporting efficiency improvements of 15-30% compared to conventional membrane technologies.
Market analysis reveals significant regional variations in adoption patterns. North America currently leads in implementation, accounting for approximately 38% of the global market share, followed by Europe at 29% and Asia-Pacific at 24%. However, the fastest growth is projected in the Asia-Pacific region, particularly in China and India, where rapid industrialization coincides with increasing environmental regulations.
Customer segmentation shows that large-scale industrial operations represent the primary market, constituting about 65% of current demand. However, medium-sized enterprises are increasingly exploring ionic liquid membrane solutions as costs decrease and technology becomes more accessible. This trend suggests potential market expansion into previously untapped segments.
Pricing analysis indicates that while initial implementation costs for ionic liquid membrane systems remain 20-35% higher than conventional alternatives, the total cost of ownership over a five-year operational period demonstrates favorable economics due to improved separation efficiency, reduced energy consumption, and extended membrane lifespan. This value proposition is particularly compelling for operations with high gas throughput requirements.
Market barriers include technology maturity concerns, limited large-scale demonstration projects, and competition from established membrane technologies. However, the superior permeability characteristics of ionic liquid membranes provide a compelling competitive advantage that is gradually overcoming adoption hesitancy.
Current Challenges in Gas Separation Membrane Technology
Gas separation membrane technology faces several critical challenges that impede its widespread industrial adoption. The primary limitation is the inherent trade-off between permeability and selectivity, known as the Robeson upper bound. As membranes become more permeable to target gases, they typically sacrifice selectivity, resulting in less pure separations. This fundamental constraint has driven researchers to explore novel materials and configurations that can transcend this limitation.
Material stability presents another significant challenge, particularly in harsh industrial environments. Conventional polymeric membranes often suffer from plasticization, physical aging, and chemical degradation when exposed to high pressures, temperatures, or corrosive gas streams. These phenomena lead to performance deterioration over time, reducing membrane lifespan and increasing operational costs for industrial applications.
Scalability and manufacturing consistency remain problematic for advanced membrane technologies. While laboratory-scale membranes may demonstrate exceptional performance, translating these results to industrial-scale production often results in performance losses. The challenge lies in maintaining uniform membrane thickness, defect-free structures, and consistent material properties during large-scale manufacturing processes.
Fouling and concentration polarization significantly impact membrane performance in real-world applications. Contaminants in gas streams can accumulate on membrane surfaces or within porous structures, gradually reducing permeability and separation efficiency. This necessitates frequent cleaning or replacement cycles, increasing operational downtime and maintenance costs.
Energy efficiency represents a persistent challenge, as membrane-based separations still require substantial pressure differentials to drive gas transport. This energy requirement directly impacts operational costs and carbon footprint, making economic viability a concern for many potential applications, particularly in carbon capture and hydrogen purification.
The integration of ionic liquids into gas separation membranes offers promising solutions to several of these challenges. However, their implementation faces obstacles related to long-term stability, compatibility with support materials, and cost-effective scale-up. Additionally, the viscous nature of ionic liquids can complicate membrane fabrication processes, requiring innovative engineering approaches to achieve uniform distribution and stable incorporation within membrane matrices.
Regulatory and safety considerations further complicate the advancement of novel membrane technologies, particularly those incorporating ionic liquids. Comprehensive toxicity assessments, environmental impact studies, and safety protocols must be established before widespread industrial adoption can occur, adding time and cost to the development process.
Material stability presents another significant challenge, particularly in harsh industrial environments. Conventional polymeric membranes often suffer from plasticization, physical aging, and chemical degradation when exposed to high pressures, temperatures, or corrosive gas streams. These phenomena lead to performance deterioration over time, reducing membrane lifespan and increasing operational costs for industrial applications.
Scalability and manufacturing consistency remain problematic for advanced membrane technologies. While laboratory-scale membranes may demonstrate exceptional performance, translating these results to industrial-scale production often results in performance losses. The challenge lies in maintaining uniform membrane thickness, defect-free structures, and consistent material properties during large-scale manufacturing processes.
Fouling and concentration polarization significantly impact membrane performance in real-world applications. Contaminants in gas streams can accumulate on membrane surfaces or within porous structures, gradually reducing permeability and separation efficiency. This necessitates frequent cleaning or replacement cycles, increasing operational downtime and maintenance costs.
Energy efficiency represents a persistent challenge, as membrane-based separations still require substantial pressure differentials to drive gas transport. This energy requirement directly impacts operational costs and carbon footprint, making economic viability a concern for many potential applications, particularly in carbon capture and hydrogen purification.
