What are ionic liquids: structure, properties, and safety
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Liquids Evolution and Research Objectives
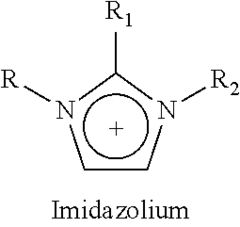
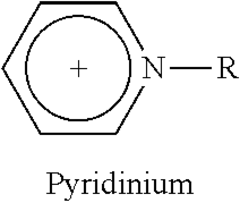
Ionic liquids represent a revolutionary class of materials that have evolved significantly over the past century. Initially discovered in 1914 by Paul Walden with ethylammonium nitrate, these substances remained largely academic curiosities until the late 20th century. The breakthrough came in the 1990s with the development of air and water-stable ionic liquids, particularly those based on imidazolium and pyridinium cations, which catalyzed widespread research interest.
The fundamental characteristic of ionic liquids is their composition of ions with melting points below 100°C, distinguishing them from conventional molten salts. Their unique structural features—asymmetric organic cations paired with various anions—create disrupted crystalline lattices that remain liquid at ambient temperatures. This structural peculiarity underpins their remarkable physicochemical properties.
Current research objectives in the ionic liquids field focus on several interconnected areas. Structure-property relationship studies aim to establish predictive models correlating molecular structure with macroscopic properties, enabling rational design of task-specific ionic liquids. Computational modeling has become increasingly important, with quantum mechanical calculations and molecular dynamics simulations providing insights into ionic interactions and behavior at molecular levels.
Safety assessment represents another critical research objective, particularly as ionic liquids transition from laboratory curiosities to industrial applications. Despite their non-volatility reducing explosion risks, concerns about toxicity and environmental persistence require systematic investigation. Standardized toxicological protocols and lifecycle assessments are being developed to ensure responsible deployment of these materials.
Application-driven research constitutes a major thrust in the field, with efforts to optimize ionic liquids for specific industrial processes. This includes their use as green solvents in chemical synthesis, electrolytes in energy storage devices, lubricants in mechanical systems, and separation media in various industrial processes. Each application demands tailored properties, driving research into structure modification and performance enhancement.
The synthesis of novel ionic liquids with enhanced properties represents another key objective. Researchers are exploring functionalized ionic liquids incorporating specific chemical groups to impart targeted properties, such as metal-binding capabilities, catalytic activity, or biocompatibility. Additionally, the development of dual-functional ionic liquids that can serve multiple roles simultaneously (e.g., as both solvent and catalyst) is gaining momentum.
Scaling production technologies for industrial implementation completes the research landscape, focusing on cost-effective, environmentally benign synthesis routes and purification methods to facilitate broader adoption of ionic liquid technologies across various sectors.
The fundamental characteristic of ionic liquids is their composition of ions with melting points below 100°C, distinguishing them from conventional molten salts. Their unique structural features—asymmetric organic cations paired with various anions—create disrupted crystalline lattices that remain liquid at ambient temperatures. This structural peculiarity underpins their remarkable physicochemical properties.
Current research objectives in the ionic liquids field focus on several interconnected areas. Structure-property relationship studies aim to establish predictive models correlating molecular structure with macroscopic properties, enabling rational design of task-specific ionic liquids. Computational modeling has become increasingly important, with quantum mechanical calculations and molecular dynamics simulations providing insights into ionic interactions and behavior at molecular levels.
Safety assessment represents another critical research objective, particularly as ionic liquids transition from laboratory curiosities to industrial applications. Despite their non-volatility reducing explosion risks, concerns about toxicity and environmental persistence require systematic investigation. Standardized toxicological protocols and lifecycle assessments are being developed to ensure responsible deployment of these materials.
Application-driven research constitutes a major thrust in the field, with efforts to optimize ionic liquids for specific industrial processes. This includes their use as green solvents in chemical synthesis, electrolytes in energy storage devices, lubricants in mechanical systems, and separation media in various industrial processes. Each application demands tailored properties, driving research into structure modification and performance enhancement.
