Ionic liquids in lithium metal batteries: dendrite control
AUG 25, 20259 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Ionic Liquids in LMB: Background and Objectives
Lithium metal batteries (LMBs) have emerged as a promising next-generation energy storage technology due to their exceptionally high theoretical energy density (3860 mAh/g), which significantly surpasses that of conventional lithium-ion batteries. This remarkable potential positions LMBs as a critical technology for addressing the growing demand for high-energy-density storage solutions in electric vehicles, portable electronics, and renewable energy systems.
The evolution of LMB technology can be traced back to the 1970s when the first commercial lithium batteries were developed. However, these early attempts faced severe safety issues primarily due to dendrite formation—irregular, branch-like lithium metal structures that grow during charging cycles. These dendrites can penetrate the separator, causing short circuits, thermal runaway, and potentially catastrophic battery failure. This fundamental challenge led to the temporary abandonment of LMB technology in favor of safer lithium-ion alternatives.
Recent years have witnessed a resurgence of interest in LMBs, driven by the limitations of current lithium-ion technology in meeting increasingly demanding energy storage requirements. The technical evolution has focused on addressing the dendrite formation issue through various approaches, including advanced electrolyte systems, engineered separators, and novel cell architectures.
Ionic liquids (ILs) have emerged as a particularly promising solution for dendrite control in LMBs. These molten salts, which remain liquid at room temperature, offer unique properties including negligible volatility, non-flammability, high ionic conductivity, and wide electrochemical stability windows. The distinctive solvation environment and interfacial chemistry provided by ionic liquids can significantly alter lithium deposition behavior, potentially suppressing dendritic growth.
The primary technical objective in this field is to develop ionic liquid-based electrolyte systems that effectively prevent dendrite formation while maintaining high ionic conductivity, favorable interfacial properties, and compatibility with lithium metal anodes. This involves understanding the fundamental mechanisms of lithium electrodeposition in ionic liquid environments and engineering IL compositions that promote uniform lithium plating.
Additional objectives include enhancing the cycling stability of lithium metal anodes in IL electrolytes, reducing interfacial resistance, improving rate capability, and ensuring long-term electrochemical performance. The ultimate goal is to enable safe, high-energy-density LMBs with long cycle life and reliable operation across a wide temperature range, thereby unlocking the full potential of lithium metal as an anode material.
The technological trajectory suggests a gradual transition from conventional organic electrolytes to hybrid systems incorporating ionic liquids, and eventually to optimized IL-based electrolytes specifically designed for dendrite-free lithium metal batteries. This evolution represents a critical pathway toward next-generation energy storage solutions with significantly improved performance metrics.
The evolution of LMB technology can be traced back to the 1970s when the first commercial lithium batteries were developed. However, these early attempts faced severe safety issues primarily due to dendrite formation—irregular, branch-like lithium metal structures that grow during charging cycles. These dendrites can penetrate the separator, causing short circuits, thermal runaway, and potentially catastrophic battery failure. This fundamental challenge led to the temporary abandonment of LMB technology in favor of safer lithium-ion alternatives.
Recent years have witnessed a resurgence of interest in LMBs, driven by the limitations of current lithium-ion technology in meeting increasingly demanding energy storage requirements. The technical evolution has focused on addressing the dendrite formation issue through various approaches, including advanced electrolyte systems, engineered separators, and novel cell architectures.
Ionic liquids (ILs) have emerged as a particularly promising solution for dendrite control in LMBs. These molten salts, which remain liquid at room temperature, offer unique properties including negligible volatility, non-flammability, high ionic conductivity, and wide electrochemical stability windows. The distinctive solvation environment and interfacial chemistry provided by ionic liquids can significantly alter lithium deposition behavior, potentially suppressing dendritic growth.
The primary technical objective in this field is to develop ionic liquid-based electrolyte systems that effectively prevent dendrite formation while maintaining high ionic conductivity, favorable interfacial properties, and compatibility with lithium metal anodes. This involves understanding the fundamental mechanisms of lithium electrodeposition in ionic liquid environments and engineering IL compositions that promote uniform lithium plating.
