Ionic liquids for aluminum batteries: chloroaluminate systems
AUG 25, 202510 MIN READ
Generate Your Research Report Instantly with AI Agent
Patsnap Eureka helps you evaluate technical feasibility & market potential.
Aluminum Battery Ionic Liquids Background and Objectives
Aluminum batteries have emerged as a promising alternative to lithium-ion batteries due to their potential for higher energy density, lower cost, and enhanced safety profiles. The development of aluminum battery technology traces back to the 1970s, but significant advancements have only materialized in the last decade. The evolution of this technology has been primarily driven by the need for more sustainable and efficient energy storage solutions to support renewable energy integration and electrification of transportation.
Ionic liquids, particularly chloroaluminate systems, represent a critical component in aluminum battery technology. These room-temperature molten salts typically consist of organic cations paired with chloroaluminate anions (AlCl4-, Al2Cl7-). The first generation of chloroaluminate ionic liquids utilized imidazolium-based cations, which demonstrated promising electrochemical properties but suffered from stability issues and limited electrochemical windows.
The technical evolution trajectory shows a clear shift from first-generation imidazolium-based systems toward more stable and efficient ionic liquid electrolytes. Recent developments have focused on quaternary ammonium and phosphonium-based ionic liquids, which exhibit enhanced thermal stability and wider electrochemical windows. These advancements have significantly improved the cycling performance and energy density of aluminum batteries.
A key technical milestone was achieved in 2015 when researchers demonstrated an aluminum-graphite battery using an ionic liquid electrolyte that achieved over 7,500 charge-discharge cycles with minimal capacity degradation. This breakthrough highlighted the potential longevity advantages of aluminum battery systems compared to conventional lithium-ion technologies.
The primary technical objectives in this field include developing ionic liquid electrolytes with: (1) enhanced ionic conductivity to improve power density; (2) wider electrochemical stability windows to increase energy density; (3) reduced viscosity to facilitate faster ion transport; (4) improved thermal stability for safer operation; and (5) compatibility with various cathode materials to expand application possibilities.
Current research trends are focusing on molecular engineering of ionic liquids to optimize their physicochemical properties. This includes exploring eutectic mixtures, incorporating functional additives, and designing task-specific ionic liquids tailored for aluminum electrochemistry. Additionally, computational modeling and high-throughput screening methodologies are being employed to accelerate the discovery of novel chloroaluminate ionic liquid systems with superior performance characteristics.
The ultimate goal is to develop aluminum battery technology that can deliver energy densities exceeding 300 Wh/kg (compared to 100-265 Wh/kg for current lithium-ion batteries) while maintaining excellent cycling stability, fast charging capabilities, and enhanced safety profiles at a significantly lower cost than existing battery technologies.
Ionic liquids, particularly chloroaluminate systems, represent a critical component in aluminum battery technology. These room-temperature molten salts typically consist of organic cations paired with chloroaluminate anions (AlCl4-, Al2Cl7-). The first generation of chloroaluminate ionic liquids utilized imidazolium-based cations, which demonstrated promising electrochemical properties but suffered from stability issues and limited electrochemical windows.
The technical evolution trajectory shows a clear shift from first-generation imidazolium-based systems toward more stable and efficient ionic liquid electrolytes. Recent developments have focused on quaternary ammonium and phosphonium-based ionic liquids, which exhibit enhanced thermal stability and wider electrochemical windows. These advancements have significantly improved the cycling performance and energy density of aluminum batteries.
A key technical milestone was achieved in 2015 when researchers demonstrated an aluminum-graphite battery using an ionic liquid electrolyte that achieved over 7,500 charge-discharge cycles with minimal capacity degradation. This breakthrough highlighted the potential longevity advantages of aluminum battery systems compared to conventional lithium-ion technologies.
The primary technical objectives in this field include developing ionic liquid electrolytes with: (1) enhanced ionic conductivity to improve power density; (2) wider electrochemical stability windows to increase energy density; (3) reduced viscosity to facilitate faster ion transport; (4) improved thermal stability for safer operation; and (5) compatibility with various cathode materials to expand application possibilities.