The synthesis of novel ionic liquids with enhanced properties represents another key objective. Researchers are exploring functionalized ionic liquids incorporating specific chemical groups to impart targeted properties, such as metal-binding capabilities, catalytic activity, or biocompatibility. Additionally, the development of dual-functional ionic liquids that can serve multiple roles simultaneously (e.g., as both solvent and catalyst) is gaining momentum.
Scaling production technologies for industrial implementation completes the research landscape, focusing on cost-effective, environmentally benign synthesis routes and purification methods to facilitate broader adoption of ionic liquid technologies across various sectors.
Market Applications and Demand Analysis for Ionic Liquids
The global market for ionic liquids has been experiencing significant growth, driven by their unique properties and versatility across multiple industries. Current market valuations indicate that the ionic liquids market is expanding at a compound annual growth rate of approximately 8-10%, with projections suggesting it will reach several hundred million dollars by 2025. This growth trajectory reflects increasing industrial adoption and diversification of applications.
The chemical industry represents the largest market segment for ionic liquids, where they serve as green alternatives to conventional volatile organic solvents. Their negligible vapor pressure, non-flammability, and recyclability align perfectly with growing regulatory pressures and sustainability initiatives. Particularly in catalysis and separation processes, demand has increased as companies seek to reduce environmental footprints while maintaining or improving process efficiency.
Energy storage applications constitute another rapidly expanding market segment. The electrochemical stability and high ionic conductivity of ionic liquids make them excellent candidates for next-generation electrolytes in batteries and supercapacitors. With the electric vehicle market expanding at double-digit rates annually, the demand for advanced energy storage solutions incorporating ionic liquids is projected to grow substantially.
Biomass processing represents an emerging application with significant potential. As industries transition toward renewable feedstocks, ionic liquids' ability to dissolve cellulose and lignin efficiently positions them as key enablers in biorefinery processes. Market analysts predict this segment could experience growth rates exceeding 12% annually as bioeconomy initiatives gain momentum globally.
Pharmaceutical and biotechnology sectors are increasingly exploring ionic liquids for drug delivery systems, protein stabilization, and as reaction media for enzyme-catalyzed processes. The market demand in these high-value applications is driven by the need for improved drug formulations and more efficient biocatalytic processes.
Geographical analysis reveals that North America and Europe currently dominate market consumption, primarily due to stringent environmental regulations and advanced research infrastructure. However, Asia-Pacific regions, particularly China, Japan, and South Korea, are showing the fastest growth rates as their chemical and electronics industries rapidly adopt these advanced materials.
Customer demand patterns indicate a growing preference for task-specific ionic liquids designed for particular applications rather than general-purpose formulations. This trend is driving increased research into structure-property relationships to develop optimized ionic liquids with enhanced performance characteristics for specific industrial processes.
The chemical industry represents the largest market segment for ionic liquids, where they serve as green alternatives to conventional volatile organic solvents. Their negligible vapor pressure, non-flammability, and recyclability align perfectly with growing regulatory pressures and sustainability initiatives. Particularly in catalysis and separation processes, demand has increased as companies seek to reduce environmental footprints while maintaining or improving process efficiency.
Energy storage applications constitute another rapidly expanding market segment. The electrochemical stability and high ionic conductivity of ionic liquids make them excellent candidates for next-generation electrolytes in batteries and supercapacitors. With the electric vehicle market expanding at double-digit rates annually, the demand for advanced energy storage solutions incorporating ionic liquids is projected to grow substantially.
Biomass processing represents an emerging application with significant potential. As industries transition toward renewable feedstocks, ionic liquids' ability to dissolve cellulose and lignin efficiently positions them as key enablers in biorefinery processes. Market analysts predict this segment could experience growth rates exceeding 12% annually as bioeconomy initiatives gain momentum globally.