Additional objectives include enhancing the cycling stability of lithium metal anodes in IL electrolytes, reducing interfacial resistance, improving rate capability, and ensuring long-term electrochemical performance. The ultimate goal is to enable safe, high-energy-density LMBs with long cycle life and reliable operation across a wide temperature range, thereby unlocking the full potential of lithium metal as an anode material.
The technological trajectory suggests a gradual transition from conventional organic electrolytes to hybrid systems incorporating ionic liquids, and eventually to optimized IL-based electrolytes specifically designed for dendrite-free lithium metal batteries. This evolution represents a critical pathway toward next-generation energy storage solutions with significantly improved performance metrics.
Market Analysis for Advanced Lithium Battery Technologies
The global lithium battery market is experiencing unprecedented growth, projected to reach $116 billion by 2030 with a CAGR of 19.8% from 2023. This expansion is primarily driven by increasing demand for electric vehicles (EVs), which is expected to grow tenfold by 2030 compared to 2020 levels. Within this broader market, advanced lithium metal batteries represent a particularly promising segment due to their theoretical energy density of 3860 mAh/g, significantly higher than conventional lithium-ion batteries (372 mAh/g for graphite anodes).
The market for dendrite control technologies in lithium metal batteries is emerging as a critical subsector, with ionic liquid-based solutions gaining substantial attention. Current market penetration remains limited, with less than 5% of commercial lithium batteries utilizing ionic liquid electrolytes. However, investment in this technology has increased by approximately 45% annually since 2020, indicating strong commercial interest.
Consumer electronics currently represents the largest application segment for advanced lithium batteries, accounting for approximately 40% of market share. However, automotive applications are growing at the fastest rate and are expected to become the dominant segment by 2027. Energy storage systems represent the third major application segment, growing steadily at 25% annually as grid-scale storage solutions become more prevalent.
Regionally, Asia-Pacific dominates the market with over 60% share, led by China, Japan, and South Korea. North America follows with approximately 20% market share, while Europe accounts for about 15%. The remaining 5% is distributed across other regions. China leads in manufacturing capacity, while South Korea and Japan excel in high-performance battery technology development.
Key market drivers include increasingly stringent emissions regulations worldwide, declining battery costs (decreasing at approximately 8% annually), and growing consumer acceptance of electric vehicles. The push for higher energy density batteries is particularly strong in the premium EV segment, where range anxiety remains a significant consumer concern.
Market barriers include safety concerns related to dendrite formation, high production costs of ionic liquids compared to conventional electrolytes, and scaling challenges. The cost premium for ionic liquid-based batteries currently stands at 30-40% above conventional lithium-ion batteries, though this gap is narrowing as production scales increase.
Emerging market opportunities include the premium portable electronics segment, where consumers demonstrate willingness to pay for longer battery life, and the aerospace sector, where weight reduction justifies higher battery costs. The military and defense sector also shows significant interest in high-energy-density batteries with enhanced safety profiles.
The market for dendrite control technologies in lithium metal batteries is emerging as a critical subsector, with ionic liquid-based solutions gaining substantial attention. Current market penetration remains limited, with less than 5% of commercial lithium batteries utilizing ionic liquid electrolytes. However, investment in this technology has increased by approximately 45% annually since 2020, indicating strong commercial interest.
Consumer electronics currently represents the largest application segment for advanced lithium batteries, accounting for approximately 40% of market share. However, automotive applications are growing at the fastest rate and are expected to become the dominant segment by 2027. Energy storage systems represent the third major application segment, growing steadily at 25% annually as grid-scale storage solutions become more prevalent.
Regionally, Asia-Pacific dominates the market with over 60% share, led by China, Japan, and South Korea. North America follows with approximately 20% market share, while Europe accounts for about 15%. The remaining 5% is distributed across other regions. China leads in manufacturing capacity, while South Korea and Japan excel in high-performance battery technology development.