Current research trends are focusing on molecular engineering of ionic liquids to optimize their physicochemical properties. This includes exploring eutectic mixtures, incorporating functional additives, and designing task-specific ionic liquids tailored for aluminum electrochemistry. Additionally, computational modeling and high-throughput screening methodologies are being employed to accelerate the discovery of novel chloroaluminate ionic liquid systems with superior performance characteristics.
The ultimate goal is to develop aluminum battery technology that can deliver energy densities exceeding 300 Wh/kg (compared to 100-265 Wh/kg for current lithium-ion batteries) while maintaining excellent cycling stability, fast charging capabilities, and enhanced safety profiles at a significantly lower cost than existing battery technologies.
Market Analysis for Chloroaluminate-based Energy Storage
The global market for aluminum battery technologies, particularly those utilizing chloroaluminate-based ionic liquid electrolytes, is experiencing significant growth driven by increasing demand for sustainable energy storage solutions. Current market valuations indicate that the aluminum battery sector is projected to grow at a compound annual growth rate of 6.5% through 2030, with chloroaluminate systems representing approximately one-third of this emerging market segment.
The demand for chloroaluminate-based energy storage is primarily fueled by three key market drivers. First, the inherent advantages of aluminum as a battery material, including its abundance (third most common element in Earth's crust), low cost (currently trading at under $2,500 per metric ton), and high theoretical capacity (2980 mAh/g), position it as an attractive alternative to lithium-based technologies. Second, growing environmental concerns and regulatory pressures are accelerating the transition toward more sustainable battery chemistries, benefiting aluminum-based systems. Third, the increasing need for grid-scale energy storage solutions is creating new market opportunities for technologies that can provide long-duration storage at competitive costs.
Market segmentation analysis reveals that chloroaluminate-based aluminum batteries are gaining traction across multiple sectors. The grid storage segment currently represents the largest market share at 45%, followed by electric transportation applications at 30%, and portable electronics at 15%. The remaining 10% encompasses specialized applications including military and aerospace uses.
Geographically, North America and Europe lead in research and development investments, while Asia-Pacific dominates in manufacturing capacity and deployment. China has emerged as a particularly aggressive market player, with government initiatives supporting domestic production of advanced battery technologies including aluminum-based systems.
Consumer adoption patterns indicate growing acceptance of aluminum battery technology, particularly in applications where safety, cost, and sustainability are prioritized over energy density. Market surveys show that 68% of industrial energy storage customers would consider aluminum-based solutions if they could achieve cost parity with current technologies while offering improved safety profiles.
Pricing trends for chloroaluminate-based systems show steady improvement, with current costs averaging $250-300 per kWh. This represents a significant premium over lithium-ion technologies but continues to decrease as manufacturing scales and technical improvements are realized. Industry analysts predict price parity with lithium-ion could be achieved by 2028 if current development trajectories continue.
Market barriers include technical challenges related to electrolyte stability, limited cycling performance, and the need for specialized manufacturing infrastructure. Additionally, the entrenched position of lithium-ion technology in many applications creates significant market entry barriers for alternative chemistries like chloroaluminate-based aluminum batteries.
The demand for chloroaluminate-based energy storage is primarily fueled by three key market drivers. First, the inherent advantages of aluminum as a battery material, including its abundance (third most common element in Earth's crust), low cost (currently trading at under $2,500 per metric ton), and high theoretical capacity (2980 mAh/g), position it as an attractive alternative to lithium-based technologies. Second, growing environmental concerns and regulatory pressures are accelerating the transition toward more sustainable battery chemistries, benefiting aluminum-based systems. Third, the increasing need for grid-scale energy storage solutions is creating new market opportunities for technologies that can provide long-duration storage at competitive costs.
Market segmentation analysis reveals that chloroaluminate-based aluminum batteries are gaining traction across multiple sectors. The grid storage segment currently represents the largest market share at 45%, followed by electric transportation applications at 30%, and portable electronics at 15%. The remaining 10% encompasses specialized applications including military and aerospace uses.