Pharmaceutical and biotechnology sectors are increasingly exploring ionic liquids for drug delivery systems, protein stabilization, and as reaction media for enzyme-catalyzed processes. The market demand in these high-value applications is driven by the need for improved drug formulations and more efficient biocatalytic processes.
Geographical analysis reveals that North America and Europe currently dominate market consumption, primarily due to stringent environmental regulations and advanced research infrastructure. However, Asia-Pacific regions, particularly China, Japan, and South Korea, are showing the fastest growth rates as their chemical and electronics industries rapidly adopt these advanced materials.
Customer demand patterns indicate a growing preference for task-specific ionic liquids designed for particular applications rather than general-purpose formulations. This trend is driving increased research into structure-property relationships to develop optimized ionic liquids with enhanced performance characteristics for specific industrial processes.
Current Development Status and Technical Barriers
Ionic liquids (ILs) have emerged as a significant area of research in green chemistry over the past two decades. Currently, more than 10,000 different ionic liquids have been synthesized and characterized, with applications spanning catalysis, electrochemistry, separation processes, and materials science. The global market for ionic liquids was valued at approximately $39.6 million in 2021 and is projected to reach $72.3 million by 2027, growing at a CAGR of 8.2%.
Research institutions across North America, Europe, and Asia have established dedicated centers for ionic liquid research, with China, the United States, and Germany leading in publication output. Industrial adoption has accelerated, particularly in pharmaceutical processing, where companies like BASF and Merck have implemented IL-based technologies for synthesis and purification processes.
Despite this progress, several significant technical barriers impede the widespread implementation of ionic liquids. Cost remains a primary concern, with most ILs being 5-20 times more expensive than conventional solvents, limiting their industrial scalability. The synthesis of high-purity ILs often requires multiple purification steps, increasing production complexity and cost.
Toxicity and environmental impact assessments present another major challenge. While ILs are often marketed as "green" alternatives, comprehensive toxicological profiles are available for only about 5% of known ionic liquids. Recent studies have revealed that certain imidazolium and pyridinium-based ILs exhibit significant aquatic toxicity and poor biodegradability, contradicting earlier assumptions about their environmental benignity.
Stability issues also persist, particularly for task-specific ionic liquids. Many functionalized ILs demonstrate thermal or chemical degradation under industrial processing conditions, limiting their application range. For instance, fluorinated anions can decompose to release toxic hydrogen fluoride when exposed to moisture at elevated temperatures.
Standardization represents another significant barrier. The lack of unified protocols for characterization, purity assessment, and performance testing hampers comparative analysis and technology transfer. The International Union of Pure and Applied Chemistry (IUPAC) has initiated projects to address this gap, but comprehensive standards remain under development.
Scalability challenges further complicate industrial implementation. Laboratory-scale synthesis methods often prove inefficient or economically unfeasible at production scale. Continuous flow processes for IL synthesis have shown promise but require further optimization to achieve consistent quality and yield at industrial volumes.
Research institutions across North America, Europe, and Asia have established dedicated centers for ionic liquid research, with China, the United States, and Germany leading in publication output. Industrial adoption has accelerated, particularly in pharmaceutical processing, where companies like BASF and Merck have implemented IL-based technologies for synthesis and purification processes.
Despite this progress, several significant technical barriers impede the widespread implementation of ionic liquids. Cost remains a primary concern, with most ILs being 5-20 times more expensive than conventional solvents, limiting their industrial scalability. The synthesis of high-purity ILs often requires multiple purification steps, increasing production complexity and cost.
Toxicity and environmental impact assessments present another major challenge. While ILs are often marketed as "green" alternatives, comprehensive toxicological profiles are available for only about 5% of known ionic liquids. Recent studies have revealed that certain imidazolium and pyridinium-based ILs exhibit significant aquatic toxicity and poor biodegradability, contradicting earlier assumptions about their environmental benignity.