Key market drivers include increasingly stringent emissions regulations worldwide, declining battery costs (decreasing at approximately 8% annually), and growing consumer acceptance of electric vehicles. The push for higher energy density batteries is particularly strong in the premium EV segment, where range anxiety remains a significant consumer concern.
Market barriers include safety concerns related to dendrite formation, high production costs of ionic liquids compared to conventional electrolytes, and scaling challenges. The cost premium for ionic liquid-based batteries currently stands at 30-40% above conventional lithium-ion batteries, though this gap is narrowing as production scales increase.
Emerging market opportunities include the premium portable electronics segment, where consumers demonstrate willingness to pay for longer battery life, and the aerospace sector, where weight reduction justifies higher battery costs. The military and defense sector also shows significant interest in high-energy-density batteries with enhanced safety profiles.
Current Challenges in Dendrite Control Technologies
Despite significant advancements in lithium-ion battery technology, lithium metal batteries face persistent challenges in dendrite control. The formation of lithium dendrites during charging cycles represents one of the most critical barriers to commercial viability. These needle-like structures grow from the anode surface, potentially penetrating the separator and causing catastrophic short circuits, thermal runaway, and safety hazards. Current dendrite control technologies struggle with fundamental limitations in both effectiveness and practicality.
Electrolyte engineering approaches, while promising, face stability issues at high current densities. Conventional organic electrolytes decompose at the lithium metal interface, forming unstable SEI (Solid Electrolyte Interphase) layers that cannot effectively suppress dendrite nucleation. Ionic liquid-based electrolytes show improved thermal stability but often suffer from high viscosity and limited ionic conductivity, restricting their performance in fast-charging scenarios.
Physical barrier methods, including artificial SEI layers and structured current collectors, demonstrate limited long-term durability. These engineered interfaces typically degrade after repeated cycling, losing their dendrite suppression capabilities. Additionally, manufacturing these specialized interfaces at scale presents significant cost and process integration challenges that impede industrial adoption.
Pressure-based dendrite control strategies require complex mechanical systems that add weight, volume, and cost to battery designs. The non-uniform pressure distribution across large-format cells often results in inconsistent dendrite suppression, creating reliability concerns for commercial applications.
Temperature management approaches show promise in laboratory settings but translate poorly to real-world conditions where ambient temperatures fluctuate. The narrow operational window for optimal dendrite suppression conflicts with the broad temperature range required for practical applications, particularly in automotive and outdoor environments.
Current computational models for dendrite growth prediction lack sufficient accuracy for practical implementation. The complex electrochemical processes governing dendrite formation involve multiple variables that existing models cannot fully capture, limiting the effectiveness of predictive control strategies.
The integration of multiple dendrite control technologies often creates compatibility issues. Combining ionic liquid electrolytes with physical barriers or pressure systems introduces interface challenges that can actually accelerate dendrite formation at boundary regions. This system-level integration problem represents a significant hurdle for comprehensive dendrite control solutions.
Economic viability remains a critical challenge, as many promising dendrite control technologies require expensive materials or complex manufacturing processes. The cost-performance balance has not yet reached the threshold necessary for mass-market adoption, particularly in price-sensitive applications like electric vehicles and grid storage.
Electrolyte engineering approaches, while promising, face stability issues at high current densities. Conventional organic electrolytes decompose at the lithium metal interface, forming unstable SEI (Solid Electrolyte Interphase) layers that cannot effectively suppress dendrite nucleation. Ionic liquid-based electrolytes show improved thermal stability but often suffer from high viscosity and limited ionic conductivity, restricting their performance in fast-charging scenarios.
Physical barrier methods, including artificial SEI layers and structured current collectors, demonstrate limited long-term durability. These engineered interfaces typically degrade after repeated cycling, losing their dendrite suppression capabilities. Additionally, manufacturing these specialized interfaces at scale presents significant cost and process integration challenges that impede industrial adoption.
Pressure-based dendrite control strategies require complex mechanical systems that add weight, volume, and cost to battery designs. The non-uniform pressure distribution across large-format cells often results in inconsistent dendrite suppression, creating reliability concerns for commercial applications.