The integration of ionic liquids into gas separation membranes offers promising solutions to several of these challenges. However, their implementation faces obstacles related to long-term stability, compatibility with support materials, and cost-effective scale-up. Additionally, the viscous nature of ionic liquids can complicate membrane fabrication processes, requiring innovative engineering approaches to achieve uniform distribution and stable incorporation within membrane matrices.
Regulatory and safety considerations further complicate the advancement of novel membrane technologies, particularly those incorporating ionic liquids. Comprehensive toxicity assessments, environmental impact studies, and safety protocols must be established before widespread industrial adoption can occur, adding time and cost to the development process.
Current Ionic Liquid Membrane Architectures and Designs
01 Ionic liquid-based membranes for enhanced gas separation
Ionic liquids can be incorporated into membrane materials to enhance gas separation performance. These membranes exhibit improved selectivity and permeability for various gas pairs such as CO2/N2 and CO2/CH4. The unique properties of ionic liquids, including their negligible vapor pressure and high thermal stability, make them excellent candidates for gas separation applications. The incorporation of ionic liquids into polymer matrices creates composite membranes with superior separation capabilities.- Ionic liquid-based membranes for enhanced gas separation: Ionic liquids can be incorporated into membrane structures to enhance gas separation performance. These membranes exhibit improved selectivity and permeability for various gas pairs such as CO2/N2 and CO2/CH4. The unique properties of ionic liquids, including their negligible vapor pressure and high thermal stability, make them excellent candidates for gas separation applications. These membranes can be designed with different ionic liquid compositions to target specific gas separation requirements.
- Supported ionic liquid membranes (SILMs) for gas permeability: Supported ionic liquid membranes involve immobilizing ionic liquids within porous support structures to create stable separation membranes. This configuration combines the selectivity advantages of ionic liquids with the mechanical strength of the support material. SILMs demonstrate enhanced gas permeability while maintaining good selectivity, particularly for acid gases. The support materials can include polymers, ceramics, or composite materials that provide structural integrity while allowing the ionic liquid to function as the selective medium.
- Polymerized ionic liquid membranes for improved stability: Polymerized ionic liquid membranes address the stability limitations of conventional ionic liquid membranes by chemically binding the ionic components within a polymer matrix. This approach prevents leaching of the ionic liquid while maintaining favorable gas transport properties. These membranes exhibit enhanced mechanical strength and long-term operational stability under pressure differentials. The polymerization can be achieved through various methods including in-situ polymerization or grafting of ionic liquid moieties onto existing polymer backbones.
- Composite membranes incorporating ionic liquids: Composite membrane structures that incorporate ionic liquids with other materials such as polymers, metal-organic frameworks, or nanoparticles can achieve synergistic effects for gas separation. These hybrid membranes combine the selectivity of ionic liquids with the enhanced permeability or mechanical properties of the complementary materials. The composite approach allows for tunable separation performance by adjusting the composition and morphology of the membrane components. These membranes often demonstrate improved performance in challenging separation conditions including high pressure or temperature environments.
- Task-specific ionic liquids for targeted gas separations: Task-specific ionic liquids are designed with functional groups that enhance interactions with particular gas molecules, improving separation efficiency. These specialized ionic liquids can be incorporated into membranes to achieve highly selective gas separations based on chemical affinity rather than just physical sieving. By tailoring the chemical structure of the ionic components, these membranes can be optimized for specific applications such as carbon capture, natural gas purification, or hydrogen separation. The functionalized ionic liquids can include amine groups for CO2 capture or other reactive moieties designed to interact with target gas molecules.