Stability issues also persist, particularly for task-specific ionic liquids. Many functionalized ILs demonstrate thermal or chemical degradation under industrial processing conditions, limiting their application range. For instance, fluorinated anions can decompose to release toxic hydrogen fluoride when exposed to moisture at elevated temperatures.
Standardization represents another significant barrier. The lack of unified protocols for characterization, purity assessment, and performance testing hampers comparative analysis and technology transfer. The International Union of Pure and Applied Chemistry (IUPAC) has initiated projects to address this gap, but comprehensive standards remain under development.
Scalability challenges further complicate industrial implementation. Laboratory-scale synthesis methods often prove inefficient or economically unfeasible at production scale. Continuous flow processes for IL synthesis have shown promise but require further optimization to achieve consistent quality and yield at industrial volumes.
Contemporary Ionic Liquid Structure-Property Relationship Models
01 Structure and composition of ionic liquids
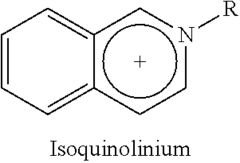
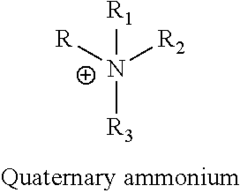
Ionic liquids are composed of organic cations and inorganic or organic anions that remain in liquid state at relatively low temperatures. Their structure typically includes asymmetric organic cations such as imidazolium, pyridinium, or quaternary ammonium, paired with anions like tetrafluoroborate, hexafluorophosphate, or bis(trifluoromethylsulfonyl)imide. This unique structure contributes to their low melting points and stability. The structural design can be tailored by modifying the cation or anion to achieve specific physicochemical properties for various applications.- Structure and composition of ionic liquids: Ionic liquids are salts that remain in liquid state at relatively low temperatures, typically composed of organic cations and inorganic or organic anions. Their structure can be tailored by selecting different cation-anion combinations, allowing for customization of properties. Common cations include imidazolium, pyridinium, and quaternary ammonium, while anions may include halides, tetrafluoroborate, and hexafluorophosphate. The asymmetry and size of these ions prevent efficient packing, resulting in their liquid state at room temperature.
- Physical and chemical properties of ionic liquids: Ionic liquids possess unique properties including negligible vapor pressure, high thermal stability, wide liquid temperature range, and excellent solvation capabilities for various compounds. They exhibit good electrical conductivity, making them suitable for electrochemical applications. Their viscosity and density can be adjusted by modifying their chemical structure. These liquids also demonstrate remarkable chemical stability, resistance to oxidation, and the ability to dissolve both organic and inorganic materials, making them versatile for numerous applications.
- Safety and toxicological aspects of ionic liquids: The safety profile of ionic liquids varies widely depending on their specific composition. While often marketed as environmentally friendly alternatives to volatile organic solvents due to their low volatility, some ionic liquids can exhibit toxicity to aquatic organisms and potential bioaccumulation. Toxicity is generally related to the cation structure, with longer alkyl chains typically increasing toxicity. Safety assessments include biodegradability, ecotoxicity, and cytotoxicity studies. Proper handling protocols and risk assessments are essential when working with these compounds.
- Applications of ionic liquids in various industries: Ionic liquids find applications across numerous industries due to their unique properties. They are used as electrolytes in batteries and capacitors, as solvents for chemical reactions and extractions, and as lubricants in mechanical systems. In the pharmaceutical industry, they serve as reaction media and for drug delivery systems. Their application extends to catalysis, gas absorption, biomass processing, and separation technologies. The tunability of their properties allows for specialized applications in electrochemistry, materials science, and green chemistry processes.