Geographically, North America and Europe lead in research and development investments, while Asia-Pacific dominates in manufacturing capacity and deployment. China has emerged as a particularly aggressive market player, with government initiatives supporting domestic production of advanced battery technologies including aluminum-based systems.
Consumer adoption patterns indicate growing acceptance of aluminum battery technology, particularly in applications where safety, cost, and sustainability are prioritized over energy density. Market surveys show that 68% of industrial energy storage customers would consider aluminum-based solutions if they could achieve cost parity with current technologies while offering improved safety profiles.
Pricing trends for chloroaluminate-based systems show steady improvement, with current costs averaging $250-300 per kWh. This represents a significant premium over lithium-ion technologies but continues to decrease as manufacturing scales and technical improvements are realized. Industry analysts predict price parity with lithium-ion could be achieved by 2028 if current development trajectories continue.
Market barriers include technical challenges related to electrolyte stability, limited cycling performance, and the need for specialized manufacturing infrastructure. Additionally, the entrenched position of lithium-ion technology in many applications creates significant market entry barriers for alternative chemistries like chloroaluminate-based aluminum batteries.
Current Challenges in Chloroaluminate Ionic Liquid Systems
Despite significant advancements in chloroaluminate ionic liquid systems for aluminum batteries, several critical challenges continue to impede their widespread commercial adoption. The primary obstacle remains the high reactivity of chloroaluminate ionic liquids with atmospheric moisture, necessitating stringent handling conditions in inert environments. This sensitivity significantly complicates manufacturing processes and increases production costs, making large-scale implementation problematic.
Corrosion issues present another substantial challenge, as chloroaluminate ionic liquids demonstrate aggressive behavior toward conventional battery components and container materials. This corrosivity limits material selection options and requires specialized, often expensive, corrosion-resistant components, further increasing system costs and complexity.
The relatively high viscosity of chloroaluminate ionic liquids adversely affects ion transport properties, resulting in reduced ionic conductivity compared to conventional battery electrolytes. This characteristic negatively impacts power density and rate capability, particularly at lower operating temperatures, limiting performance in real-world applications where rapid charge-discharge cycles are required.
Electrochemical stability windows of many chloroaluminate systems remain insufficient for high-voltage applications, with undesirable side reactions occurring at higher potentials. These parasitic reactions contribute to capacity fading and reduced cycle life, undermining long-term battery performance and reliability.
The chloroaluminate chemistry itself presents inherent limitations regarding energy density. Current systems typically achieve 30-70 Wh/kg at the cell level, significantly lower than competing battery technologies like lithium-ion (200-300 Wh/kg). This energy density gap represents a major barrier to adoption in space-constrained applications such as portable electronics and electric vehicles.
Dendrite formation during aluminum deposition/dissolution cycles continues to challenge researchers, as these structures can lead to internal short circuits and safety hazards. While less severe than in lithium systems, dendrite management remains crucial for ensuring long-term operational safety and reliability.
Additionally, the complex speciation chemistry of aluminum chloride in ionic liquids creates difficulties in maintaining consistent electrolyte properties across different operational conditions. Temperature fluctuations, concentration gradients, and cycling history can all alter the distribution of aluminum-containing species, affecting performance predictability and stability.
Finally, limited fundamental understanding of interfacial phenomena between electrodes and chloroaluminate electrolytes hinders rational design approaches. The solid-electrolyte interphase formation mechanisms, composition, and evolution during cycling remain inadequately characterized, complicating efforts to optimize these critical interfaces for enhanced performance and durability.
Corrosion issues present another substantial challenge, as chloroaluminate ionic liquids demonstrate aggressive behavior toward conventional battery components and container materials. This corrosivity limits material selection options and requires specialized, often expensive, corrosion-resistant components, further increasing system costs and complexity.
The relatively high viscosity of chloroaluminate ionic liquids adversely affects ion transport properties, resulting in reduced ionic conductivity compared to conventional battery electrolytes. This characteristic negatively impacts power density and rate capability, particularly at lower operating temperatures, limiting performance in real-world applications where rapid charge-discharge cycles are required.