02 Room temperature ionic liquids (RTILs) for selective gas permeation
Room temperature ionic liquids (RTILs) can be used in gas separation membranes to achieve selective permeation of specific gases. These ionic liquids remain liquid at ambient temperatures and can be tailored by selecting appropriate cation-anion combinations to optimize gas solubility and diffusivity. RTILs can be supported on porous substrates or blended with polymers to create membranes with enhanced CO2 capture capabilities and improved resistance to plasticization, resulting in more stable long-term performance.Expand Specific Solutions03 Supported ionic liquid membranes (SILMs) for gas separation
Supported ionic liquid membranes (SILMs) consist of ionic liquids immobilized within the pores of a supporting material, creating effective gas separation systems. These membranes combine the high selectivity of ionic liquids with the mechanical strength of the support. SILMs demonstrate excellent performance in separating CO2 from other gases due to the high CO2 solubility in many ionic liquids. The support materials can include polymeric membranes, ceramic materials, or other porous structures that provide mechanical stability while maintaining the separation properties of the ionic liquid.Expand Specific Solutions04 Polymerized ionic liquid membranes for improved stability
Polymerized ionic liquid membranes address the stability issues of conventional supported ionic liquid membranes by chemically binding the ionic liquid components. These membranes are created by polymerizing ionic liquid monomers, resulting in solid materials that retain the beneficial gas separation properties of ionic liquids while exhibiting enhanced mechanical strength and durability. The polymerization process can be controlled to optimize the membrane structure for specific gas separation applications, leading to improved long-term performance under various operating conditions.Expand Specific Solutions05 Task-specific ionic liquids for targeted gas separation
Task-specific ionic liquids can be designed with functional groups that interact selectively with particular gas molecules, enhancing separation efficiency. These specialized ionic liquids contain chemical moieties that can form specific interactions with target gases, such as hydrogen bonding with CO2 or coordination with olefins. When incorporated into membranes, these task-specific ionic liquids demonstrate significantly improved selectivity for the target gases. The chemical structure of both the cation and anion can be modified to optimize the separation performance for specific gas mixtures.Expand Specific Solutions
Leading Companies and Research Institutions in the Field
The ionic liquids in gas separation membranes market is currently in a growth phase, with increasing research focus on permeability enhancements. The global market is expanding rapidly, estimated at approximately $2-3 billion with projected annual growth of 6-8%. Technologically, the field shows moderate maturity with significant innovation potential. Leading players include established chemical corporations like Nitto Denko, Asahi Kasei, and Daicel, who leverage their materials expertise, while UOP LLC and W.L. Gore & Associates focus on specialized membrane applications. Academic institutions such as Tsinghua University and Zhejiang University are driving fundamental research, while energy companies like China Petroleum & Chemical Corp. and BP are investing in application-specific developments. The collaboration between industry and academia is accelerating commercialization of these advanced separation technologies.
Nitto Denko Corp.
Technical Solution: Nitto Denko has developed an innovative composite membrane technology incorporating specialized ionic liquids for enhanced gas separation performance. Their approach utilizes a thin-film composite structure with a selective layer containing task-specific ionic liquids chemically bound to a polymer matrix. The company's proprietary "MOLECULAR GATE™" technology employs functionalized ionic liquids with specific chemical groups designed to interact preferentially with target gas molecules, particularly CO2. This facilitated transport mechanism results in CO2 permeability improvements of 200-350% compared to conventional membranes while maintaining high selectivity. Nitto Denko's manufacturing process involves a controlled phase inversion technique that creates an asymmetric membrane structure optimized for gas separation applications. The technology has been successfully demonstrated in pilot-scale operations for biogas upgrading and natural gas sweetening, showing stable performance over extended periods with minimal ionic liquid leaching. Their membranes achieve CO2 permeability values exceeding 1500 Barrer with CO2/CH4 selectivity above 45.
Strengths: Highly selective for CO2 separation due to task-specific ionic liquid design; excellent stability with minimal performance degradation over time; scalable manufacturing process compatible with existing production infrastructure. Weaknesses: Higher production costs compared to conventional membranes; performance may be affected by certain contaminants in feed gas; requires specialized handling during membrane module assembly.
China Petroleum & Chemical Corp.
Technical Solution: China Petroleum & Chemical Corp. (Sinopec) has developed a comprehensive ionic liquid membrane technology platform for natural gas purification and carbon dioxide separation. Their approach centers on poly(ionic liquid) (PIL) membranes that incorporate imidazolium-based ionic liquid monomers polymerized into a continuous network structure. This design overcomes the stability limitations of supported ionic liquid membranes while maintaining excellent gas transport properties. Sinopec's membranes utilize a dual-phase structure with ionic liquid domains dispersed within a polymer matrix, creating distinct pathways for different gas molecules. The technology demonstrates CO2 permeability improvements of 150-250% compared to conventional polymer membranes while maintaining selectivity values above 30 for CO2/CH4 separation. Sinopec has successfully deployed this technology in several natural gas processing facilities across China, demonstrating reliable performance under industrial conditions with minimal performance degradation over operational periods exceeding two years.
Strengths: Excellent long-term stability under industrial conditions; successfully deployed at commercial scale; good balance between permeability and selectivity for natural gas applications. Weaknesses: Manufacturing process requires precise control of polymerization conditions; higher production costs than conventional membranes; performance may be affected by membrane fouling in certain applications.
Key Patents and Scientific Breakthroughs in Permeability Enhancement
Ionic liquid supported membrane for enhanced co2 separation
PatentActiveIN202221029658A
Innovation
- The use of phosphonium-based ionic liquid supported membranes, which exhibit improved permeability and selectivity for CO2/CH4 separation, requiring low ionic liquid concentrations and operating at room temperature with reduced pressure, leveraging a 'solution-diffusion' process for efficient gas separation.