- Synthesis and purification methods for ionic liquids: The synthesis of ionic liquids typically involves quaternization reactions followed by anion exchange. Common methods include direct combination of halide salts with metal salts, acid-base neutralization, and metathesis reactions. Purification is crucial as impurities significantly affect properties and performance. Techniques include column chromatography, extraction, recrystallization, and vacuum distillation. Advanced methods such as supercritical fluid extraction and membrane filtration are also employed. Quality control measures include spectroscopic analysis, thermal analysis, and electrochemical characterization to ensure purity and consistency.
02 Physicochemical properties of ionic liquids
Ionic liquids exhibit remarkable physicochemical properties including negligible vapor pressure, high thermal stability, wide liquid temperature range, and excellent solvation capabilities for both organic and inorganic compounds. They possess tunable properties such as viscosity, conductivity, and polarity that can be adjusted by modifying their chemical structure. Their non-volatility makes them environmentally advantageous compared to conventional volatile organic solvents. Additionally, many ionic liquids demonstrate good electrochemical stability, making them suitable for applications in electrochemistry and energy storage systems.Expand Specific Solutions03 Safety and toxicological aspects of ionic liquids
The safety profile of ionic liquids varies significantly depending on their specific chemical composition. While often marketed as environmentally friendly alternatives to volatile organic solvents due to their negligible vapor pressure, certain ionic liquids may exhibit toxicity to aquatic organisms or demonstrate cytotoxicity. Safety assessments typically evaluate parameters such as acute toxicity, biodegradability, bioaccumulation potential, and ecotoxicity. Research indicates that ionic liquids with shorter alkyl chains generally show lower toxicity. Proper handling protocols and safety measures should be implemented when working with ionic liquids, particularly for novel compositions where toxicological data may be limited.Expand Specific Solutions04 Applications of ionic liquids in various industries
Ionic liquids find diverse applications across multiple industries due to their unique properties. In chemical processing, they serve as green solvents for reactions, separations, and extractions. The electrochemical industry utilizes them in batteries, fuel cells, and capacitors due to their ionic conductivity and wide electrochemical window. In pharmaceuticals, ionic liquids function as drug delivery systems and active pharmaceutical ingredients. They also play roles in biomass processing, lubricants, thermal fluids, and gas capture technologies. Their tailorable nature allows for specific optimization for each application, making them versatile materials for solving complex industrial challenges.Expand Specific Solutions05 Synthesis and preparation methods of ionic liquids
Various methods exist for synthesizing ionic liquids, with the most common being quaternization reactions followed by anion exchange. Direct combination of halide salts with metal salts containing the desired anion is another prevalent approach. Microwave-assisted synthesis has gained popularity for its efficiency and reduced reaction times. Purification techniques are crucial in ionic liquid preparation, as impurities can significantly affect their properties. Methods include column chromatography, recrystallization, and washing with appropriate solvents. Recent advances focus on developing greener synthesis routes with fewer steps, higher yields, and reduced waste generation to enhance the environmental credentials of ionic liquids.Expand Specific Solutions
Leading Research Institutions and Industrial Manufacturers
The ionic liquids market is currently in a growth phase, characterized by increasing research and commercial applications across various industries. The global market size is expanding steadily, driven by the unique properties of ionic liquids as green solvents and their versatility in chemical processes. From a technological maturity perspective, companies like BASF, DuPont, and Merck Patent GmbH are leading commercial development with established product lines, while academic institutions such as Peking University, Zhejiang University, and the Institute of Process Engineering (CAS) are advancing fundamental research on structure-property relationships. The Chemours Co. and Idemitsu Kosan are focusing on specialized applications, particularly in industrial processes. Safety considerations remain a key focus area, with collaborative efforts between industry players and research institutions addressing toxicity and environmental impact concerns.
