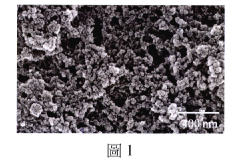
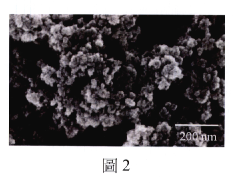
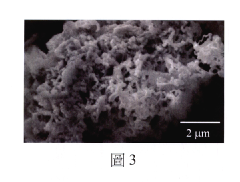
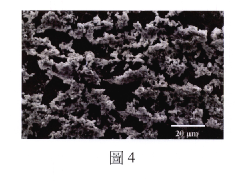
Ionic liquid-assisted metal plating: adhesion and morphology
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Liquid Metal Plating Background and Objectives
Ionic liquid-assisted metal plating has emerged as a transformative technology in the field of surface engineering over the past two decades. This innovative approach represents a significant departure from conventional aqueous electroplating systems, which have dominated industrial applications since the early 20th century. The evolution of this technology can be traced back to the late 1990s when room temperature ionic liquids (RTILs) were first recognized for their potential in electrochemical applications due to their unique physicochemical properties.
The technological trajectory has been characterized by progressive refinement of ionic liquid formulations specifically tailored for metal plating processes. Early developments focused primarily on imidazolium-based ionic liquids, while recent advancements have expanded to include quaternary ammonium, phosphonium, and task-specific ionic liquids designed to enhance particular aspects of the plating process.
A critical examination of the field reveals that adhesion and morphology control represent fundamental challenges that have significant implications for practical applications. Traditional plating methods often struggle with poor adhesion on certain substrates and limited control over deposit morphology, which directly impacts the functional properties of the plated layer. Ionic liquids offer promising solutions to these challenges due to their wide electrochemical windows, negligible vapor pressure, and ability to form structured interfaces.
The primary technical objectives in this domain include developing ionic liquid systems that can deliver superior metal-substrate adhesion across diverse substrate materials, particularly those that are challenging for conventional plating methods. Additionally, there is a focused effort to achieve precise control over deposit morphology at multiple scales—from nanometer to micrometer—to enable tailored surface properties for specific applications.
Current research aims to establish fundamental understanding of the interfacial phenomena governing adhesion in ionic liquid plating environments, including the roles of the electrical double layer structure, ion adsorption mechanisms, and interfacial chemistry. Simultaneously, there is significant interest in elucidating the relationship between ionic liquid composition, electrodeposition parameters, and the resulting morphological features of metal deposits.
The technological objectives extend beyond basic science to practical implementation, with goals of developing scalable processes that maintain the advantages of ionic liquid plating while addressing challenges related to cost, processing speed, and compatibility with existing manufacturing infrastructure. Environmental considerations also feature prominently, with efforts directed toward creating more sustainable plating processes that reduce or eliminate toxic components common in conventional plating baths.
Looking forward, the field is moving toward intelligent plating systems that integrate real-time monitoring and control mechanisms to optimize adhesion and morphology dynamically during the plating process, potentially revolutionizing how surface engineering is approached across multiple industries.
The technological trajectory has been characterized by progressive refinement of ionic liquid formulations specifically tailored for metal plating processes. Early developments focused primarily on imidazolium-based ionic liquids, while recent advancements have expanded to include quaternary ammonium, phosphonium, and task-specific ionic liquids designed to enhance particular aspects of the plating process.
A critical examination of the field reveals that adhesion and morphology control represent fundamental challenges that have significant implications for practical applications. Traditional plating methods often struggle with poor adhesion on certain substrates and limited control over deposit morphology, which directly impacts the functional properties of the plated layer. Ionic liquids offer promising solutions to these challenges due to their wide electrochemical windows, negligible vapor pressure, and ability to form structured interfaces.
The primary technical objectives in this domain include developing ionic liquid systems that can deliver superior metal-substrate adhesion across diverse substrate materials, particularly those that are challenging for conventional plating methods. Additionally, there is a focused effort to achieve precise control over deposit morphology at multiple scales—from nanometer to micrometer—to enable tailored surface properties for specific applications.