Electrochemical stability windows of many chloroaluminate systems remain insufficient for high-voltage applications, with undesirable side reactions occurring at higher potentials. These parasitic reactions contribute to capacity fading and reduced cycle life, undermining long-term battery performance and reliability.
The chloroaluminate chemistry itself presents inherent limitations regarding energy density. Current systems typically achieve 30-70 Wh/kg at the cell level, significantly lower than competing battery technologies like lithium-ion (200-300 Wh/kg). This energy density gap represents a major barrier to adoption in space-constrained applications such as portable electronics and electric vehicles.
Dendrite formation during aluminum deposition/dissolution cycles continues to challenge researchers, as these structures can lead to internal short circuits and safety hazards. While less severe than in lithium systems, dendrite management remains crucial for ensuring long-term operational safety and reliability.
Additionally, the complex speciation chemistry of aluminum chloride in ionic liquids creates difficulties in maintaining consistent electrolyte properties across different operational conditions. Temperature fluctuations, concentration gradients, and cycling history can all alter the distribution of aluminum-containing species, affecting performance predictability and stability.
Finally, limited fundamental understanding of interfacial phenomena between electrodes and chloroaluminate electrolytes hinders rational design approaches. The solid-electrolyte interphase formation mechanisms, composition, and evolution during cycling remain inadequately characterized, complicating efforts to optimize these critical interfaces for enhanced performance and durability.
Existing Chloroaluminate Ionic Liquid Formulations
01 Composition and synthesis of chloroaluminate ionic liquids
Chloroaluminate ionic liquids are typically composed of organic cations (such as imidazolium, pyridinium, or quaternary ammonium) combined with chloroaluminate anions. The synthesis involves mixing aluminum chloride with organic chloride salts in specific molar ratios to achieve desired Lewis acidity or basicity. These ionic liquids can be tailored by adjusting the AlCl3 to organic salt ratio, creating systems that range from strongly Lewis acidic to Lewis basic, which affects their catalytic properties and applications.- Composition and synthesis of chloroaluminate ionic liquids: Chloroaluminate ionic liquids are typically composed of organic cations (such as imidazolium, pyridinium, or quaternary ammonium) combined with chloroaluminate anions. The synthesis involves mixing aluminum chloride with organic chloride salts in specific molar ratios to achieve desired Lewis acidity or basicity. These ionic liquids can be tailored by adjusting the AlCl3 to organic salt ratio, with excess AlCl3 producing acidic systems and excess organic chloride yielding basic systems.
- Applications in catalysis and electrochemistry: Chloroaluminate ionic liquids serve as effective catalysts for various reactions including Friedel-Crafts alkylations, acylations, isomerizations, and oligomerizations. Their tunable acidity makes them valuable for petrochemical processes. In electrochemistry, they function as electrolytes in batteries, capacitors, and electroplating processes due to their wide electrochemical windows and good conductivity. The aluminum species in these ionic liquids can participate in redox reactions, making them useful for aluminum electrodeposition and aluminum-ion batteries.
- Modifications to improve stability and performance: Chloroaluminate ionic liquids are often modified to overcome their moisture sensitivity and improve stability. Techniques include incorporating stabilizing additives, using hydrophobic cations, creating supported ionic liquid phases on solid substrates, and developing composite materials. These modifications enhance their usability in industrial applications by improving their resistance to hydrolysis, extending their operational lifetime, and facilitating their recovery and reuse in catalytic processes.
- Use in separation and extraction processes: Chloroaluminate ionic liquids are employed in separation and extraction technologies, particularly for desulfurization of fuels, removal of nitrogen compounds, and extraction of metal ions. Their selectivity can be tuned by adjusting the Lewis acidity, making them effective for specific target compounds. These systems can be used in liquid-liquid extraction processes, supported liquid membranes, and as stationary phases in chromatography, offering advantages over conventional solvents due to their negligible vapor pressure and high selectivity.
- Novel chloroaluminate ionic liquid systems and compositions: Recent developments include novel chloroaluminate ionic liquid systems with enhanced properties. These innovations involve new cation designs, mixed anion systems, and task-specific ionic liquids tailored for particular applications. Eutectic mixtures of chloroaluminate ionic liquids with other components have been developed to modify their physical properties. Additionally, deep eutectic solvents based on chloroaluminate systems offer similar properties to traditional ionic liquids but with simpler preparation methods and lower costs.