Merck Patent GmbH
Technical Solution: Merck has established itself as a pioneer in ionic liquid research with their IOLITEC division specializing in custom-designed ionic liquids. Their approach centers on structure-property relationships, developing over 400 different ionic liquids with precisely engineered properties[1]. Merck's research has yielded significant breakthroughs in understanding how structural modifications to cations (imidazolium, pyridinium, ammonium) and anions (tetrafluoroborate, hexafluorophosphate, bis(trifluoromethylsulfonyl)imide) affect physical properties including viscosity, conductivity, and thermal stability[2]. Their proprietary synthesis methods enable production of ultra-high purity ionic liquids (>99.9%) essential for electrochemical applications. Merck has developed specialized characterization techniques for ionic liquid safety assessment, including comprehensive toxicity profiling and environmental persistence evaluation[3]. Their research extends to application-specific ionic liquids for pharmaceuticals, including API-ILs (Active Pharmaceutical Ingredient Ionic Liquids) that enhance drug solubility and bioavailability.
Strengths: Exceptional purity standards exceeding industry norms; comprehensive analytical capabilities for structure-property correlation; established global distribution network for research-grade ionic liquids. Weaknesses: Higher cost structure compared to bulk chemical producers; primarily focused on research-scale production rather than industrial volumes; limited presence in certain application sectors like petroleum processing.
DuPont de Nemours, Inc.
Technical Solution: DuPont has developed significant expertise in fluorinated ionic liquids, leveraging their fluoropolymer technology background. Their research focuses on perfluoroalkyl-containing ionic liquids with exceptional chemical stability and unique solvation properties[1]. DuPont's proprietary synthesis routes yield ionic liquids with hydrophobic domains from fluorinated chains alongside hydrophilic ionic regions, creating amphiphilic structures with self-assembly capabilities at nanoscale[2]. Their ionic liquid portfolio includes compounds with thermal stability exceeding 350°C and electrochemical windows approaching 6V, making them suitable for extreme condition applications. DuPont has pioneered safety assessment protocols specifically for fluorinated ionic liquids, addressing bioaccumulation concerns through structure modification and degradation pathway engineering[3]. Their research extends to applications in fluoropolymer processing, where ionic liquids serve as specialized reaction media enabling previously impossible polymerization conditions and polymer architectures.
Strengths: Unparalleled expertise in fluorinated ionic liquid chemistry; established safety testing protocols specific to fluorinated compounds; integration with broader fluoropolymer technology portfolio. Weaknesses: Higher production costs for fluorinated ionic liquids; potential environmental persistence concerns with some fluorinated structures; narrower application focus compared to general ionic liquid producers.
Breakthrough Patents and Scientific Literature in Ionic Liquid Design
Halogen-free ionic liquids in naphtha desulfurization and their recovery
PatentInactiveUS20150090639A1
Innovation
- Development of halogen-free ionic liquids with aliphatic or aromatic carboxylate anions, specifically synthesized using alkyl, alkenyl, or benzyl-functionalized halides and imidazoles, pyridines, or tertiary amines, which can be reused through heating, reduced pressure, and solvent washing for deep desulfurization of naphtha via liquid-liquid extraction at room temperature and pressure.
Lubricating oil
PatentInactiveEP1672051A8
Innovation
- Employing an ionic liquid as the base oil, specifically formulated with a cation and anion combination that provides a high ion concentration, controlled acid value, and specific anion species to achieve the desired properties, along with the option of mixing ionic liquid species to enhance physical characteristics and incorporating additives for improved performance.
Environmental Impact and Sustainability Considerations
The environmental impact of ionic liquids (ILs) represents a critical dimension in their research and application landscape. Initially promoted as "green solvents" due to their negligible vapor pressure and reduced air pollution potential compared to volatile organic compounds, subsequent research has revealed a more nuanced environmental profile. Many conventional ILs demonstrate significant aquatic toxicity, with imidazolium and pyridinium-based ILs showing particular ecotoxicological concerns in various aquatic organisms including algae, daphnia, and fish.