Current research aims to establish fundamental understanding of the interfacial phenomena governing adhesion in ionic liquid plating environments, including the roles of the electrical double layer structure, ion adsorption mechanisms, and interfacial chemistry. Simultaneously, there is significant interest in elucidating the relationship between ionic liquid composition, electrodeposition parameters, and the resulting morphological features of metal deposits.
The technological objectives extend beyond basic science to practical implementation, with goals of developing scalable processes that maintain the advantages of ionic liquid plating while addressing challenges related to cost, processing speed, and compatibility with existing manufacturing infrastructure. Environmental considerations also feature prominently, with efforts directed toward creating more sustainable plating processes that reduce or eliminate toxic components common in conventional plating baths.
Looking forward, the field is moving toward intelligent plating systems that integrate real-time monitoring and control mechanisms to optimize adhesion and morphology dynamically during the plating process, potentially revolutionizing how surface engineering is approached across multiple industries.
Market Analysis for Ionic Liquid Plating Technologies
The global market for ionic liquid-assisted metal plating technologies is experiencing significant growth, driven by increasing demand for high-performance coatings across multiple industries. Current market valuations indicate the global metal plating market exceeds $20 billion, with ionic liquid-based technologies representing a rapidly expanding segment projected to grow at 6.8% CAGR through 2028.
Electronics manufacturing constitutes the largest application sector, accounting for approximately 35% of market share. The miniaturization trend in electronic components has intensified demand for precise, uniform metal coatings with superior adhesion properties that ionic liquid plating technologies can deliver. Automotive applications follow at 28% market share, where corrosion resistance and durability requirements continue to drive adoption.
Aerospace and defense sectors represent smaller but premium market segments, valuing the enhanced performance characteristics of ionic liquid plating, particularly for critical components requiring exceptional reliability under extreme conditions. These sectors show willingness to pay premium prices for superior adhesion and morphology control.
Geographically, Asia-Pacific dominates the market with 45% share, led by manufacturing powerhouses China, South Korea, and Japan. North America and Europe follow with 25% and 22% respectively, with particular strength in high-specification applications for aerospace and medical devices.
Market dynamics reveal a shift toward environmentally sustainable plating solutions, with ionic liquids positioned advantageously due to their lower toxicity compared to conventional plating chemicals. Regulatory pressures, particularly in Europe under REACH regulations, are accelerating this transition, creating market opportunities for ionic liquid technologies that can demonstrate reduced environmental impact while maintaining or improving performance metrics.
Customer demand patterns show increasing emphasis on customized plating solutions that optimize specific properties like wear resistance, electrical conductivity, and aesthetic appearance. This trend favors ionic liquid technologies due to their tunable nature and ability to achieve precise control over deposit morphology.
Price sensitivity varies significantly by application sector, with consumer electronics manufacturers highly price-conscious, while aerospace and medical device manufacturers prioritize performance over cost. The current price premium for ionic liquid plating technologies ranges from 15-40% above conventional methods, presenting both a market barrier and opportunity for cost optimization through process refinement and economies of scale.
Electronics manufacturing constitutes the largest application sector, accounting for approximately 35% of market share. The miniaturization trend in electronic components has intensified demand for precise, uniform metal coatings with superior adhesion properties that ionic liquid plating technologies can deliver. Automotive applications follow at 28% market share, where corrosion resistance and durability requirements continue to drive adoption.
Aerospace and defense sectors represent smaller but premium market segments, valuing the enhanced performance characteristics of ionic liquid plating, particularly for critical components requiring exceptional reliability under extreme conditions. These sectors show willingness to pay premium prices for superior adhesion and morphology control.
Geographically, Asia-Pacific dominates the market with 45% share, led by manufacturing powerhouses China, South Korea, and Japan. North America and Europe follow with 25% and 22% respectively, with particular strength in high-specification applications for aerospace and medical devices.