Temperature management approaches show promise in laboratory settings but translate poorly to real-world conditions where ambient temperatures fluctuate. The narrow operational window for optimal dendrite suppression conflicts with the broad temperature range required for practical applications, particularly in automotive and outdoor environments.
Current computational models for dendrite growth prediction lack sufficient accuracy for practical implementation. The complex electrochemical processes governing dendrite formation involve multiple variables that existing models cannot fully capture, limiting the effectiveness of predictive control strategies.
The integration of multiple dendrite control technologies often creates compatibility issues. Combining ionic liquid electrolytes with physical barriers or pressure systems introduces interface challenges that can actually accelerate dendrite formation at boundary regions. This system-level integration problem represents a significant hurdle for comprehensive dendrite control solutions.
Economic viability remains a critical challenge, as many promising dendrite control technologies require expensive materials or complex manufacturing processes. The cost-performance balance has not yet reached the threshold necessary for mass-market adoption, particularly in price-sensitive applications like electric vehicles and grid storage.
Current Ionic Liquid Solutions for Dendrite Suppression
01 Ionic liquid electrolytes for dendrite suppression
Ionic liquids can be used as electrolytes in lithium metal batteries to suppress dendrite formation. These non-flammable, low-volatility electrolytes create a stable solid electrolyte interphase (SEI) layer on the lithium metal surface, which helps to achieve uniform lithium deposition. The unique properties of ionic liquids, including high ionic conductivity and wide electrochemical windows, make them effective in controlling dendrite growth during charging and discharging cycles.- Ionic liquid electrolytes for dendrite suppression: Ionic liquids can be used as electrolytes in lithium metal batteries to suppress dendrite formation. These electrolytes have unique properties such as high ionic conductivity, low volatility, and wide electrochemical stability windows. The ionic liquid electrolytes create a stable solid electrolyte interphase (SEI) on the lithium metal surface, which helps to prevent dendrite growth and improve cycling stability of the battery.
- Ionic liquid additives in conventional electrolytes: Incorporating ionic liquids as additives in conventional electrolytes can effectively control lithium dendrite growth. These additives modify the properties of the electrolyte system, enhancing the uniformity of lithium deposition and reducing dendrite formation. The ionic liquid additives can be used in small concentrations to improve the performance and safety of lithium metal batteries without significantly altering other electrolyte properties.
- Ionic liquid-based composite electrolytes: Composite electrolytes combining ionic liquids with polymers, ceramic materials, or other components can effectively control dendrite growth in lithium metal batteries. These composite systems leverage the benefits of ionic liquids while addressing their limitations. The resulting electrolytes provide mechanical strength to physically block dendrites while maintaining good ionic conductivity and electrochemical stability, leading to improved battery safety and cycle life.
- Surface modification of lithium metal with ionic liquids: Treating the surface of lithium metal electrodes with ionic liquids can create protective layers that inhibit dendrite formation. These treatments modify the surface chemistry and morphology of the lithium metal, promoting uniform lithium deposition during cycling. The ionic liquid-derived protective layers help regulate the lithium ion flux and create a more stable interface between the electrode and electrolyte.
- Functionalized ionic liquids for enhanced dendrite control: Specially designed and functionalized ionic liquids can provide superior dendrite suppression in lithium metal batteries. These ionic liquids contain functional groups that interact specifically with lithium ions or the electrode surface to regulate deposition behavior. By tailoring the chemical structure of the ionic liquid components, researchers can optimize properties such as lithium ion transport, interfacial stability, and dendrite inhibition capabilities.