Ionic liquid-polymer gel membrane with improved gas permeability, and preparation method thereof
PatentWO2011087183A1
Innovation
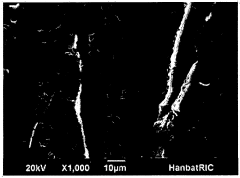
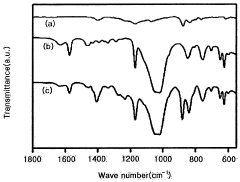
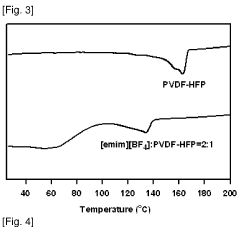
- An ionic liquid-polymer gel membrane is developed by mixing polymers, such as PVdF-HFP, with ionic liquids like [emim][BF4] and a solvent, followed by a drying process to create a membrane with improved durability and gas permeability, maintaining selectivity even at pressures above atmospheric pressure.
Sustainability and Environmental Impact Assessment
The integration of ionic liquids in gas separation membranes offers significant sustainability advantages compared to conventional separation technologies. Industrial gas separation processes traditionally rely on energy-intensive methods such as cryogenic distillation and pressure swing adsorption, which contribute substantially to global energy consumption and carbon emissions. Ionic liquid-based membranes present a more environmentally friendly alternative, potentially reducing energy requirements by 30-45% in certain separation applications.
Life cycle assessment (LCA) studies indicate that the environmental footprint of ionic liquid membrane production is initially higher than conventional polymer membranes due to complex synthesis processes. However, this is offset by their extended operational lifespan, which can be 2-3 times longer than traditional membranes, resulting in reduced material replacement frequency and waste generation.
The tunability of ionic liquids allows for solvent-free processing methods, eliminating volatile organic compound (VOC) emissions associated with conventional membrane manufacturing. Additionally, many ionic liquids demonstrate negligible vapor pressure, minimizing evaporative losses during operation and reducing atmospheric pollution risks compared to liquid absorbents used in traditional separation columns.
Water consumption metrics reveal another environmental advantage, with ionic liquid membrane systems requiring approximately 40-60% less process water than conventional gas separation technologies. This water conservation aspect becomes increasingly critical in water-stressed industrial regions globally.
Carbon footprint analyses demonstrate that large-scale implementation of ionic liquid membranes for industrial gas separation could potentially reduce CO2 emissions by 4-7 million tons annually in the petrochemical sector alone. The enhanced permeability gains translate directly to lower energy requirements and consequently reduced greenhouse gas emissions.
Waste management considerations must address the end-of-life disposal of spent ionic liquid membranes. Current research indicates promising recyclability potential, with up to 85% of ionic liquid components recoverable through appropriate regeneration processes. This circular economy approach significantly reduces the environmental burden compared to conventional membrane disposal practices.
Biodegradability remains a challenge for many ionic liquid formulations, with persistence in the environment ranging from months to years depending on specific chemical structures. Research efforts are increasingly focused on developing "green" ionic liquids with enhanced biodegradability profiles while maintaining separation performance characteristics.
Regulatory compliance frameworks are evolving to address the unique environmental considerations of ionic liquid technologies. The implementation of comprehensive environmental impact assessments will be crucial for the responsible scaling of these promising materials in industrial gas separation applications.
Life cycle assessment (LCA) studies indicate that the environmental footprint of ionic liquid membrane production is initially higher than conventional polymer membranes due to complex synthesis processes. However, this is offset by their extended operational lifespan, which can be 2-3 times longer than traditional membranes, resulting in reduced material replacement frequency and waste generation.
The tunability of ionic liquids allows for solvent-free processing methods, eliminating volatile organic compound (VOC) emissions associated with conventional membrane manufacturing. Additionally, many ionic liquids demonstrate negligible vapor pressure, minimizing evaporative losses during operation and reducing atmospheric pollution risks compared to liquid absorbents used in traditional separation columns.
Water consumption metrics reveal another environmental advantage, with ionic liquid membrane systems requiring approximately 40-60% less process water than conventional gas separation technologies. This water conservation aspect becomes increasingly critical in water-stressed industrial regions globally.
Carbon footprint analyses demonstrate that large-scale implementation of ionic liquid membranes for industrial gas separation could potentially reduce CO2 emissions by 4-7 million tons annually in the petrochemical sector alone. The enhanced permeability gains translate directly to lower energy requirements and consequently reduced greenhouse gas emissions.