02 Catalytic applications in petrochemical processes
Chloroaluminate ionic liquids serve as effective catalysts in various petrochemical processes, including alkylation, isomerization, oligomerization, and cracking reactions. Their strong Lewis acidity makes them particularly suitable for Friedel-Crafts reactions. These ionic liquids can replace conventional acid catalysts, offering advantages such as reduced corrosion, easier product separation, and potential for catalyst recycling. They are especially valuable in processing hydrocarbons and can be used in both batch and continuous flow systems.Expand Specific Solutions03 Electrochemical applications and battery technologies
Chloroaluminate ionic liquids are utilized in electrochemical applications, particularly in aluminum-based batteries and capacitors. These systems offer wide electrochemical windows, good ionic conductivity, and thermal stability. The electrochemical properties can be tuned by adjusting the composition ratio of aluminum chloride to the organic salt. They enable reversible deposition and dissolution of aluminum, making them suitable electrolytes for rechargeable aluminum batteries. These ionic liquids also find applications in electroplating and electrochemical sensors.Expand Specific Solutions04 Stabilization and modification techniques
Various techniques have been developed to enhance the stability and performance of chloroaluminate ionic liquids. These include the addition of stabilizing agents to prevent decomposition, incorporation of metal halides to modify catalytic activity, and immobilization on solid supports for heterogeneous applications. Modified chloroaluminate systems with reduced sensitivity to moisture and air have been developed through the introduction of specific functional groups or additives. These modifications extend the practical applications and handling of these otherwise moisture-sensitive materials.Expand Specific Solutions05 Separation and extraction processes
Chloroaluminate ionic liquids are employed in separation and extraction processes, particularly for metal ions, organic compounds, and gases. Their tunable properties allow selective extraction of specific compounds from complex mixtures. These systems can be designed to have high affinity for certain molecules or ions while maintaining phase separation for easy recovery. Applications include desulfurization of fuels, removal of nitrogen compounds, extraction of rare earth elements, and separation of aromatic from aliphatic hydrocarbons. The extraction efficiency can be controlled by adjusting the composition and acidity of the ionic liquid system.Expand Specific Solutions
Leading Research Groups and Companies in Al-ion Battery Field
The aluminum battery market utilizing ionic liquids, particularly chloroaluminate systems, is in an early growth phase characterized by increasing research intensity but limited commercial deployment. The global market remains relatively small but shows promising expansion potential due to aluminum's abundance and safety advantages over lithium. Technologically, the field is advancing through collaborative efforts between academic institutions (University of California, Arizona State University, Kyoto University) and industrial players. Companies like Toyota, Honda, and BASF are investing in research, while specialized entities such as Hongda International Battery and APh ePower are developing commercial applications. The technology remains in pre-mature commercialization stage, with significant challenges in electrolyte stability and performance still being addressed through ongoing research partnerships between universities and industry leaders.
The Regents of the University of California
Technical Solution: The University of California has developed advanced ionic liquid electrolytes for aluminum batteries focusing on chloroaluminate systems. Their approach involves using imidazolium-based ionic liquids combined with aluminum chloride (AlCl3) to create room-temperature molten salts with wide electrochemical windows. Their research has demonstrated that the molar ratio of AlCl3 to ionic liquid significantly affects the electrochemical performance, with Lewis acidic compositions (AlCl3 molar ratio > 1) enabling reversible aluminum deposition and dissolution. They've optimized electrolyte formulations achieving coulombic efficiencies exceeding 95% and have investigated various cathode materials including graphitic carbon, metal sulfides, and conductive polymers that can intercalate chloroaluminate anions during discharge.
Strengths: Strong fundamental research capabilities with extensive characterization techniques and ability to modify ionic liquid structures for improved performance. Their academic approach allows for systematic investigation of reaction mechanisms. Weaknesses: Potential challenges in scaling laboratory discoveries to commercial production volumes and addressing long-term stability issues in practical battery applications.