02 Ionic liquid additives in conventional electrolytes
Adding ionic liquids as additives to conventional carbonate-based or ether-based electrolytes can significantly improve dendrite suppression in lithium metal batteries. These additives modify the SEI composition and morphology, leading to more uniform lithium deposition. The synergistic effect between ionic liquids and conventional solvents enhances both the electrochemical performance and safety of lithium metal batteries by reducing dendrite formation without sacrificing ionic conductivity.Expand Specific Solutions03 Polymer-ionic liquid composite electrolytes
Combining ionic liquids with polymers creates composite electrolytes that effectively control lithium dendrite growth. These composites benefit from the mechanical strength of the polymer matrix and the electrochemical stability of ionic liquids. The polymer component physically blocks dendrite penetration while the ionic liquid ensures high lithium-ion conductivity. This approach results in safer lithium metal batteries with improved cycle life and reduced risk of short circuits caused by dendrite formation.Expand Specific Solutions04 Ionic liquid-based solid-state electrolytes
Solid-state electrolytes incorporating ionic liquids offer a promising solution for dendrite control in lithium metal batteries. These electrolytes combine the non-flammability and high electrochemical stability of ionic liquids with the mechanical robustness of solid-state systems. The high interfacial stability between the electrolyte and lithium metal anode prevents dendrite penetration, while maintaining good ionic conductivity at room temperature, addressing key challenges in lithium metal battery technology.Expand Specific Solutions05 Surface modification of lithium metal using ionic liquids
Treating lithium metal surfaces with ionic liquids creates protective layers that inhibit dendrite formation. This surface modification approach alters the lithium deposition behavior by forming a uniform and stable interface between the lithium metal and the electrolyte. The ionic liquid treatment can be applied either as a pre-treatment step or as an in-situ process during battery operation, resulting in smoother lithium deposition morphology and reduced dendrite growth over extended cycling.Expand Specific Solutions
Key Industry Players in Ionic Liquid Battery Research
The lithium metal battery market with ionic liquids for dendrite control is in a growth phase, characterized by increasing R&D investments and emerging commercial applications. The global market is projected to expand significantly as electric vehicle adoption accelerates, with estimates suggesting a multi-billion dollar opportunity by 2030. Technologically, the field shows moderate maturity with key players at different development stages. Leading automotive manufacturers (Honda, Nissan, Mitsubishi) are actively pursuing this technology to enhance battery safety and performance. Research institutions (KAIST, Wuhan University, Northwestern Polytechnical University) are advancing fundamental understanding, while specialized battery manufacturers (LG Energy Solution, GS Yuasa, SVOLT) are developing commercial implementations. Chemical companies (Solvay, Arkema, Nippon Shokubai) are providing essential materials support, creating a diverse ecosystem of stakeholders working to overcome dendrite formation challenges.
LG Energy Solution Ltd.
Technical Solution: LG Energy Solution has developed advanced ionic liquid-based electrolyte systems specifically designed to control lithium dendrite growth in lithium metal batteries. Their approach incorporates fluorinated ionic liquids with high electrochemical stability windows (>5V vs Li/Li+) and excellent thermal stability up to 300°C. The company's proprietary formulation combines pyrrolidinium-based cations with TFSI anions, creating a robust SEI (Solid Electrolyte Interphase) layer that effectively suppresses dendrite formation. Their technology employs dual-salt systems where ionic liquids are mixed with conventional lithium salts (LiPF6, LiFSI) in optimized ratios to maintain high ionic conductivity (>3 mS/cm at room temperature) while preventing uneven lithium deposition. LG's solution also incorporates nano-engineered additives that promote uniform Li+ flux across the electrode surface, resulting in smooth, dendrite-free lithium plating even at high current densities (>3 mA/cm²).
Strengths: Superior thermal stability and safety compared to conventional electrolytes; enables higher energy density (>400 Wh/kg) by supporting stable lithium metal anodes; excellent cycle life with >1000 cycles demonstrated at 80% capacity retention. Weaknesses: Higher production costs compared to conventional electrolytes; slightly lower ionic conductivity at low temperatures; requires specialized manufacturing processes for quality control.