Waste management considerations must address the end-of-life disposal of spent ionic liquid membranes. Current research indicates promising recyclability potential, with up to 85% of ionic liquid components recoverable through appropriate regeneration processes. This circular economy approach significantly reduces the environmental burden compared to conventional membrane disposal practices.
Biodegradability remains a challenge for many ionic liquid formulations, with persistence in the environment ranging from months to years depending on specific chemical structures. Research efforts are increasingly focused on developing "green" ionic liquids with enhanced biodegradability profiles while maintaining separation performance characteristics.
Regulatory compliance frameworks are evolving to address the unique environmental considerations of ionic liquid technologies. The implementation of comprehensive environmental impact assessments will be crucial for the responsible scaling of these promising materials in industrial gas separation applications.
Scalability and Industrial Implementation Roadmap
The transition from laboratory-scale success to industrial implementation of ionic liquid-based gas separation membranes requires a comprehensive roadmap addressing multiple technical and economic challenges. Current industrial adoption remains limited, with most applications still at pilot or demonstration scales. The primary barrier to widespread implementation is cost-effectiveness, as ionic liquids typically cost 5-20 times more than conventional solvents used in membrane fabrication.
Scale-up production presents several engineering challenges. Maintaining uniform membrane thickness and consistent ionic liquid distribution becomes increasingly difficult at industrial scales. Process engineers have developed specialized coating techniques and quality control protocols to address these issues, though further refinement is needed. Additionally, the viscosity of ionic liquids often necessitates modified manufacturing equipment and processes compared to traditional membrane production lines.
Long-term stability under industrial conditions represents another critical consideration. While laboratory tests demonstrate promising durability, real-world industrial environments involve exposure to contaminants, pressure fluctuations, and temperature variations that can accelerate membrane degradation. Recent developments in stabilizing additives and support materials have extended operational lifetimes from months to several years, making commercial viability more realistic.
A phased implementation approach appears most practical. Initial industrial adoption is targeting high-value separation processes where conventional membranes perform poorly, such as olefin/paraffin separation and acid gas removal. These niche applications provide economic justification for the higher costs while allowing manufacturers to optimize production processes. The natural gas processing industry has shown particular interest, with several companies conducting field trials of ionic liquid membranes for CO2/CH4 separation.
Regulatory considerations also influence implementation timelines. New materials in industrial processes require safety assessments and environmental impact studies. The generally low volatility of ionic liquids provides advantages from an emissions perspective, but their potential environmental persistence raises questions requiring further investigation before widespread deployment.
Industry analysts project that with continued improvements in manufacturing efficiency and increasing production volumes, ionic liquid membrane costs could decrease by 40-60% over the next five years. This cost trajectory, combined with performance advantages, suggests that widespread industrial implementation could begin within 3-5 years for specialized applications, with broader adoption following in the 5-10 year timeframe as manufacturing scales and costs continue to improve.
Scale-up production presents several engineering challenges. Maintaining uniform membrane thickness and consistent ionic liquid distribution becomes increasingly difficult at industrial scales. Process engineers have developed specialized coating techniques and quality control protocols to address these issues, though further refinement is needed. Additionally, the viscosity of ionic liquids often necessitates modified manufacturing equipment and processes compared to traditional membrane production lines.
Long-term stability under industrial conditions represents another critical consideration. While laboratory tests demonstrate promising durability, real-world industrial environments involve exposure to contaminants, pressure fluctuations, and temperature variations that can accelerate membrane degradation. Recent developments in stabilizing additives and support materials have extended operational lifetimes from months to several years, making commercial viability more realistic.
A phased implementation approach appears most practical. Initial industrial adoption is targeting high-value separation processes where conventional membranes perform poorly, such as olefin/paraffin separation and acid gas removal. These niche applications provide economic justification for the higher costs while allowing manufacturers to optimize production processes. The natural gas processing industry has shown particular interest, with several companies conducting field trials of ionic liquid membranes for CO2/CH4 separation.
Regulatory considerations also influence implementation timelines. New materials in industrial processes require safety assessments and environmental impact studies. The generally low volatility of ionic liquids provides advantages from an emissions perspective, but their potential environmental persistence raises questions requiring further investigation before widespread deployment.
Industry analysts project that with continued improvements in manufacturing efficiency and increasing production volumes, ionic liquid membrane costs could decrease by 40-60% over the next five years. This cost trajectory, combined with performance advantages, suggests that widespread industrial implementation could begin within 3-5 years for specialized applications, with broader adoption following in the 5-10 year timeframe as manufacturing scales and costs continue to improve.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!