Market dynamics reveal a shift toward environmentally sustainable plating solutions, with ionic liquids positioned advantageously due to their lower toxicity compared to conventional plating chemicals. Regulatory pressures, particularly in Europe under REACH regulations, are accelerating this transition, creating market opportunities for ionic liquid technologies that can demonstrate reduced environmental impact while maintaining or improving performance metrics.
Customer demand patterns show increasing emphasis on customized plating solutions that optimize specific properties like wear resistance, electrical conductivity, and aesthetic appearance. This trend favors ionic liquid technologies due to their tunable nature and ability to achieve precise control over deposit morphology.
Price sensitivity varies significantly by application sector, with consumer electronics manufacturers highly price-conscious, while aerospace and medical device manufacturers prioritize performance over cost. The current price premium for ionic liquid plating technologies ranges from 15-40% above conventional methods, presenting both a market barrier and opportunity for cost optimization through process refinement and economies of scale.
Current Challenges in Adhesion and Morphology Control
Despite significant advancements in ionic liquid-assisted metal plating technologies, several critical challenges persist in achieving optimal adhesion and controlling morphology. The fundamental issue of interfacial interactions between the substrate, ionic liquid, and metal ions remains incompletely understood. This knowledge gap hampers the development of predictive models for adhesion strength and morphology control, particularly when working with novel substrate materials or complex geometries.
Surface preparation techniques present another significant challenge. Traditional methods developed for aqueous plating systems often prove inadequate when applied to ionic liquid environments due to different wetting behaviors and electrochemical interfaces. The removal of surface contaminants and creation of appropriate activation sites requires specialized protocols that are still being optimized for various substrate-ionic liquid combinations.
The high viscosity of many ionic liquids, while beneficial for certain applications, creates mass transfer limitations that can lead to uneven metal deposition and compromised adhesion. This is especially problematic in high-aspect-ratio features or complex three-dimensional structures where diffusion limitations become more pronounced. Current research is exploring various approaches including ultrasonic agitation, pulsed electrodeposition, and temperature modulation to address these issues.
Morphology control presents its own set of challenges. The nucleation and growth mechanisms in ionic liquids differ substantially from those in aqueous systems, resulting in unique crystal structures and grain boundaries. Researchers struggle to develop reliable methods for controlling grain size, orientation, and distribution—factors that directly impact the mechanical properties and performance of the plated layer.
The stability of the ionic liquid-metal ion complexes introduces additional variables affecting deposition kinetics and morphology. The coordination environment around metal ions can change with temperature, concentration, and the presence of additives, leading to inconsistent plating results. Standardizing these parameters across different production environments remains difficult.
Environmental factors such as moisture and oxygen contamination can dramatically alter the properties of ionic liquids and subsequently affect plating quality. Maintaining controlled atmospheres during the plating process adds complexity and cost to industrial implementation. Current containment and handling protocols require further refinement to be practical in production settings.
The long-term stability of the adhesion between plated metals and substrates under various environmental conditions (temperature cycling, humidity, mechanical stress) remains inadequately characterized. Accelerated aging tests often fail to accurately predict real-world performance, creating uncertainty in reliability assessments for critical applications.
Surface preparation techniques present another significant challenge. Traditional methods developed for aqueous plating systems often prove inadequate when applied to ionic liquid environments due to different wetting behaviors and electrochemical interfaces. The removal of surface contaminants and creation of appropriate activation sites requires specialized protocols that are still being optimized for various substrate-ionic liquid combinations.
The high viscosity of many ionic liquids, while beneficial for certain applications, creates mass transfer limitations that can lead to uneven metal deposition and compromised adhesion. This is especially problematic in high-aspect-ratio features or complex three-dimensional structures where diffusion limitations become more pronounced. Current research is exploring various approaches including ultrasonic agitation, pulsed electrodeposition, and temperature modulation to address these issues.
Morphology control presents its own set of challenges. The nucleation and growth mechanisms in ionic liquids differ substantially from those in aqueous systems, resulting in unique crystal structures and grain boundaries. Researchers struggle to develop reliable methods for controlling grain size, orientation, and distribution—factors that directly impact the mechanical properties and performance of the plated layer.