Hydro-Québec
Technical Solution: Hydro-Québec has developed an innovative ionic liquid-based electrolyte system specifically engineered to address lithium dendrite growth in lithium metal batteries. Their approach centers on a novel class of phosphonium-based ionic liquids with asymmetric cation structures that promote uniform lithium deposition. The company's proprietary formulation combines these ionic liquids with lithium bis(fluorosulfonyl)imide (LiFSI) salt at optimized concentrations (0.8-1.2M) to create a stable and dendrite-resistant interface. Hydro-Québec's technology incorporates a dual-phase electrolyte architecture where the ionic liquid forms a protective layer directly on the lithium surface, while maintaining high bulk ionic conductivity (>2 mS/cm at room temperature). Their research has demonstrated that this approach creates a uniform and robust SEI layer approximately 20-30nm thick that effectively distributes lithium ions during plating and prevents the formation of high-surface-area dendritic structures. The company has validated this technology through extensive cycling tests showing stable performance over 300+ cycles with minimal capacity degradation and no dendrite-induced short circuits.
Strengths: Exceptional dendrite suppression even at high current densities (>2 mA/cm²); compatible with existing manufacturing processes; significantly improved safety profile with reduced thermal runaway risk. Weaknesses: Higher cost compared to conventional carbonate electrolytes; slightly lower ionic conductivity at sub-zero temperatures; requires careful moisture control during cell assembly.
Critical Patents and Research on Ionic Liquid Electrolytes
Dendrite-Free, Wide Temperature Range Lithium Metal Batteries Enabled by Hybrid Network Ionic Liquids
PatentPendingUS20240243356A1
Innovation
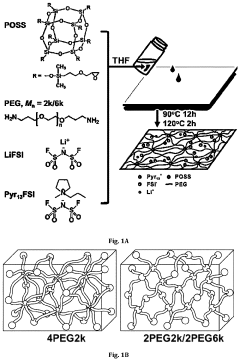
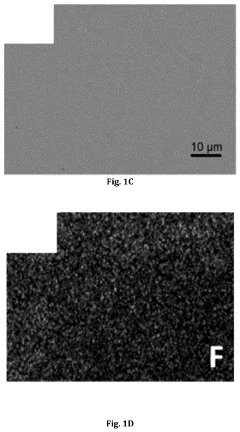
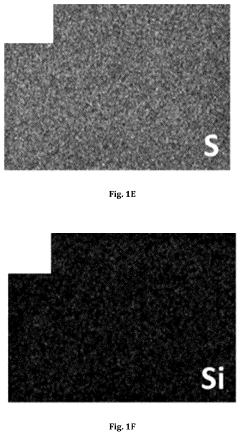
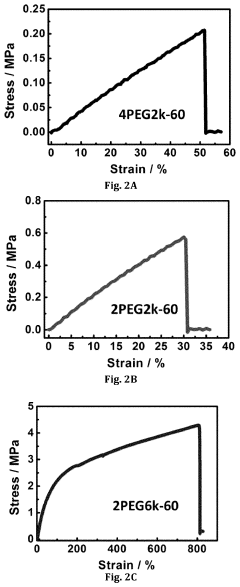
- A lithium gel polymer electrolyte composition is developed, featuring a crosslinked network formed by reacting inorganic polyhedral oligomeric silsesquioxane with functionalized poly(ethylene glycol or poly(ethylene oxide) and incorporating an ionic liquid and lithium salts, creating a mechanically stable hybrid network with enhanced ionic conductivity.
Electrolyte and Lithium Metal Electrode Comprising the Same
PatentActiveKR1020190124505A
Innovation
- An electrolyte for lithium metal batteries comprising an ionic liquid with a pyrrolidinium cation and bis(fluorosulfonyl)imide anion is used to inhibit dendrite growth by dispersing charge aggregation and forming a LiF-rich film on the lithium metal surface.
Safety and Performance Metrics for Ionic Liquid Electrolytes
The evaluation of ionic liquid electrolytes for lithium metal batteries requires comprehensive safety and performance metrics to ensure their viability in commercial applications. Safety considerations are paramount, with thermal stability being a critical factor. Ionic liquids demonstrate exceptional thermal stability, typically maintaining integrity at temperatures exceeding 300°C, significantly outperforming conventional carbonate-based electrolytes that decompose around 80-100°C. This enhanced thermal resistance substantially reduces thermal runaway risks in lithium metal batteries.