Biodegradability assessments indicate that many traditional ILs, especially those with fluorinated anions like [PF6]- and [BF4]-, exhibit poor degradation rates in natural environments. This persistence raises concerns about long-term accumulation in ecosystems. The environmental fate of ILs is further complicated by their high water solubility, which facilitates potential contamination of aquatic systems through industrial discharge or accidental release.
Recent sustainability efforts have focused on developing environmentally benign ILs through strategic molecular design. Bio-based ILs derived from renewable resources such as amino acids, carbohydrates, and choline represent promising alternatives with improved biodegradability profiles. These "third-generation" ILs often demonstrate reduced toxicity while maintaining desirable physicochemical properties for industrial applications.
Life cycle assessment (LCA) studies comparing ILs with conventional solvents reveal complex sustainability trade-offs. While ILs offer advantages in reduced emissions and recyclability, their synthesis often involves energy-intensive processes and precursors with significant environmental footprints. The environmental benefits of ILs are most pronounced in applications enabling multiple reuse cycles, thereby amortizing the initial production impacts.
Regulatory frameworks for IL environmental management continue to evolve globally. The European REACH regulation has begun addressing ILs as potential substances of concern, while researchers advocate for standardized ecotoxicological testing protocols specific to these compounds. Predictive models using quantitative structure-activity relationships (QSARs) are being developed to screen IL candidates for environmental compatibility before synthesis.
Industrial implementation of ILs increasingly incorporates environmental considerations through green chemistry principles. Strategies include designing ILs with biodegradable components, optimizing recycling processes to minimize waste, and developing efficient recovery methods from aqueous streams. These approaches aim to balance the technological advantages of ILs with environmental responsibility, ensuring their sustainable integration into chemical processes and products.
Biodegradability assessments indicate that many traditional ILs, especially those with fluorinated anions like [PF6]- and [BF4]-, exhibit poor degradation rates in natural environments. This persistence raises concerns about long-term accumulation in ecosystems. The environmental fate of ILs is further complicated by their high water solubility, which facilitates potential contamination of aquatic systems through industrial discharge or accidental release.
Recent sustainability efforts have focused on developing environmentally benign ILs through strategic molecular design. Bio-based ILs derived from renewable resources such as amino acids, carbohydrates, and choline represent promising alternatives with improved biodegradability profiles. These "third-generation" ILs often demonstrate reduced toxicity while maintaining desirable physicochemical properties for industrial applications.
Life cycle assessment (LCA) studies comparing ILs with conventional solvents reveal complex sustainability trade-offs. While ILs offer advantages in reduced emissions and recyclability, their synthesis often involves energy-intensive processes and precursors with significant environmental footprints. The environmental benefits of ILs are most pronounced in applications enabling multiple reuse cycles, thereby amortizing the initial production impacts.
Regulatory frameworks for IL environmental management continue to evolve globally. The European REACH regulation has begun addressing ILs as potential substances of concern, while researchers advocate for standardized ecotoxicological testing protocols specific to these compounds. Predictive models using quantitative structure-activity relationships (QSARs) are being developed to screen IL candidates for environmental compatibility before synthesis.
Industrial implementation of ILs increasingly incorporates environmental considerations through green chemistry principles. Strategies include designing ILs with biodegradable components, optimizing recycling processes to minimize waste, and developing efficient recovery methods from aqueous streams. These approaches aim to balance the technological advantages of ILs with environmental responsibility, ensuring their sustainable integration into chemical processes and products.
Toxicological Assessment and Safety Protocols
The toxicological assessment of ionic liquids (ILs) has become increasingly important as these compounds gain prominence in industrial applications. Current research indicates that while ILs are often marketed as "green solvents," their toxicity profiles vary significantly based on their structural components. Cationic moieties, particularly those containing imidazolium and pyridinium groups, demonstrate dose-dependent cytotoxicity in multiple cell lines, with longer alkyl chain lengths generally correlating with increased toxicity.