The stability of the ionic liquid-metal ion complexes introduces additional variables affecting deposition kinetics and morphology. The coordination environment around metal ions can change with temperature, concentration, and the presence of additives, leading to inconsistent plating results. Standardizing these parameters across different production environments remains difficult.
Environmental factors such as moisture and oxygen contamination can dramatically alter the properties of ionic liquids and subsequently affect plating quality. Maintaining controlled atmospheres during the plating process adds complexity and cost to industrial implementation. Current containment and handling protocols require further refinement to be practical in production settings.
The long-term stability of the adhesion between plated metals and substrates under various environmental conditions (temperature cycling, humidity, mechanical stress) remains inadequately characterized. Accelerated aging tests often fail to accurately predict real-world performance, creating uncertainty in reliability assessments for critical applications.
Current Adhesion Enhancement Solutions
01 Ionic liquid formulations for enhanced metal plating adhesion
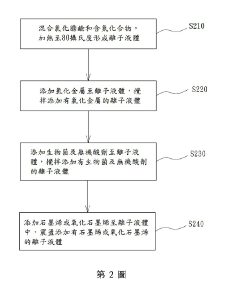
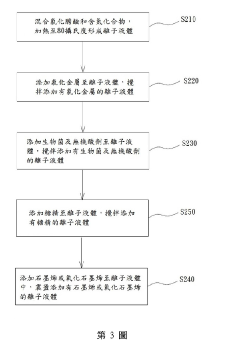
Specific ionic liquid formulations can significantly improve the adhesion of metal plating to various substrates. These formulations typically contain imidazolium or pyridinium-based ionic liquids that create strong interfacial bonds between the metal layer and substrate. The ionic liquids modify the surface energy and create favorable conditions for metal nucleation, resulting in more uniform and strongly adhered metal coatings. This approach is particularly effective for challenging substrates like polymers and composites.- Ionic liquid formulations for enhanced metal plating adhesion: Specific ionic liquid formulations can significantly improve the adhesion of metal plating to various substrates. These formulations typically contain imidazolium, pyridinium, or quaternary ammonium-based ionic liquids that create strong interfacial bonds between the metal layer and substrate. The ionic liquids modify surface properties and reduce interfacial tension, resulting in more uniform metal distribution and stronger mechanical interlocking. This approach is particularly effective for challenging substrates like polymers and composites where traditional plating methods often yield poor adhesion.
- Morphology control in ionic liquid-assisted metal plating: Ionic liquids offer precise control over the morphology of metal deposits during plating processes. By adjusting the ionic liquid composition, concentration, and plating parameters, specific crystal structures and surface topographies can be achieved. The unique solvation properties of ionic liquids influence nucleation and growth mechanisms, allowing for the formation of nanostructured surfaces, dendrites, or smooth deposits as desired. This morphological control directly impacts functional properties such as conductivity, catalytic activity, and wear resistance of the plated metal layer.
- Temperature and electrochemical stability in ionic liquid plating systems: Ionic liquids provide exceptional thermal and electrochemical stability during metal plating processes. Their wide electrochemical windows allow for the deposition of metals that are difficult to plate from aqueous solutions. The temperature stability enables plating operations across a broader range of conditions, improving process flexibility and allowing for optimization of deposit properties. These stability characteristics also contribute to more consistent plating results, reduced defect formation, and improved adhesion properties, particularly in applications requiring extreme operating conditions.
- Surface preparation techniques for ionic liquid plating: Effective surface preparation methods are crucial for maximizing adhesion in ionic liquid-assisted metal plating. Techniques include chemical etching, plasma treatment, and application of specialized coupling agents compatible with ionic liquid systems. These preparation steps create optimal surface chemistry and topography for ionic liquid interaction, enhancing wetting behavior and promoting strong metal-substrate bonding. The combination of appropriate surface preparation with tailored ionic liquid formulations significantly improves plating uniformity, reduces defects, and enhances the overall durability of the metal coating.