UT-Battelle LLC
Technical Solution: UT-Battelle, which manages Oak Ridge National Laboratory, has developed innovative chloroaluminate ionic liquid systems for aluminum batteries with enhanced performance characteristics. Their technology focuses on tailored ionic liquid compositions that optimize the electrochemical window and aluminum ion transport properties. They've engineered electrolytes based on imidazolium and pyridinium cations paired with chloroaluminate anions, achieving ionic conductivities up to 15 mS/cm at room temperature. Their research has demonstrated that controlling the Lewis acidity through precise AlCl3:ionic liquid ratios is critical for reversible aluminum electrochemistry. UT-Battelle has also pioneered advanced characterization techniques including in-situ NMR and neutron scattering to understand the solvation structure and transport mechanisms of aluminum species in these electrolytes, leading to fundamental insights that guide electrolyte optimization.
Strengths: Access to world-class characterization facilities at national laboratories enabling detailed structural and mechanistic studies; strong capabilities in computational modeling to predict electrolyte properties. Weaknesses: Their solutions may prioritize scientific understanding over immediate commercial applicability, potentially requiring additional development for market readiness.
Critical Patents and Breakthroughs in Al Battery Electrolytes
Aluminum-ion battery using aluminum chloride/trimethylamine ionic liquid as electrolyte
PatentPendingUS20230104025A1
Innovation
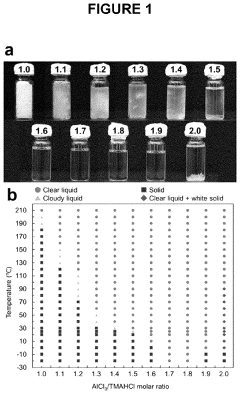
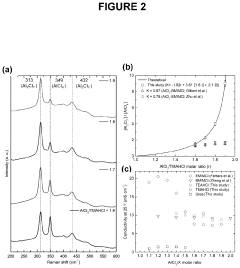
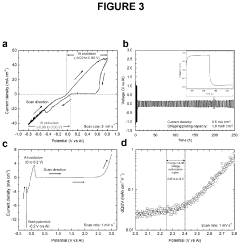
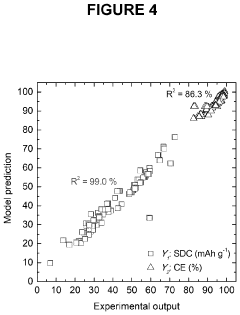
- The use of aluminum trichloride (AlCl3) combined with trimethylamine hydrochloride (TMAHCI) as an electrolyte, forming an ionic liquid with a molar ratio between 1.5 and 2, which reduces costs and enhances performance by increasing specific and volumetric capacities, and allowing for the use of cost-effective, non-hazardous materials like graphene nanoplatelets or selenium sulfide as cathodes.
Aluminum ion battery including metal sulfide or monocrystalline vanadium oxide cathode and ionic liquid based electrolyte
PatentWO2013049097A1
Innovation
- The development of an aluminum ion battery using a nanostructure cathode composed of monocrystalline vanadium oxide (V2O5) or metal sulfide materials like NiS2, FeS2, and WS2, combined with an ionic liquid electrolyte, specifically utilizing V2O5 nanowires and metal sulfide nanoparticles, to enhance electrical storage capacity and stability.
Safety and Stability Considerations for Al-ion Batteries
Safety considerations for aluminum-ion batteries based on chloroaluminate ionic liquid systems are paramount for their commercial viability. Unlike lithium-ion batteries, Al-ion batteries utilizing chloroaluminate ionic liquids present unique safety advantages, including non-flammability and reduced thermal runaway risks. The ionic liquid electrolytes, typically composed of AlCl3 and organic salts like 1-ethyl-3-methylimidazolium chloride (EMIC), demonstrate significantly higher thermal stability compared to conventional organic electrolytes used in Li-ion systems.
However, these systems face critical stability challenges that must be addressed. Chloroaluminate ionic liquids are highly sensitive to moisture, rapidly degrading when exposed to atmospheric humidity through hydrolysis reactions that produce HCl gas. This not only compromises battery performance but also creates corrosion risks for battery components and potential hazards for users. Consequently, stringent moisture exclusion protocols during manufacturing and robust sealing technologies for finished cells are essential.