Acute toxicity studies reveal that many ILs exhibit EC50 values comparable to conventional organic solvents, challenging their universal classification as environmentally benign alternatives. Particularly concerning are findings that certain ILs can penetrate cell membranes due to their amphiphilic nature, potentially disrupting cellular functions and triggering apoptotic pathways. Recent investigations using zebrafish models have demonstrated developmental toxicity at concentrations as low as 1-5 mg/L for some imidazolium-based ILs.
Environmental persistence represents another critical safety concern, as many ILs resist conventional biodegradation processes. Studies indicate half-lives exceeding 30 days in standard environmental conditions for numerous commercially relevant ILs, particularly those containing fluorinated anions such as [BF4]- and [PF6]-. This persistence, coupled with water solubility, raises significant concerns about potential bioaccumulation in aquatic ecosystems.
Standardized safety protocols for handling ILs have evolved significantly in recent years. Current best practices include mandatory use of nitrile gloves rather than latex (which offers insufficient protection against IL penetration), face shields during transfer operations, and specialized containment systems for storage. Workplace exposure limits remain under development, though interim guidelines suggest maintaining airborne concentrations below 1 mg/m³ for most common ILs, with more stringent limits for those containing known toxic elements.
Risk assessment frameworks specifically designed for ILs have been developed by several regulatory bodies, incorporating both structural alert systems and experimental toxicity data. The European Chemicals Agency has recently implemented a tiered approach for IL evaluation, requiring comprehensive toxicological profiling for novel ILs before commercial deployment. This includes mandatory acute toxicity, genotoxicity, and ecotoxicity assessments following OECD guidelines.
Detoxification and spill management protocols for ILs differ substantially from those for conventional solvents. Current recommendations emphasize adsorption using activated carbon or specialized polymeric materials rather than dilution, as the latter may actually enhance mobility and environmental distribution of these persistent compounds. Emergency response guidelines now include IL-specific neutralization procedures based on counter-ion precipitation techniques.
Acute toxicity studies reveal that many ILs exhibit EC50 values comparable to conventional organic solvents, challenging their universal classification as environmentally benign alternatives. Particularly concerning are findings that certain ILs can penetrate cell membranes due to their amphiphilic nature, potentially disrupting cellular functions and triggering apoptotic pathways. Recent investigations using zebrafish models have demonstrated developmental toxicity at concentrations as low as 1-5 mg/L for some imidazolium-based ILs.
Environmental persistence represents another critical safety concern, as many ILs resist conventional biodegradation processes. Studies indicate half-lives exceeding 30 days in standard environmental conditions for numerous commercially relevant ILs, particularly those containing fluorinated anions such as [BF4]- and [PF6]-. This persistence, coupled with water solubility, raises significant concerns about potential bioaccumulation in aquatic ecosystems.
Standardized safety protocols for handling ILs have evolved significantly in recent years. Current best practices include mandatory use of nitrile gloves rather than latex (which offers insufficient protection against IL penetration), face shields during transfer operations, and specialized containment systems for storage. Workplace exposure limits remain under development, though interim guidelines suggest maintaining airborne concentrations below 1 mg/m³ for most common ILs, with more stringent limits for those containing known toxic elements.
Risk assessment frameworks specifically designed for ILs have been developed by several regulatory bodies, incorporating both structural alert systems and experimental toxicity data. The European Chemicals Agency has recently implemented a tiered approach for IL evaluation, requiring comprehensive toxicological profiling for novel ILs before commercial deployment. This includes mandatory acute toxicity, genotoxicity, and ecotoxicity assessments following OECD guidelines.
Detoxification and spill management protocols for ILs differ substantially from those for conventional solvents. Current recommendations emphasize adsorption using activated carbon or specialized polymeric materials rather than dilution, as the latter may actually enhance mobility and environmental distribution of these persistent compounds. Emergency response guidelines now include IL-specific neutralization procedures based on counter-ion precipitation techniques.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!