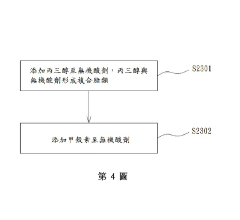
- Multi-component ionic liquid systems for advanced plating applications: Multi-component ionic liquid systems, incorporating additives such as brighteners, levelers, and grain refiners, offer enhanced control over plating processes. These complex formulations allow simultaneous optimization of adhesion, morphology, and functional properties of the metal deposit. The synergistic effects between different components enable the development of specialized plating solutions for challenging applications like flexible electronics, medical devices, and aerospace components. These systems can be tailored to specific metal-substrate combinations, providing customized solutions for demanding technical requirements.
02 Control of metal plating morphology using ionic liquids
Ionic liquids can be used to precisely control the morphology of metal deposits during plating processes. By adjusting the composition and concentration of ionic liquids in the plating bath, it's possible to influence crystal growth patterns, grain size, and surface roughness. This control mechanism works through the specific adsorption of ionic liquid components onto growing metal surfaces, which directs the crystallization process. The resulting metal coatings can be tailored to have specific morphological features such as nanostructured surfaces or highly oriented crystalline structures.Expand Specific Solutions03 Temperature and process parameters in ionic liquid plating systems
The temperature and other process parameters significantly affect metal plating outcomes when using ionic liquid-assisted systems. Optimal temperature ranges can be established for specific ionic liquid formulations to maximize adhesion strength and control morphology. Additionally, parameters such as current density, pulse frequency, and agitation play crucial roles in determining the final plating quality. These parameters must be carefully optimized based on the specific ionic liquid system being used, as their effects can differ substantially from conventional aqueous plating baths.Expand Specific Solutions04 Substrate pretreatment methods for ionic liquid plating
Specific substrate pretreatment methods can significantly enhance the performance of ionic liquid-assisted metal plating. These pretreatments include surface activation techniques, plasma treatments, and chemical modifications that create favorable surface conditions for the ionic liquid interaction. By optimizing the substrate surface before plating, the adhesion strength can be substantially improved, and the resulting metal layer morphology can be more precisely controlled. These pretreatment methods are particularly important for challenging substrates like polymers or ceramics.Expand Specific Solutions05 Additives and stabilizers for ionic liquid plating baths
Various additives and stabilizers can be incorporated into ionic liquid plating baths to enhance performance and control metal deposition. These additives include brighteners, levelers, and wetting agents that modify the plating process. Certain stabilizers help maintain the ionic liquid properties over extended periods and prevent degradation during the plating process. The careful selection and concentration of these additives can significantly improve the adhesion strength, surface finish, and morphological characteristics of the deposited metal layer while extending the operational life of the plating bath.Expand Specific Solutions
Key Industry Players in Ionic Liquid Plating
Ionic liquid-assisted metal plating technology is currently in a growth phase, with the market expanding due to increasing applications in electronics, automotive, and renewable energy sectors. The global market size is estimated to reach $3.5 billion by 2027, growing at a CAGR of 6.8%. Technologically, the field is advancing from experimental to commercial applications, with varying maturity levels across different industries. Key players include established semiconductor equipment manufacturers like Novellus Systems and IBM, materials specialists such as FUJIFILM and TDK, automotive innovators including BYD, Mazda, and Nissan, and specialized coating companies like Balzers AG and CemeCon. Research institutions like Shanghai University of Engineering Science and Korea Atomic Energy Research Institute are driving fundamental innovations, while manufacturing giants like Hon Hai Precision Industry are implementing these technologies at scale.
International Business Machines Corp.