Flammability metrics represent another crucial safety parameter. Unlike traditional organic electrolytes with flash points below 30°C, most ionic liquids are non-flammable or exhibit flash points above 200°C. This characteristic dramatically improves the safety profile of lithium metal batteries, particularly in high-temperature environments or during mechanical abuse scenarios.
Electrochemical stability windows (ESWs) serve as both safety and performance indicators. High-quality ionic liquid electrolytes typically demonstrate ESWs of 4.0-5.5V, providing sufficient oxidative stability for high-voltage cathode materials while maintaining reductive stability at the lithium metal interface. This wide electrochemical window prevents parasitic reactions that could lead to thermal events or performance degradation.
From a performance perspective, ionic conductivity remains a critical metric, with values ranging from 0.1 to 10 mS/cm at room temperature depending on the ionic liquid composition. While this conductivity is generally lower than conventional liquid electrolytes, it can be optimized through molecular design and additives to achieve acceptable power performance.
Interfacial resistance measurements, particularly through electrochemical impedance spectroscopy, provide insights into the ionic liquid's ability to form stable solid electrolyte interphases (SEI) on lithium metal. Lower interfacial resistances correlate with improved dendrite suppression capabilities and enhanced cycling efficiency.
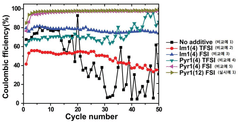
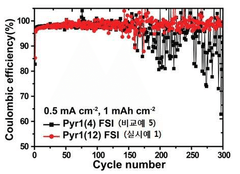
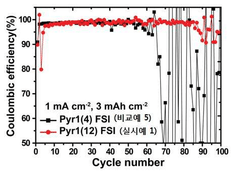
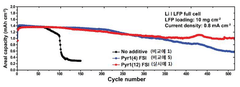
Coulombic efficiency serves as a definitive performance metric for lithium metal anodes, with state-of-the-art ionic liquid formulations achieving values exceeding 99.5% over hundreds of cycles. This high efficiency indicates minimal irreversible lithium consumption and correlates strongly with dendrite suppression capabilities.
Viscosity measurements, typically ranging from 20 to 500 cP at room temperature, impact both safety and performance aspects. While higher viscosity can mechanically suppress dendrite formation, excessive viscosity limits ion transport and power capability, necessitating careful optimization for specific application requirements.
Flammability metrics represent another crucial safety parameter. Unlike traditional organic electrolytes with flash points below 30°C, most ionic liquids are non-flammable or exhibit flash points above 200°C. This characteristic dramatically improves the safety profile of lithium metal batteries, particularly in high-temperature environments or during mechanical abuse scenarios.
Electrochemical stability windows (ESWs) serve as both safety and performance indicators. High-quality ionic liquid electrolytes typically demonstrate ESWs of 4.0-5.5V, providing sufficient oxidative stability for high-voltage cathode materials while maintaining reductive stability at the lithium metal interface. This wide electrochemical window prevents parasitic reactions that could lead to thermal events or performance degradation.
From a performance perspective, ionic conductivity remains a critical metric, with values ranging from 0.1 to 10 mS/cm at room temperature depending on the ionic liquid composition. While this conductivity is generally lower than conventional liquid electrolytes, it can be optimized through molecular design and additives to achieve acceptable power performance.
Interfacial resistance measurements, particularly through electrochemical impedance spectroscopy, provide insights into the ionic liquid's ability to form stable solid electrolyte interphases (SEI) on lithium metal. Lower interfacial resistances correlate with improved dendrite suppression capabilities and enhanced cycling efficiency.
Coulombic efficiency serves as a definitive performance metric for lithium metal anodes, with state-of-the-art ionic liquid formulations achieving values exceeding 99.5% over hundreds of cycles. This high efficiency indicates minimal irreversible lithium consumption and correlates strongly with dendrite suppression capabilities.
Viscosity measurements, typically ranging from 20 to 500 cP at room temperature, impact both safety and performance aspects. While higher viscosity can mechanically suppress dendrite formation, excessive viscosity limits ion transport and power capability, necessitating careful optimization for specific application requirements.