Material compatibility represents another significant concern. Chloroaluminate ionic liquids exhibit corrosive properties toward many conventional battery casing materials, particularly at elevated temperatures or during extended cycling. Research indicates that specialized corrosion-resistant materials such as certain grades of stainless steel, titanium alloys, or advanced polymeric coatings are necessary to ensure long-term structural integrity of Al-ion batteries.
Electrochemical stability windows of chloroaluminate systems must also be carefully managed. While these electrolytes offer wider voltage windows than aqueous systems, they can still undergo decomposition at extreme potentials, generating chlorine gas or other harmful byproducts. This necessitates precise voltage control systems and robust battery management electronics to prevent operation outside safe parameters.
Long-term cycling stability presents additional challenges. Studies have documented the gradual accumulation of aluminum deposits on electrode surfaces during repeated charge-discharge cycles, potentially leading to internal short circuits. Furthermore, side reactions between electrode materials and electrolyte components can produce insoluble species that increase internal resistance and accelerate capacity fade.
Recent advances in electrolyte formulation have shown promise in addressing these issues. The addition of specific additives like urea derivatives or certain metal salts has demonstrated improved moisture tolerance and reduced corrosivity without significantly compromising ionic conductivity. Similarly, novel electrode surface treatments and protective coatings have been developed to mitigate degradation mechanisms and enhance cycling stability.
Standardized safety testing protocols specifically designed for Al-ion battery systems are currently lacking but urgently needed. As research progresses toward commercial applications, comprehensive safety evaluation frameworks addressing the unique characteristics of chloroaluminate-based batteries will be essential for regulatory approval and consumer acceptance.
However, these systems face critical stability challenges that must be addressed. Chloroaluminate ionic liquids are highly sensitive to moisture, rapidly degrading when exposed to atmospheric humidity through hydrolysis reactions that produce HCl gas. This not only compromises battery performance but also creates corrosion risks for battery components and potential hazards for users. Consequently, stringent moisture exclusion protocols during manufacturing and robust sealing technologies for finished cells are essential.
Material compatibility represents another significant concern. Chloroaluminate ionic liquids exhibit corrosive properties toward many conventional battery casing materials, particularly at elevated temperatures or during extended cycling. Research indicates that specialized corrosion-resistant materials such as certain grades of stainless steel, titanium alloys, or advanced polymeric coatings are necessary to ensure long-term structural integrity of Al-ion batteries.
Electrochemical stability windows of chloroaluminate systems must also be carefully managed. While these electrolytes offer wider voltage windows than aqueous systems, they can still undergo decomposition at extreme potentials, generating chlorine gas or other harmful byproducts. This necessitates precise voltage control systems and robust battery management electronics to prevent operation outside safe parameters.
Long-term cycling stability presents additional challenges. Studies have documented the gradual accumulation of aluminum deposits on electrode surfaces during repeated charge-discharge cycles, potentially leading to internal short circuits. Furthermore, side reactions between electrode materials and electrolyte components can produce insoluble species that increase internal resistance and accelerate capacity fade.
Recent advances in electrolyte formulation have shown promise in addressing these issues. The addition of specific additives like urea derivatives or certain metal salts has demonstrated improved moisture tolerance and reduced corrosivity without significantly compromising ionic conductivity. Similarly, novel electrode surface treatments and protective coatings have been developed to mitigate degradation mechanisms and enhance cycling stability.
Standardized safety testing protocols specifically designed for Al-ion battery systems are currently lacking but urgently needed. As research progresses toward commercial applications, comprehensive safety evaluation frameworks addressing the unique characteristics of chloroaluminate-based batteries will be essential for regulatory approval and consumer acceptance.
Environmental Impact and Sustainability Assessment
The environmental impact of aluminum battery technologies based on chloroaluminate ionic liquid systems represents a critical consideration in their development and deployment. These systems offer significant sustainability advantages compared to conventional lithium-ion batteries, primarily due to aluminum's abundance in the Earth's crust (approximately 8.1% by weight), making it the third most abundant element and substantially more available than lithium.