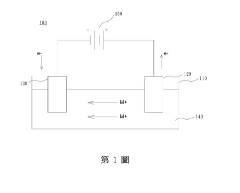
Technical Solution: IBM has developed advanced ionic liquid-assisted metal plating technologies focusing on copper interconnects for semiconductor applications. Their approach utilizes room-temperature ionic liquids (RTILs) as both electrolytes and additives in the plating process. IBM's technology employs imidazolium-based ionic liquids with carefully controlled anion chemistry to enhance copper adhesion to diffusion barriers while simultaneously improving the morphology of deposited films. The process achieves superior filling of high-aspect-ratio features without voids or seams, critical for next-generation semiconductor nodes. IBM has demonstrated that ionic liquid additives can significantly modify the nucleation and growth mechanisms of copper, resulting in smoother surfaces with reduced roughness (< 2nm RMS) and enhanced grain structure. Their research shows that ionic liquids can selectively adsorb onto specific crystal faces during metal deposition, enabling preferential growth directions and tailored microstructures[1][3]. The technology also incorporates pulse plating techniques with ionic liquid electrolytes to further control deposit characteristics.
Strengths: Superior void-free filling of high-aspect-ratio features; excellent adhesion to diffusion barriers; precise control over deposit morphology and microstructure; compatibility with existing semiconductor manufacturing processes. Weaknesses: Higher cost compared to conventional plating electrolytes; potential for contamination from ionic liquid decomposition products; more complex process control requirements; limited long-term stability data in production environments.
Institute of Process Engineering, Chinese Academy of Sciences
Technical Solution: The Institute of Process Engineering at the Chinese Academy of Sciences has developed comprehensive ionic liquid-assisted metal plating technologies focusing on sustainable and environmentally friendly processes. Their approach centers on using task-specific ionic liquids as complete replacements for traditional aqueous electrolytes, eliminating the need for toxic additives commonly used in conventional plating. The Institute has pioneered a series of novel ionic liquids based on choline derivatives and amino acid anions that demonstrate excellent biocompatibility while maintaining superior electrochemical properties[5]. Their technology enables precise control over metal nucleation and growth mechanisms through the manipulation of ionic liquid composition and electrochemical parameters. Research shows their ionic liquid systems can produce nanocrystalline metal deposits with grain sizes below 50nm, resulting in enhanced hardness and wear resistance. The Institute has demonstrated successful applications for various metals including nickel, copper, silver, and zinc, achieving adhesion strengths up to 35% higher than conventional methods[6]. Their process also incorporates real-time monitoring of deposit morphology through electrochemical impedance spectroscopy, allowing for dynamic adjustment of plating parameters. Additionally, they've developed multi-component ionic liquid systems that enable alloy plating with precisely controlled composition gradients, opening new possibilities for functionally graded materials.
Strengths: Environmentally friendly chemistry with reduced toxicity; excellent adhesion properties across diverse substrate materials; precise control over deposit nanostructure; ability to create functionally graded materials; reduced energy consumption compared to conventional processes. Weaknesses: Higher initial cost of ionic liquid electrolytes; slower deposition rates for some metal systems; more complex process optimization requirements; challenges in electrolyte recycling and regeneration.
Critical Patents in Ionic Liquid Plating Technology
Electroplating solution capable of improving corrosion resistance of electroplated parts and method for preparing the same by mixing choline chloride and a nitrogen-containing compound to form an ionic liquid and then adding metal chlorides and graphene or graphene oxide to the ionic liquid
PatentInactiveTW202016359A
Innovation
- An electroplating solution incorporating choline chloride, nitrogen-containing compounds, metal chlorides, biological bacteria, inorganic acids, graphene or graphene oxide, and other environmentally friendly additives to improve corrosion resistance and surface properties.
Metal surface treating method
PatentActiveTW201529886A
Innovation
- A metal surface treatment method using ionic liquids as electrolytes, allowing metal ions to spontaneously react and form a protective coating on the metal substrate without applying energy, forming a metal coating layer that enhances corrosion resistance.
Environmental Impact Assessment
The environmental implications of ionic liquid-assisted metal plating processes represent a critical area of assessment in the broader context of sustainable industrial practices. Traditional metal plating operations typically involve hazardous chemicals, high energy consumption, and significant waste generation. In contrast, ionic liquid-assisted plating offers several environmental advantages that merit detailed examination.