Scalability and Manufacturing Considerations for Commercial Implementation
The commercial implementation of ionic liquid technologies for dendrite control in lithium metal batteries faces significant manufacturing challenges that must be addressed to achieve market viability. Current production methods for ionic liquids typically involve small-batch synthesis in laboratory settings, which results in high costs ranging from $50-200 per kilogram—substantially higher than conventional electrolyte components. This cost barrier represents a major impediment to widespread adoption in commercial battery production.
Scaling up ionic liquid production requires the development of continuous flow processes to replace batch methods. Several chemical companies have begun implementing microreactor technologies that allow for more precise control of reaction conditions while increasing throughput. These advanced manufacturing approaches have demonstrated potential to reduce production costs by 40-60% at pilot scale, though further optimization is needed for full commercial implementation.
Integration of ionic liquids into existing battery manufacturing lines presents another critical challenge. The higher viscosity of many ionic liquid formulations may require modifications to electrode coating equipment and processes. Manufacturers must recalibrate coating parameters, drying protocols, and cell assembly procedures to accommodate these unique electrolyte properties while maintaining production speeds comparable to conventional systems.
Quality control represents a third major consideration, as ionic liquid purity significantly impacts electrochemical performance and dendrite suppression capabilities. Trace impurities, particularly water content, can dramatically alter ionic conductivity and electrochemical stability windows. Implementing inline analytical techniques such as spectroscopic methods for real-time purity assessment will be essential for maintaining consistent product quality at industrial scales.
Environmental and safety considerations must also be addressed in manufacturing scale-up. While ionic liquids generally exhibit lower volatility than conventional organic electrolytes, their synthesis often involves precursors that require careful handling. Developing green chemistry approaches that reduce solvent usage and hazardous waste generation will be crucial for sustainable large-scale production.
Economic viability ultimately depends on achieving cost parity with existing technologies while delivering superior performance. Current projections suggest that with optimized manufacturing processes and economies of scale, ionic liquid costs could potentially decrease to $15-30 per kilogram within 5-7 years. This price point, combined with the extended cycle life and improved safety profile they enable, could create a compelling value proposition for commercial implementation, particularly in premium battery applications where performance and safety command price premiums.
Scaling up ionic liquid production requires the development of continuous flow processes to replace batch methods. Several chemical companies have begun implementing microreactor technologies that allow for more precise control of reaction conditions while increasing throughput. These advanced manufacturing approaches have demonstrated potential to reduce production costs by 40-60% at pilot scale, though further optimization is needed for full commercial implementation.
Integration of ionic liquids into existing battery manufacturing lines presents another critical challenge. The higher viscosity of many ionic liquid formulations may require modifications to electrode coating equipment and processes. Manufacturers must recalibrate coating parameters, drying protocols, and cell assembly procedures to accommodate these unique electrolyte properties while maintaining production speeds comparable to conventional systems.
Quality control represents a third major consideration, as ionic liquid purity significantly impacts electrochemical performance and dendrite suppression capabilities. Trace impurities, particularly water content, can dramatically alter ionic conductivity and electrochemical stability windows. Implementing inline analytical techniques such as spectroscopic methods for real-time purity assessment will be essential for maintaining consistent product quality at industrial scales.
Environmental and safety considerations must also be addressed in manufacturing scale-up. While ionic liquids generally exhibit lower volatility than conventional organic electrolytes, their synthesis often involves precursors that require careful handling. Developing green chemistry approaches that reduce solvent usage and hazardous waste generation will be crucial for sustainable large-scale production.
Economic viability ultimately depends on achieving cost parity with existing technologies while delivering superior performance. Current projections suggest that with optimized manufacturing processes and economies of scale, ionic liquid costs could potentially decrease to $15-30 per kilogram within 5-7 years. This price point, combined with the extended cycle life and improved safety profile they enable, could create a compelling value proposition for commercial implementation, particularly in premium battery applications where performance and safety command price premiums.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!