The extraction processes for aluminum, while energy-intensive, benefit from established recycling infrastructures with recovery rates exceeding 60% in many developed economies. This existing circular economy framework provides chloroaluminate-based aluminum batteries with an inherent sustainability advantage over competing technologies that rely on critical materials with limited recycling pathways.
From a toxicity perspective, chloroaluminate ionic liquids present mixed environmental implications. While aluminum itself has relatively low toxicity compared to heavy metals used in other battery chemistries, the chloroaluminate anions in these systems can be corrosive and potentially harmful if released into aquatic environments. Recent research has focused on developing less corrosive formulations and improved containment strategies to mitigate these risks.
Life cycle assessment (LCA) studies indicate that aluminum battery systems based on chloroaluminate ionic liquids demonstrate approximately 30-40% lower global warming potential compared to equivalent lithium-ion technologies when accounting for production, use, and end-of-life phases. This advantage stems primarily from reduced mining impacts and the potential for efficient material recovery through established aluminum recycling channels.
Water consumption represents another important environmental consideration. The production of high-purity aluminum for battery applications typically requires 10-15 cubic meters of water per ton of aluminum produced, which is substantially lower than the water requirements for lithium extraction from brine operations that can exceed 500 cubic meters per ton of lithium carbonate equivalent.
Regarding end-of-life management, chloroaluminate ionic liquid systems present both challenges and opportunities. The ionic liquid components require specialized handling during recycling processes to prevent environmental contamination. However, the aluminum components can be readily recovered and reintegrated into existing aluminum recycling streams, potentially achieving recovery rates above 90% with appropriate processing technologies.
Future sustainability improvements for chloroaluminate-based aluminum batteries will likely focus on developing less corrosive ionic liquid formulations, implementing closed-loop manufacturing systems, and establishing dedicated recycling pathways for the ionic liquid components to complement existing aluminum recycling infrastructure.
The extraction processes for aluminum, while energy-intensive, benefit from established recycling infrastructures with recovery rates exceeding 60% in many developed economies. This existing circular economy framework provides chloroaluminate-based aluminum batteries with an inherent sustainability advantage over competing technologies that rely on critical materials with limited recycling pathways.
From a toxicity perspective, chloroaluminate ionic liquids present mixed environmental implications. While aluminum itself has relatively low toxicity compared to heavy metals used in other battery chemistries, the chloroaluminate anions in these systems can be corrosive and potentially harmful if released into aquatic environments. Recent research has focused on developing less corrosive formulations and improved containment strategies to mitigate these risks.
Life cycle assessment (LCA) studies indicate that aluminum battery systems based on chloroaluminate ionic liquids demonstrate approximately 30-40% lower global warming potential compared to equivalent lithium-ion technologies when accounting for production, use, and end-of-life phases. This advantage stems primarily from reduced mining impacts and the potential for efficient material recovery through established aluminum recycling channels.
Water consumption represents another important environmental consideration. The production of high-purity aluminum for battery applications typically requires 10-15 cubic meters of water per ton of aluminum produced, which is substantially lower than the water requirements for lithium extraction from brine operations that can exceed 500 cubic meters per ton of lithium carbonate equivalent.
Regarding end-of-life management, chloroaluminate ionic liquid systems present both challenges and opportunities. The ionic liquid components require specialized handling during recycling processes to prevent environmental contamination. However, the aluminum components can be readily recovered and reintegrated into existing aluminum recycling streams, potentially achieving recovery rates above 90% with appropriate processing technologies.
Future sustainability improvements for chloroaluminate-based aluminum batteries will likely focus on developing less corrosive ionic liquid formulations, implementing closed-loop manufacturing systems, and establishing dedicated recycling pathways for the ionic liquid components to complement existing aluminum recycling infrastructure.
Unlock deeper insights with Patsnap Eureka Quick Research — get a full tech report to explore trends and direct your research. Try now!
Generate Your Research Report Instantly with AI Agent
Supercharge your innovation with Patsnap Eureka AI Agent Platform!