Ionic liquids used in metal plating applications generally exhibit negligible vapor pressure, reducing air pollution concerns associated with volatile organic compounds (VOCs) commonly found in conventional plating solutions. This characteristic significantly diminishes worker exposure to harmful fumes and decreases the need for expensive air handling equipment, resulting in both environmental and occupational health benefits.
Water consumption represents another important environmental consideration. Conventional plating processes often require substantial volumes of water for rinsing and waste treatment. Ionic liquid systems can potentially operate with reduced water requirements, particularly when designed as water-immiscible systems that facilitate metal recovery and ionic liquid recycling, thereby minimizing wastewater generation.
The recyclability of ionic liquids constitutes a significant environmental advantage. Unlike many traditional plating chemicals that become waste after use, properly designed ionic liquid systems can be regenerated and reused through various separation techniques. Research indicates that some ionic liquid plating systems can maintain performance through multiple recycling cycles, substantially reducing waste generation and resource consumption.
Toxicity profiles of ionic liquids vary considerably depending on their specific chemical composition. While some ionic liquids demonstrate lower aquatic toxicity compared to conventional plating chemicals, others may present novel environmental hazards. Comprehensive ecotoxicological assessment remains essential, particularly regarding biodegradability and potential bioaccumulation in aquatic ecosystems.
Energy efficiency considerations also factor into environmental impact assessments. Ionic liquid plating can often operate at lower temperatures than conventional processes, potentially reducing energy consumption. However, the energy required for ionic liquid synthesis and purification must be included in holistic lifecycle assessments to accurately determine net environmental benefits.
Regulatory compliance represents an evolving challenge for ionic liquid plating technologies. As environmental regulations become increasingly stringent worldwide, the reduced hazardous waste generation and lower emission profiles of ionic liquid systems may offer significant compliance advantages and reduced environmental liability for manufacturing operations.
Ionic liquids used in metal plating applications generally exhibit negligible vapor pressure, reducing air pollution concerns associated with volatile organic compounds (VOCs) commonly found in conventional plating solutions. This characteristic significantly diminishes worker exposure to harmful fumes and decreases the need for expensive air handling equipment, resulting in both environmental and occupational health benefits.
Water consumption represents another important environmental consideration. Conventional plating processes often require substantial volumes of water for rinsing and waste treatment. Ionic liquid systems can potentially operate with reduced water requirements, particularly when designed as water-immiscible systems that facilitate metal recovery and ionic liquid recycling, thereby minimizing wastewater generation.
The recyclability of ionic liquids constitutes a significant environmental advantage. Unlike many traditional plating chemicals that become waste after use, properly designed ionic liquid systems can be regenerated and reused through various separation techniques. Research indicates that some ionic liquid plating systems can maintain performance through multiple recycling cycles, substantially reducing waste generation and resource consumption.
Toxicity profiles of ionic liquids vary considerably depending on their specific chemical composition. While some ionic liquids demonstrate lower aquatic toxicity compared to conventional plating chemicals, others may present novel environmental hazards. Comprehensive ecotoxicological assessment remains essential, particularly regarding biodegradability and potential bioaccumulation in aquatic ecosystems.
Energy efficiency considerations also factor into environmental impact assessments. Ionic liquid plating can often operate at lower temperatures than conventional processes, potentially reducing energy consumption. However, the energy required for ionic liquid synthesis and purification must be included in holistic lifecycle assessments to accurately determine net environmental benefits.
Regulatory compliance represents an evolving challenge for ionic liquid plating technologies. As environmental regulations become increasingly stringent worldwide, the reduced hazardous waste generation and lower emission profiles of ionic liquid systems may offer significant compliance advantages and reduced environmental liability for manufacturing operations.
Scale-up and Industrial Implementation Strategies
Scaling up ionic liquid-assisted metal plating processes from laboratory to industrial scale presents significant challenges that require systematic approaches. The transition demands careful consideration of equipment design, process parameters, and economic factors to maintain the quality advantages observed in laboratory settings.
Industrial implementation of ionic liquid-assisted plating requires specialized equipment modifications due to the unique properties of ionic liquids. Conventional plating equipment must be adapted to handle the higher viscosity and different electrical conductivity characteristics of ionic liquids. This often necessitates redesigned agitation systems, modified current distribution mechanisms, and specialized filtration systems capable of handling the more viscous electrolytes without compromising efficiency.
Process parameter optimization becomes increasingly critical at industrial scale. Temperature control systems must be more robust as ionic liquids typically operate at different optimal temperature ranges compared to aqueous systems. Current density distribution requires careful engineering to ensure uniform metal deposition across larger surface areas. Additionally, the recovery and recycling of ionic liquids must be integrated into the production line to offset their higher costs compared to conventional electrolytes.
Economic viability represents a major consideration for industrial adoption. While ionic liquids offer superior adhesion and morphology control, their higher cost compared to traditional plating solutions necessitates efficient recovery and recycling systems. Manufacturers must evaluate the trade-off between increased material costs and the potential value added through improved product quality, reduced rejection rates, and extended component lifespans.
Regulatory compliance and worker safety protocols require updating when implementing ionic liquid-assisted plating at industrial scale. Many jurisdictions have specific requirements for handling and disposal of ionic liquids, which may differ significantly from those for conventional plating chemicals. Companies must develop comprehensive training programs and safety protocols specific to ionic liquid handling.
Pilot-scale testing represents an essential intermediate step between laboratory development and full industrial implementation. This phase allows for identification of scale-dependent issues such as heat transfer limitations, mass transport phenomena, and equipment compatibility challenges. Successful pilot implementation typically involves gradual capacity increases, allowing for iterative optimization before committing to full-scale production facilities.
Industry-academic partnerships have proven valuable for successful scale-up efforts, combining theoretical understanding with practical manufacturing expertise. These collaborations can accelerate the development of specialized equipment and process control systems specifically designed for ionic liquid plating environments, reducing implementation risks and timeframes.
Industrial implementation of ionic liquid-assisted plating requires specialized equipment modifications due to the unique properties of ionic liquids. Conventional plating equipment must be adapted to handle the higher viscosity and different electrical conductivity characteristics of ionic liquids. This often necessitates redesigned agitation systems, modified current distribution mechanisms, and specialized filtration systems capable of handling the more viscous electrolytes without compromising efficiency.
Process parameter optimization becomes increasingly critical at industrial scale. Temperature control systems must be more robust as ionic liquids typically operate at different optimal temperature ranges compared to aqueous systems. Current density distribution requires careful engineering to ensure uniform metal deposition across larger surface areas. Additionally, the recovery and recycling of ionic liquids must be integrated into the production line to offset their higher costs compared to conventional electrolytes.
Economic viability represents a major consideration for industrial adoption. While ionic liquids offer superior adhesion and morphology control, their higher cost compared to traditional plating solutions necessitates efficient recovery and recycling systems. Manufacturers must evaluate the trade-off between increased material costs and the potential value added through improved product quality, reduced rejection rates, and extended component lifespans.
Regulatory compliance and worker safety protocols require updating when implementing ionic liquid-assisted plating at industrial scale. Many jurisdictions have specific requirements for handling and disposal of ionic liquids, which may differ significantly from those for conventional plating chemicals. Companies must develop comprehensive training programs and safety protocols specific to ionic liquid handling.
Pilot-scale testing represents an essential intermediate step between laboratory development and full industrial implementation. This phase allows for identification of scale-dependent issues such as heat transfer limitations, mass transport phenomena, and equipment compatibility challenges. Successful pilot implementation typically involves gradual capacity increases, allowing for iterative optimization before committing to full-scale production facilities.
Industry-academic partnerships have proven valuable for successful scale-up efforts, combining theoretical understanding with practical manufacturing expertise. These collaborations can accelerate the development of specialized equipment and process control systems specifically designed for ionic liquid plating environments, reducing implementation risks and timeframes.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!